Abstract
With the increasing environmental issues caused by sulfur compound emissions from fossil fuels, the development of green and efficient desulfurization technologies has become a research hotspot. Deep eutectic solvents (DESs), as emerging low-cost, customizable green solvents, show great potential in fuel desulfurization. This article provides an in-depth analysis of the desulfurization mechanism of DESs, including hydrogen bonding, enhanced π–π and CH–π interactions, and synergistic effects of metal ions 12. In addition, oxidation–extraction coupling strategies and process enhancement techniques such as ultrasonic-assisted extraction and microchannel mass transfer further improve desulfurization efficiency. In addition, oxidation–extraction coupling strategies and process enhancement techniques (such as ultrasonic-assisted extraction and microchannel mass transfer) further improve desulfurization efficiency. Overall, DES has broad application prospects in fuel extraction desulfurization and is expected to serve as an alternative or complementary method to hydrodesulfurization technology.
Graphical abstract
1 Introduction
The global environmental air pollution problem is becoming increasingly serious, and people are continuously striving for high-quality energy. When burned, fuel sulfur compounds release SO x . These emissions cause acid rain and photochemical smog and contribute to PM2.5 formation [1–5]. According to the World Health Organization (WHO), approximately 91% of the global population is exposed to harmful levels of air pollution over the long term, with sulfur pollution contributing to 23% of this total [6,7]. Reducing the sulfur content of fuels is one of the important methods to decrease sulfur oxides in the atmosphere [8]. Fuels produced through fluid catalytic cracking (FCC) of crude oil contain a considerable amount of sulfur in the form of thiophene sulfur, mercaptans, and sulfides [9–11]. Among them, thiophene-type sulfur compounds account for the majority of the total sulfur content in FCC fuels. Desulfurization of fuels requires the deep processing of fuel, disrupting the original structure of sulfur-containing compounds, altering their properties, and producing sulfur-containing solid, liquid, or gaseous substances that are easier to handle [12–14]. The key to deep desulfurization of fuel is to efficiently remove thiophene-type sulfur compounds in an appropriate manner. In this context, desulfurization technologies such as hydrodesulfurization (HDS) and green solvent-based methods (e.g., deep eutectic solvents [DESs]) have been widely explored, yet critical limitations remain to be addressed.
As a critical pathway to obtain clean fuels, existing desulfurization methods primarily fall into two categories: HDS and non-hydrodesulfurization (NHDS) technologies. Although significant progress has been made with conventional approaches, their industrial applicability remains constrained by persistent technical and economic challenges, as detailed below.
2 Fuel desulfurization technologies
Currently, desulfurization technologies for fuels are mainly divided into HDS and NHDS methods.
HDS refers to the method in which organic sulfur in fuel reacts with hydrogen gas under the action of a hydrogenation catalyst to generate hydrogen sulfide, thereby removing sulfur [15–17]. Traditional HDS can eliminate sulfur-containing compounds, but for cyclic organic sulfur compounds such as thiophene (T), benzothiophene (BT), dibenzothiophene (DBT), and their derivatives, the effectiveness of HDS is not significant [18–20]. Additionally, this process requires a high-temperature heat source of over 330°C and expensive cobalt–molybdenum catalysts [21]. The equipment investment is substantial, operational costs are high, and a considerable amount of hydrogen is needed, making it difficult to recycle and limiting its application scope [22,23].
NHDS technologies mainly include extraction desulfurization (EDS), oxidation desulfurization (ODS), adsorption desulfurization (ADS), and biological desulfurization (BDS), among others [15].
EDS is a method that separates sulfur compounds from fuel using the different solubilities of sulfides and fuel in an extractant [15]. The reaction conditions of EDS are mild, and the extractant containing organic sulfur compounds can be recycled and reused. The EDS process conducted at low temperatures and low pressures has high desulfurization efficiency, virtually no impact on the octane rating of the oil, low cost, and simple operation. Moreover, the investment and operational costs are lower. However, the extractant containing organic sulfur compounds can be recycled and reused. On the downside, single-pass desulfurization efficiency is low, and energy consumption is high. It needs to be combined with other technologies [24–27]. Currently, common desulfurization solvents mainly include the following categories: organic solvents (such as pyridine, N-methylpyrrolidone [NMP], dimethylformamide [DMF]), physical solvents (such as sulfolane, methanol), as well as emerging ionic liquids (ILs, such as imidazolium-based, phosphonium-based) and DESs. While traditional organic solvents have high selectivity for thiophenic sulfur compounds (e.g., the partition coefficient of NMP for DBT reaches 1.2–1.5), they are increasingly restricted by environmental regulations (such as EU REACH regulations) due to issues like toxicity (pyridine is carcinogenic), high volatility (DMF releases VOCs), and significant regeneration energy consumption (distillation costs account for over 60%) [28]. Among physical solvents, sulfolane holds a 35% market share in natural gas desulfurization due to its high sulfur capacity and stability, but it has poor biodegradability and decomposes easily at high temperatures; methanol is cost-effective but requires low-temperature operation (<−30°C), posing significant energy consumption and safety risks [29]. Ionic liquids (such as [BMIM][BF4]), with their low volatility and tunability, have shown high desulfurization rates (>90%) in small-scale trials, but their high synthesis costs and challenges in large-scale application hinder widespread adoption. In contrast, DESs have emerged as promising green solvents with low cost, low toxicity (biodegradability >80%), and customizability (e.g., acidic ChCl–oxalic acid systems achieving 98% desulfurization rate for DBT). They show potential for replacement in fields like fuel desulfurization and gas purification, especially as viscosity and cycling stability are optimized (e.g., menthol–caprylic acid systems retaining 90% efficiency over five cycles), gradually overcoming industrial bottlenecks. In the future, with stricter environmental policies and technological advancements, DES is expected to become the core direction for upgrading desulfurization solvents [30–32].
ODS utilizes oxidants such as hydrogen peroxide, molecular oxygen, and organic peroxides to oxidize organic sulfur compounds into sulfones and sulfoxides, which are then removed from the fuel [32–36]. ODS exhibits good selectivity, mild reaction conditions, simplicity in process, and high desulfurization efficiency. However, the regeneration of desulfurizing agents in ODS is challenging [37].
ADS is a method that utilizes desulfurization adsorbents to remove sulfides from fuel [38–41]. ADS is simple to operate, economically viable, and environmentally friendly. However, it has the poorest ability to adsorb aliphatic sulfur compounds, limited adsorption capacity, stringent conditions for adsorbent regeneration, and is challenging for industrialization [42].
BDS involves using bacteria capable of desulfurizing fuels, including Rhodococcus, Actinobacillus, Pseudomonas, Bacillus, and Gordonia [15,43–46]. These bacteria convert organic sulfur compounds into sulfates, which are then removed from the fuel. BDS achieves thorough removal of thiophene with mild operating conditions, but it has a long reaction time.
3 DESs
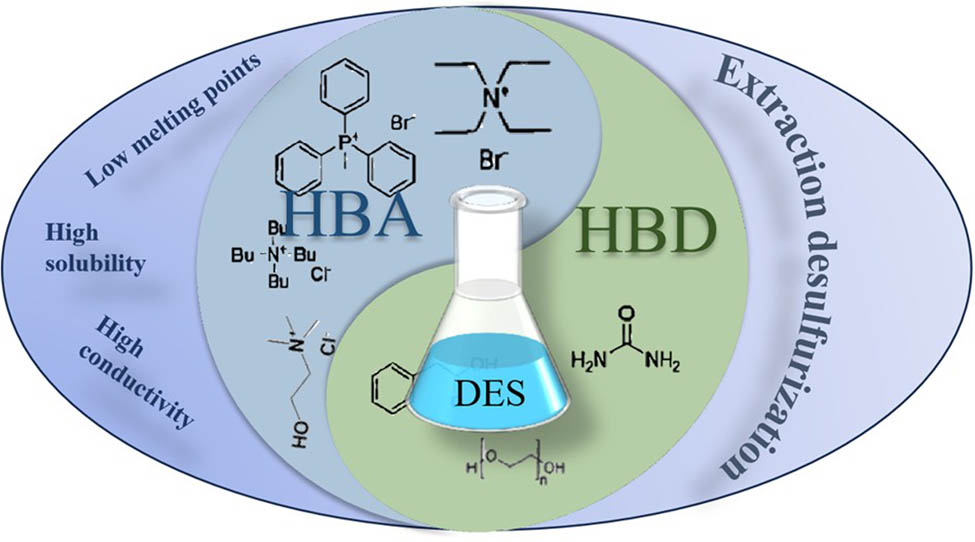
DESs, also known as eutectic solvents, low eutectic point solvents, or pseudo-ILs, have emerged as a new generation of green solvents developed alongside the in-depth study of ILs. They are considered superior to ILs and may become alternatives to them in the future [47–51].
DESs are eutectic mixtures derived from Lewis acids and bases or Brønsted acids and bases. They are composed of two or more components capable of forming intermolecular forces [26,52]. DESs are low-melting-point deep eutectic mixtures formed by the hydrogen bonding interaction between a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA). The hydrogen acceptor is typically a quaternary ammonium salt, while the hydrogen donor is typically an alcohol, organic acid, or amide compound. The formation of DES is based on the deep eutectic mixture of high-melting-point components, resulting in a lowering of the freezing point, preventing solidification from the components. The starting materials for DES are widely chosen and economically affordable, providing a cheaper synthesis method and making it a preferred approach for scale-up.
In 2003, Abbott et al. first reported a mixture based on choline chloride (ChCl) and urea, introducing the concept of deep eutectic for the first time. The melting points of ChCl and urea are 302°C and 133°C, respectively [53]. However, by combining these two components in a 1:2 molar ratio, a deep eutectic mixture was obtained with a melting point of 12°C, significantly lower than the original melting points of each component. Characterization revealed a correlation between the melting point change of choline salts and urea and the strength of hydrogen bonds. These mixtures, influenced by strong hydrogen bonding, exhibited unusual solvent properties such as low melting points, high solubility, and high conductivity. They also possessed advantages such as sustainability and biodegradability.
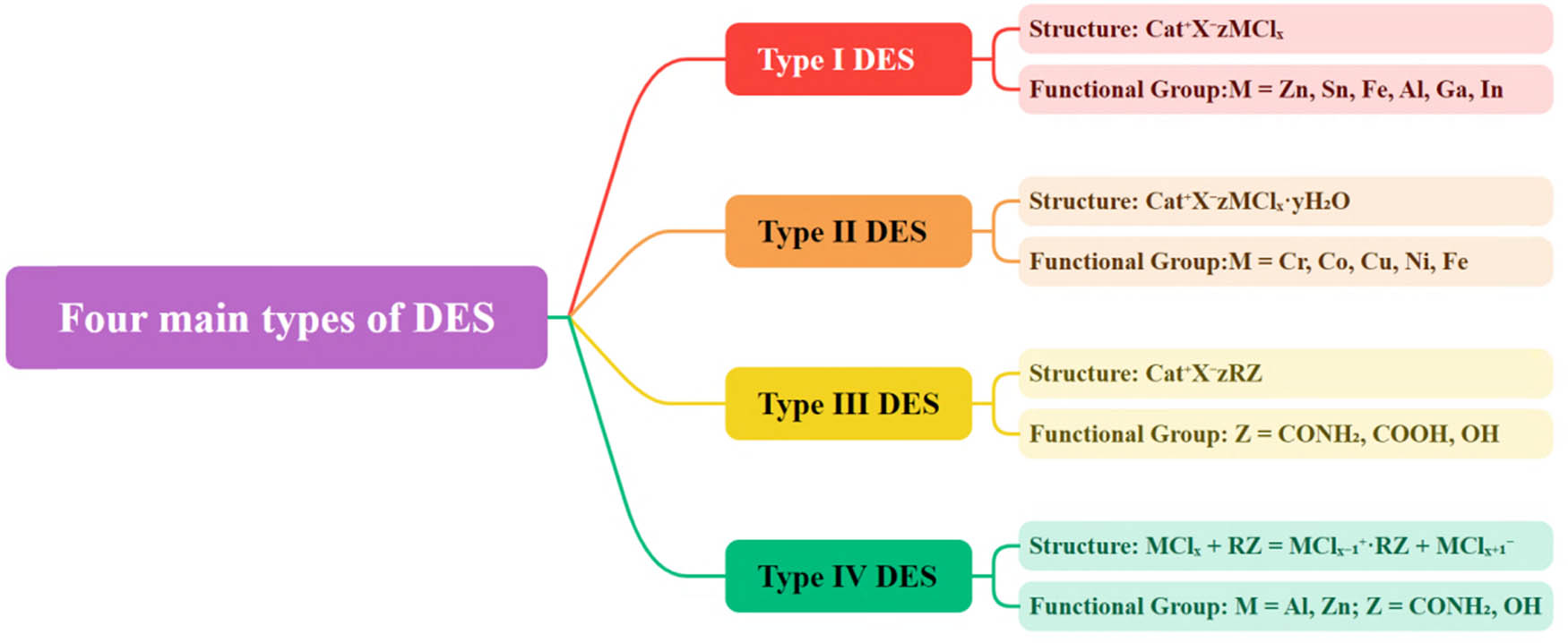
DESs are primarily classified into four types, as shown in Figure 1 [52]. They can be represented by the general formula Cat+X-zY, where Cat+ is usually an ammonium, phosphonium, or imidazolium cation, X−s is a Lewis base (typically a halide ion), Y is a Lewis acid or Brønsted acid, and z represents the number of interactions between Y and the anion.
Type I DES:
Type I DES originated from previous studies on metal halides and imidazole-based ILs. It evolved through the substitution of elements from Group 13 and transition metals. While the scope of I-type DES formed by low-melting anhydrous metal halides is limited, systems composed of hydrated metal halides and ChCl (i.e., Type II DES) can expand the range of DESs.
Type II DES:
Type II DES involves the combination of hydrated metal halides with ChCl or other organic salts. This type is insensitive to air components and humidity, exhibiting a melting point range suitable for industrial applications.
Type III DES:
Type III DES is based on ChCl salts and HBD such as amides, carboxylic acids, and alcohols. Type III DES can be formed by various combinations of HBD and HBA, allowing the dissolution of transition metals, chlorides, oxides, and other substances. They are simple to prepare, relatively cost-effective, biodegradable, non-reactive with water, and exhibit broad adaptability to HBD.
Type IV DES (Natural Deep Eutectic Solvents [NADES]):
Type IV DES refers to deep eutectic solvents formed by combining metal halides with HBD. While the formation of eutectic with inorganic cations is challenging due to their high charge density, combining urea with halides can yield eutectics. This type of DES, also known as NADES, utilizes combinations of HBD from renewable materials.
Four main types of DES.
Each type of DES has its unique characteristics and applications, ranging from industrial processes to environmentally friendly and biodegradable formulations.
Metal deep eutectic solvents (MDESs) are obtained by incorporating different metal salts in certain proportions based on the composition of DESs. They have found extensive applications in various fields [15,54,55]. The addition of metals enhances the performance of MDES compared to DES, as metal ions can participate in the formation of complexes and catalyze reactions when involved in or used as reaction solvents. MDES is typically synthesized as a three-component system, offering significantly improved design flexibility. This allows for tailored design according to specific application scenarios.
4 The development of DESs in the field of desulfurization
4.1 Ionic liquid phase
The development of DESs originated from extensive research on ILs, which were initially explored as green alternatives for desulfurization. In 2001, Bøsmann et al. proposed a method utilizing ILs as solvents for the deep EDS of diesel [56]. Since then, a number of studies have been published, discussing how to use ILs to remove sulfur in various fuel systems. These studies revealed critical structure–activity relationships:
Cationic chain length effect: Imidazolium- and pyridinium-based ILs with longer alkyl side chains (e.g., [C₈MIM][BF4]) exhibited enhanced extraction efficiency for DBT (>90%) due to strengthened π–π interactions. However, excessive chain elongation increased IL solubility in the fuel phase, complicating phase separation and offsetting selectivity gains [31,57].
Metal synergy: Incorporating Lewis acidic metal salts (e.g., FeCl3, CuCl2) into ILs significantly improved desulfurization via π-complexation. For instance, Ban et al. achieved 96% DBT removal using [BMIM]Cl/FeCl3, attributing the performance to metal-sulfur coordination and enhanced Lewis acidity [32,58]. It is noteworthy that compounds containing metal ions are more effective in removing sulfur compounds compared to those with common anions, a fact confirmed through the π-complexation of metal anions with sulfur atoms in aromatic rings.
Mechanistic evolution: Early studies emphasized aromatic π–π interactions, but later work demonstrated that non-aromatic ILs (e.g., pyrazinium-based) could also achieve high desulfurization efficiency through polarity-driven solute–solvent interactions. Li et al. further identified hydrogen bonding between IL active hydrogen and sulfur atoms as a key mechanism via ¹H NMR analysis [34,59].
Despite their potential, ILs face significant barriers to industrialization. The high costs of synthesis and purification, especially for metal-containing systems, remain a major challenge. Additionally, the toxicity and persistence of fluorinated anions (e.g., [NTf2]⁻), which exhibit poor biodegradability, raise environmental concerns under EU REACH regulations. Moreover, the high viscosity of ILs hinders mass transfer, requiring energy-intensive mixing or dilution to overcome these limitations.
By 2014, IL-related desulfurization publications gradually decreased, reflecting waning industrial interest. Nevertheless, insights from IL research, such as the roles of hydrogen bonding, metal coordination, and polarity mismatch, laid the groundwork for DES development, highlighting the need for low-cost, tunable, and environmentally benign alternatives [60].
4.2 DESs phase
To overcome industrialization bottlenecks of ILs (e.g., high costs, environmental risks, and mass transfer limitations), DESs have emerged as greener alternatives with greater industrial potential.
First, their low volatility, high thermal stability, and tunable physicochemical properties (such as polarity and viscosity) allow them to meet various operational requirements. Second, the flexible combination of HBA and HBD provides highly tunable molecular design capabilities, enabling optimization of hydrogen bonding and π–π interactions for specific sulfur compounds (such as thiophene and BT). Third, the raw materials are inexpensive and readily available (e.g., choline derivatives, carboxylic acids) and many components are biodegradable, making DES significantly superior to traditional volatile organic solvents and high-cost ILs. Fourth, the mild synthesis conditions (without the need for purification) and recyclability lower the barriers for industrial application. Based on these characteristics, researchers have increasingly focused on studying the application of DES in the field of EDS in recent decades [61].
The breakthrough in desulfurization emerged in 2013 when Li et al. pioneered the application of DESs, specifically tetrabutylammonium chloride–polyethylene glycol (TBAC/PEG), achieving 82.8% sulfur removal in a single extraction cycle and 99.5% after five cycles [62]. This marked the transition from ILs to DESs. Compared to ILs, DESs also have the following limitations in applications:
Current limitations and research advances:
Viscosity and mass transfer limits: High viscosity in some DES (e.g., ChCl/glycerol) requires optimization through HBD ratio adjustment or cosolvent addition.
Design challenges: Hydrogen-bonding networks’ impact requires mechanistic studies via computational modeling and spectroscopy.
5 Research progress on desulfurization by DES
5.1 Component regulation and optimization
The molecular design and ratio of HBA and HBD in DESs are key factors in optimizing desulfurization extraction performance. The structural characteristics of HBD and HBA have a significant impact on the overall properties of DES, and by adjusting these factors, the solvent’s structure and performance can be optimized to meet specific application requirements. The electronic donor and acceptor groups in the solvent components affect its electron density. Since the primary intermolecular interactions in the solvent are hydrogen bonding, electrostatic forces, and van der Waals forces, the type of functional groups of the HBD (such as carboxylic acids, alcohols, polyols, or carbohydrates) plays a crucial role in determining the properties of DES [65]. The following will discuss the regulation mechanisms of HBA and HBD and their impact on desulfurization efficiency.
Yan et al. investigated the desulfurization performance of single solvents and mixed solvents, such as DES (tetrabutylammonium bromide [TBAB]/sulfolane), through liquid–liquid equilibrium (LLE) experiments and molecular dynamics simulations. They used interaction energy, radial distribution functions, and self-diffusion coefficients to reveal the microscopic separation mechanisms, providing a theoretical basis for the application of mixed solvents in fuel desulfurization [66].
5.1.1 HBA modification
In fuel desulfurization, the strong polarity and tunability of the HBA can selectively disrupt the intermolecular interactions between sulfur compounds and hydrocarbons, promoting their transfer from the oil phase to the DES phase. The HBA can form a hydrogen-bonding network with the sulfur atoms of thiophene compounds, combined with π–π interactions, to achieve a reduction in sulfur content.
In the work of Zhang et al., the COSMOthermX software was used to predict the capacity (C∞) and selectivity (S∞) of T or BT in different DESs, providing a theoretical basis for selecting extractants. The calculations revealed that the longer the alkyl chain in the quaternary ammonium salt, the larger the C∞ and S∞ for T, but this also increases the cost and the miscibility with non-polar alkanes, making it less likely to form DES with carboxylic acids. By selecting hydrogen acceptors such as tetraethylammonium bromide (TEAB) and tetraethylammonium chloride (TEAC), and hydrogen donors like acetic acid (AA) and propionic acid (PrA) for the DES system, the extraction results showed that TEAC exhibited better extraction performance [67]. It is further explained that quaternary ammonium salts with longer alkyl chains (e.g., TBAC) outperformed ChCl due to enhanced hydrophobicity, which minimized fuel phase miscibility. The extraction efficiency followed TBAC > TMAC > ChCl. In Jiang's work, the same trend was observed. He prepared DESs containing hydrogen donors with different alkyl chains, and the [C12DMEA]Cl/FeCl3 with the longest carbon chain exhibited the best extraction performance [24]. In the work of Gosu et al., tetrabutylphosphonium bromide (TBPB), ChCl, and methyltriphenylphosphonium bromide (MTPB) were used as HBA, with ethylene glycol as the HBD, to prepare DESs for extracting BT from model oil. The results showed that the extraction efficiency followed the order: TBPB > MTPB > ChCl. This may be due to the presence of bromide ions in TBPB, which could facilitate the desulfurization process [68].
In the work of Xu et al., metal halides were mixed with amide compounds to prepare a series of type-IV DESs, which were then used for desulfurization extraction. The results showed that the desulfurization efficiency of the DESs formed by different metal salts followed the order: ZnCl2 > CuCl2 > CoCl2 > NiCl2. This is likely due to the soft acid characteristics of Zn2+, which result in a stronger interaction with sulfur compounds, leading to higher extraction efficiency for Zn-based DESs. Density functional theory (DFT) calculations indicated that π–π interactions and CH–π interactions are the main reasons for the effective removal of DBT from oil by DES. Electrostatic potential analysis also confirmed that these interactions promote both the stability of the DES and the desulfurization process [63].
5.1.2 HBD modification
In DES, the HBD provides protons to form a strong hydrogen-bonding network with the hydrogen acceptor, creating a polar solvent system. In EDS, the acidity and hydrogen-bonding capability of the hydrogen donor directly influence the interaction of DES with sulfur-containing compounds, promoting the efficient transfer of sulfur compounds from the oil phase to the DES phase.
Ethylene glycol exhibited superior performance over glycols or carboxylic acids (ethylene glycol > glycerol > malonic acid), attributed to its high hydroxyl density and flexible chain structure, which strengthened hydrogen bonding with sulfur compounds [69]. In the work of Kobotaeva et al., zinc chloride was used as the HBA and polyols as HBDs to synthesize a series of DESs for extracting sulfur compounds from diesel fractions. They found that the extraction efficiency followed the order: butanol > polypropylene glycol > PEG > butanediol > triethylene glycol > glycerol > ethylene glycol [70].
Zhang et al. designed a simulated asphalt solution using two sulfur-containing compounds, DBT and BT, along with n-tetracosane dissolved in cyclohexane as raw materials [71]. They employed a DES consisting of TBAB and PEG to extract DBT and BT. The study investigated the influence of PEG with different molecular weights as a donor on desulfurization efficiency, finding that PEG400 exhibited the best extraction performance for both BT and DBT, indicating that an appropriate molecular chain length is crucial for effective desulfurization extraction. The research also revealed that a moderate increase in hydrogen donor concentration facilitates the encapsulation of anions, thus providing more active hydrogen and enhancing desulfurization efficiency. However, excessive hydrogen donor concentration can lead to a reduction in desulfurization efficiency due to steric hindrance effects.
5.2 Desulfurization mechanism
5.2.1 Hydrogen bonding interaction dominance
Li et al. prepared a series of acidic DESs with TBAB as the hydrogen acceptor and HCOOH as the hydrogen donor. Fourier-transform infrared (FT-IR) and nuclear magnetic resonance (NMR) analyses indicate that hydrogen bonding formed between DES and BT, which leads to damage to the bond of the deep eutectic solvent itself. The results indicate that there is a strong interaction between formic acid and TBAB to form TBAB/HCOOH DES. This is likely a result of the proton transfer between acid and base molecules. The above analysis confirms that hydrogen bonding is the primary driving force for desulfurization extraction, as illustrated in Figure 2 [72].
![Figure 2
Extraction mechanism of DESs [72].](/document/doi/10.1515/gps-2025-0069/asset/graphic/j_gps-2025-0069_fig_002.jpg)
Extraction mechanism of DESs [72].
For instance, in Li et al.’s work, the addition of BT destroyed the hydrogen bond formed between Cl− and active hydrogen H+ in the deep eutectic solvent. As shown in Figure 3, as the concentration of BT increases, the interaction between Cl⁻ and BT intensifies, which disrupts the hydrogen bonds within the DES itself. This results in a weakening interaction between Cl⁻ and the hydrogen in propionate. This further proved that hydrogen bonding is key to the desulfurization extraction process [62]. In the work of Khan and Srivastava, FT-IR showed that compared to the v-OH absorption peak of the DES, the v-OH stretching vibration of DES-DBT shifted from around 3,200 cm−1 to about 3,400 cm−1. At the same time, as the DBT concentration increased during the extraction process, the OH peak became narrower. This suggests that the sulfur atom may form new hydrogen bonds with the active hydrogen (H+) in the DES, replacing the hydrogen bonds originally formed between Cl⁻ and H⁺ [73].
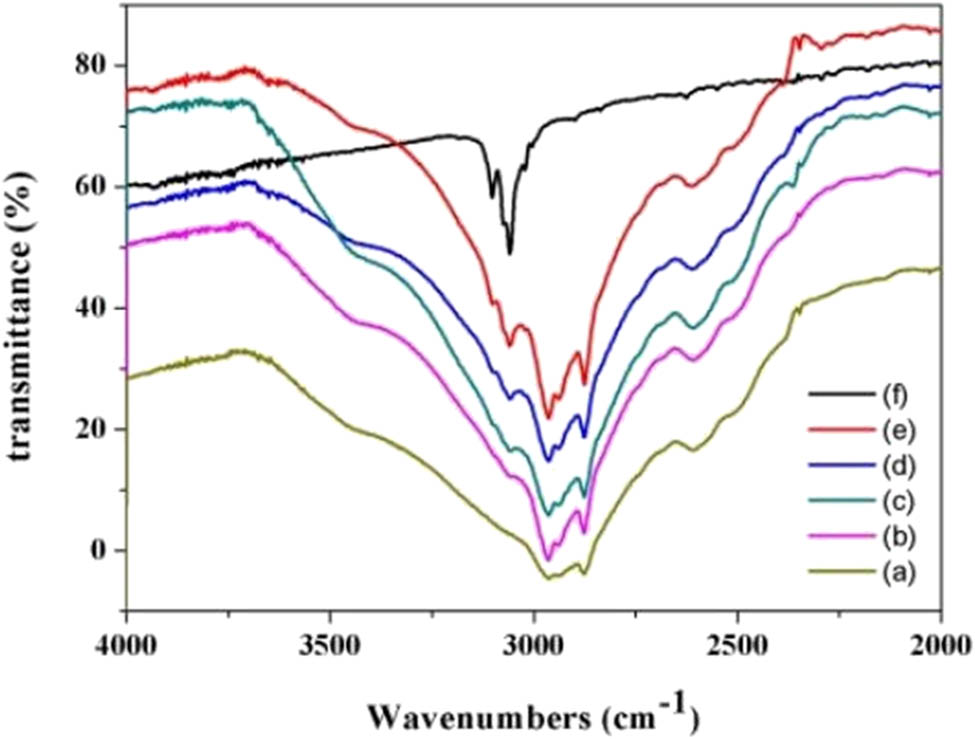
FT-IR of different molar ratio (TBAC + Pr/BT) (a) 1:0 (b) 1:0.5 (c) 1:1 (d) 1:1.5 (e) 1:4 (f) 0:1.
Based on the above analysis, the hydrogen bond-dominated mechanism occurs when the active hydrogen (H⁺) from the O–H group of the HBD forms a new S → H⁺–O hydrogen bond with the sulfur atom (S) bearing a lone pair in sulfides (e.g., DBT/BT). This disrupts the solubility of sulfides in the oil phase, enabling directional extraction. Essential conditions include low-temperature operation (25–30°C), as elevated temperatures cause hydrogen bond dissociation. Structurally, long-chain quaternary ammonium salts (e.g., TBAB/TBAC) are preferred as HBAs, while HBDs must exhibit high polarity, exemplified by carboxylic acids or alcoholic compounds. Experimental validation via ¹H NMR spectroscopy confirms the weakening of original hydrogen bonds, and FT-IR spectroscopy provides evidence for the formation of the new S → H–O bonds.
5.2.2 Enhanced π–π and CH–π bond interaction increases selectivity
In the work of Babu et al., a series of novel imidazole-based DESs were prepared, and DFT studies showed that the interactions between these DESs and sulfur elements were lower compared to traditional solvent extraction (SES) and IL combinations. The extraction performance of imidazole-based dehydroepiandrosterone can be attributed to the synergistic effect of hydrogen bonding, π–π interactions, and CH–π interactions, as well as the improved polarity of the HBA and HBD [74]. Jiang et al. reported on choline-based DESs, among which [C12DMEA]Cl/FeCl3 exhibited a desulfurization rate of 52.9% [24]. It was observed that aliphatic DESs with longer carbon chains outperformed aromatic counterparts. This enhanced performance is attributed to interactions such as π–π interactions, CH–π interactions, and hydrogen bonding between the DESs and sulfur-containing compounds. The authors suggest that in their study, CH–π interactions and coordination effects are the primary factors influencing the extraction efficiency of DESs. Therefore, the selection of DESs and the molar ratio of HBA to HBD significantly impact the efficiency of sulfur-containing compound removal.
This reveals that, aliphatic DESs with extended carbon chains leverage enhanced π–π and CH–π interactions to drive the desulfurization process. Long alkyl chains strengthen van der Waals binding to sulfur heterocycles (e.g., DBT) via CH–π interactions, while in imidazolium-based DESs containing aromatic moieties, the π–π effect cooperates with hydrogen bonding to enable synergistic adsorption.
5.2.3 Metal complexation effect
The addition of FeCl3 to TBAC/PEG (4:1:0.05 molar ratio) formed a ternary MDES, boosting DBT extraction efficiency to 89.5%. Fe3+ acted as a Lewis acid, coordinating with sulfur atoms while synergizing with hydrogen bonding, as validated by UV–Vis and X-ray absorption spectroscopy [49]. In 2016, Khezeli and Daneshfar utilized the magnetic properties of FeCl3 to prepare a magnetic ternary deep eutectic solvent (magnetic DES) for the extraction of thiophene [75]. Ultrasonic-assisted fuel desulfurization extraction was employed. Dispersing the magnetic DES in simulated oil and subjecting it to ultrasonic irradiation increased the surface area, enhancing the extraction capacity. The extraction process only required 5 min, significantly reducing the extraction time. Magnetic attraction with a magnet was used to collect and separate the micro-droplets containing the deep eutectic solvent and simulated oil. With a minimal amount of deep eutectic solvent, the extraction efficiency for thiophene was nearly elevated to 100%. This approach provided a cost-effective, simple, and efficient optimization strategy for the extraction of metal-based DESs, as illustrated in Figure 4.
![Figure 4
Ultrasonic-assisted liquid–liquid extraction schematic diagram of magnetic deep eutectic solvents (Magnetic DES) for T (Thiophene) [75].](/document/doi/10.1515/gps-2025-0069/asset/graphic/j_gps-2025-0069_fig_004.jpg)
Ultrasonic-assisted liquid–liquid extraction schematic diagram of magnetic deep eutectic solvents (Magnetic DES) for T (Thiophene) [75].
In 2016, Li et al. investigated the effectiveness of ternary mixtures of DESs composed of HBAs, HBDs, and metal components in the desulfurization process [49]. Various transition metals were employed, including iron(III) chloride (FeCl3), zinc(II) chloride (ZnCl2), nickel(II) chloride (NiCl2), cobalt(II) chloride (CoCl2), and copper(II) chloride (CuCl2). Preliminary screening results indicated that the extraction efficiency of TBAC:PEG:FeCl3 was the highest among other metal ion-based DESs (81.15%). At the optimal TBAC:PEG:FeCl3 ratio of 4:1:0.05, the extraction efficiency could be further enhanced to 89.53%. The primary factors influencing sulfur extraction were hydrogen bonding and coordination effects. Due to the higher electron density of the central metal component in such DESs, the distribution of the outer electron cloud of the metal ion affects the interaction between DBT and the deep eutectic solvent. This explains why iron exhibits higher extraction efficiency compared to other metals.
From the above analysis, it is evident that trivalent Fe3+ (e.g., FeCl3) as a metal component in DES demonstrates the most significant effect. The reason lies in its higher electron density and suitable d-orbital electron configuration, enabling it to function as a strong Lewis acid. This effectively facilitates coordination with the lone pair electrons on sulfur atoms. UV–Vis and XAS spectroscopy confirmed that the direct coordination between Fe3+ and S atoms is the core driving force in this process. It should be noted that the metal complexation effect does not occur in isolation; it can synergize with the inherent hydrogen-bonding network in DES, leading to a joint enhancement in the affinity and extraction capacity for sulfur-containing compounds.
5.3 Factors influencing performance optimization
5.3.1 Component, molar ratio regulation, and temperature control
The desulfurization performance of DESs is closely related to the chemical properties of its HBA and HBD, the molar ratio of the components, and the operating temperature. Research shows that the basicity of the HBA and the proton donation ability of the HBD together determine the hydrogen bonding network structure and the polarity characteristics of the DES. In terms of molar ratio control, DES with different molar ratios exhibit distinct physical properties, which directly affect their interaction with sulfides, thereby influencing the extraction performance. Temperature also has a certain impact on mass transfer kinetics. Typically, the extraction temperature is around room temperature. A series of literature reports suggest that DESs can be washed with deionized water or ether or regenerated through vacuum distillation for cyclic use [76,77]. Mechanistic studies indicate that, in addition to hydrogen bonding, metal ions in MDES also act as coordinating compounds, thereby enhancing desulfurization efficiency. This research provides an effective technique for the removal of sulfur compounds from fuels.
For more details on the factors on model oil EDS mentioned above, refer to Table 1.
Effects of different DES on model oil EDS at different conditions
| DES types | HBA/HBD (molar ratio) | Sulfur compounds | Model oil solvents | Extraction temperature (°C) | Extraction time (min) | Extraction efficiency (wt%) | Remark | Ref. |
|---|---|---|---|---|---|---|---|---|
| C4DMEACl/FeCl3 | N,N-dimethylethanolamine/C4H9Cl/FeCl3 (1:1:(1.1–1.3)) | DBT | n-Octane | 30 | 10 | — | N/A | [24] |
| C8DMEACl/FeCl3 | N,N-dimethylethanolamine/C8H17/Cl/FeCl3 (1:1:(1.1–1.3)) | DBT | Lwer than 52.9 | |||||
| C12DMEACl/FeCl3 | N,N-dimethylethanolamine/C12H25Cl/FeCl3 (1:1:(1.1–1.3)) | DBT | 52.90 | |||||
| BzDMEACl/FeCl3 | N,N-dimethylethanolamine/benzyl chloride/FeCl3 (1:1:(1.1–1.3)) | DBT | 39.00 | |||||
| C12DMEACl/FeCl3 | N,N-dimethylethanolamine/C12H25Cl/FeCl3 (1:1:(1.1–1.3)) | BT/3-MBT/4-MDBT/4,6-DMDBT | Not specified/not specified/not specified/35.7 | |||||
| TEAC:PrA | Tetraethylammonium chloride/propionic acid (1:3) | Thiophene (T), BT | n-Decane | 25 | 30 | 98.06, 98.53 | Three-stage | [67] |
| TBAB:FA | Tetrabutylammonium bromide/fumaric acid (1:3) | T/BT/DBT | Model oil | 25 | 30 | 98.32/98.24/97.6 | Three-stage | |
| TEAC:AA | Tetraethylammonium chloride (1:3) | T | n-Decane | 25 | 30 | 92.33 | N/A | |
| TEAC:PrA | Tetraethylammonium chloride/propionic acid (1:3) | BT | n-Decane (NDT) | 50 | 30 | 93.56 | N/A | |
| TEAC:PrA/FeCl3 | Tetraethylammonium chloride/propionic acid/FeCl3 (4:1:0.05) | T/BT | Model gasoline | 30 | 120 | 89.53 (T), not specified (BT) | N/A | |
| ChCl/DEG | Choline chloride/diethylene glycol (1:4) | DBT | n-Heptane | 25 | 60 | 67.14 | N/A | [82] |
| TBAB/DEG | Tetrabutylammonium bromide/diethylene glycol (1:4) | 60.1 | N/A | |||||
| ChCl/MEG | Choline chloride/ethylene glycol (1:4) | 57.98 | N/A | |||||
| ChCl/MEA | Choline chloride/monoethanolamine (1:4) | 48.55 | N/A | |||||
| ChCl/DEG (Regenerated) | Choline chloride/diethylene glycol (1:4) | ∼65 | After 3 cycles | |||||
| ChCl/DEG | Choline chloride/diethylene glycol (1:4) | 68.09, 52.62 | 0.1% DBT, 1% DBT | |||||
| ChCl/DEG | Choline chloride/diethylene glycol (1:2) | 30 | 47.44 | N/A | ||||
| ChCl/DEG | Choline chloride/diethylene glycol (1:1) | 40 | 28.07 | N/A | ||||
| SES:IMI | Sesamol/imidazole (1:1) | T | n-Decane + 5% Th, 5% Py, 5% Tol* | 25 | 240 | 78.1 | Four cycles | [74] |
| CRE:IMI | o-Cresol/imidazole (1:1) | n-Decane + 5% Th, 5% Py, 5% Tol | 67.7 | N/A | ||||
| GUA:IMI | Guaiacol/imidazole/(1:1) | n-Decane + 5% Th, 5% Py, 5% Tol | 66.7 | N/A | ||||
| ZnCl2/MFA | ZnCl2/N-methylformanilide (1:3) | DBT | n-Dodecane (200-800 ppm) | 35 | 30 | 85.7 | Six cycles | [63] |
| ZnCl2/MFA | ZnCl2/N-methylformanilide (1:2), (1:4) | n-Dodecane | 20, 33.4 | N/A | ||||
| CuCl2/MFA, CoCl2/MFA, NiCl2/MFA | CuCl2/N-methylformanilide (1:3), CoCl2/N-methylformanilide (1:3), NiCl2/N-methylformanilide (1:3) | n-Dodecane | 19.1, 17.3, 14.2 | N/A | ||||
| TEA/PEG200 | Triethylamine/polyethylene glycol 200(1:3) | DBT | n-Decane | 20–25 | 0.417 (25 s) | 67.81 | Regenerated (>5 cycles); micro-channel contactor | [83] |
| TEA/PEG200 | Triethylamine/polyethylene glycol 200(1:4) | 0.417 (25 s) | 62.6 | N/A | ||||
| TMAC/PEG200 | Tetramethyl ammonium chloride/polyethylene glycol 200(1:4) | 1.667 (100 s) | 60.1 | N/A | ||||
| ChCl/PEG200 | Choline chloride/polyethylene glycol 200(1:4) | 1.667 (100 s) | 57.44 | N/A | ||||
| ZC/PEG200 | Zinc chloride/polyethylene glycol 200(1:4) | 1.667 (100 s) | 59.7 | N/A | ||||
| TEA/Pr | Triethylamine/propionic acid (1:3), (1:4) | 0.417 (25 s) | 78.28 | Three cycles | ||||
| TMAC/Pr | Tetramethyl ammonium chloride/propionic acid (1:4) | 1.667 (100 s) | 35.37 | N/A | ||||
| ChCl/Pr | Choline chloride/propionic acid (1:4) | 1.667 (100 s) | 29.32 | N/A | ||||
| TBAB/EG | Tetrabutylammonium bromide/polyethylene glycol (1:2) | DBT | iso-Octane | 25 | 30 | 81 | Regenerated after five cycles; intensification using ultrasound | [84] |
| TPAB/EG | Tetrabutylammonium bromide/olyethylene glycol (1:3) | 77 | Regenerated after five cycles; intensification using ultrasound | |||||
| TPAB/G | Tetrapropylammonium bromide/glycol (1:2) | 72 | Regenerated after five cycles; intensification using ultrasound | |||||
| TBAB/HCOOH | TBAB/formic acid (not recorded) | T/BT/DBT | n-Octane | 30 | 40 | 72/81.75/80.47 | Regenerated after four cycles | [72] |
| TBAB/CH3COOH | TBAB/acetic acid (not recorded) | 55/63/60 | N/A | |||||
| TBAB/CH3CH2COOH | TBAB/propionic acid (not recorded) | 55/63/60 | N/A | |||||
| TBAB/HOOCCOOH | TBAB/oxalic acid (not recorded) | 50/50/50 | N/A | |||||
| TEA/Pr | Triethylamine/propionic acid (1:3) | DBT | n-Decane | 25 | 0.283 (17 s) | 70.8 | Three cycles (VR = 1), six cycles (VR = 3), eight cycles (VR = 5) | [85] |
| TEA/Pr | Triethylamine/propionic acid (1:3) | BT | 0.283 (17 s) | 68.7 | N/A | |||
| TEA/Pr | Triethylamine/propionic acid (1:3) | T | 0.283 (17 s) | 44.4 | N/A | |||
| ChCl/p-TsOH | Choline chloride/p-toluenesulfonic acid (2:1) | BT | n-Octane50% and toluene50% | 50 | 60 | 18.7 | N/A | [88] |
| TEAC/p-TsOH | Tetraethylammonium chloride/p-toluenesulfonic acid(2:1) | 23.9 | ||||||
| TBAC/p-TsOH | Tetrabutylammonium chloride/p-toluenesulfonic acid(2:1) | 35.9 | ||||||
| BET/p-TsOH | Betaine/p-toluenesulfonic acid(3:1) | 22.1 | ||||||
| TBAB/PEG400 | Tetrabutylammonium bromide/polyethylene Glycol400 (1:2) | BT/DBT | Cyclohexane and n-eicosane | 25 | 40 | 89.4/95.5 | Eight cycles | [71] |
| TBAB/PEG200, TBAB/PEG600, TBAB/PEG800 | Tetrabutylammonium bromide/polyethylene glycol 200 (1:2), TBAB/PEG600 (1:2), TBAB/PEG 800 (1:2) | DBT | Cyclohexane and n-eicosane | 25 | 44.9/30, 25 | N/A | ||
| TEAC/OA, TEAB/OA | TEAC/oxalic acid (1:3), TEAB/oxalic acid (1:3) | T | n-Heptane (HEP) | 30 | 30 | 28, 24 | Retained stable after six cycles | [89] |
Comprehensive analysis of influencing factors the following conclusion is drawn. The HBA/HBD molar ratio is the decisive factor in DES extractive desulfurization, governing performance by altering physicochemical properties (e.g., hydrogen bonding, polarity) that regulate sulfur compound interactions (e.g., ChCl/DEG efficiency drops from 68.09% at 1:4 to 28.07% at 1:1; TEAC:PrA at 1:3 exceeds 98%). Mass transfer enhancement drives breakthrough improvements: multistage extraction (efficiency rises to >72% in five stages), ultrasound (82%), microchannel reactors (>78% in 25 s), or oxidative coupling (>90% with H2O2) overcome mass transfer limitations, enhance efficiency, and drastically reduce time. Temperature (20–50°C) has a milder impact, with efficiency typically decreasing only ∼5% when raised from 25°C to 50°C under specific conditions, favoring energy-efficient ambient operation. Therefore, optimization should prioritize ① molar ratio tuning and ② intensification integration, treating temperature as a flexible secondary parameter.
It is also observed that model oils dominate research on DES desulfurization due to their provision of a highly controllable and simplified experimental environment. By dissolving target sulfur compounds in single-component alkane solvents, researchers can eliminate interference from real oils’ complex matrices and precisely assess DES selectivity for specific sulfurs. Such model systems significantly reduce experimental variables, enabling efficient screening of DES formulations, process parameters, and cyclic stability verification. For instance, studies using model oils rapidly demonstrated the high efficiency of TEA/PrA and TBAB/PEG400, laying the foundation for mechanistic investigation and preliminary process design. Even though these findings require validation with real oils, model oils remain a significant experimental platform for advancing DES desulfurization technology.
Table 2 presents the desulfurization performance in real fuels, revealing that complex actual oil matrices significantly suppress efficiency. Real fuels (e.g., FCC gasoline, catalytic diesel) contain multiple interferents – alkylated polycyclic sulfides (e.g., 4,6-DMDBT), nitrogen compounds, olefins, and aromatics – that compete for DES active sites or impair catalysts. This causes single-stage efficiency to plummet to 28–60% (e.g., merely 40.94% for FCC gasoline with TBAB/formic acid). To compensate, industrial setups must rely on multi-stage extraction (often four stages) coupled with oxidative intensification: adding oxidants like H2O2 and metal catalysts (e.g., FeCl3) boosts efficiency to 97.25% for actual diesel using p-TsOH/ChCl systems [78]. However, these processes substantially increase operational complexity and costs. Moreover, the long-term cycling stability of DES remains insufficiently validated in real matrices (≤4 regeneration cycles in most studies), highlighting an urgent need for industrial compatibility upgrades.
Effects of different DES on real fuel EDS at different conditions
| HBD | HBA | Oil type | Desulfurization efficiency | Extraction stages | Key condition | Ref. |
|---|---|---|---|---|---|---|
| Formic acid | TBAB | FCC gasoline | Single-stage: 40.94% | 4 | Model oil/SDES mass ratio 1:0.5, 30°C, 40 min/extraction | [15] |
| Cumulative (four-stage): 83.61% | ||||||
| Formic acid | TBAB | Diesel fuel | Single-stage: 35.27% | 4 | Real diesel/SDES mass ratio 1:0.5, 30°C, 40 min/extraction | |
| Cumulative (four-stage): 70.21% | ||||||
| p-Toluenesulfonic acid | Tetraethylammonium chloride (TEAC) | Catalytic cracking diesel | 60.00% | N/A | Oxidation assistance (H2O2), SDES/oil mass ratio 1:1, 50°C, 60 min | [88] |
| p-Toluenesulfonic acid | TEAC/FeCl3 (1:0.1 molar) | Catalytic Cracking Diesel | 69.00% | N/A | Oxidation assistance (H2O2), SDES/oil mass ratio 1:1, 50°C, 60 min | |
| p-TsOH (p-toluenesulfonic acid) | ChCl (choline chloride) | Actual diesel | 97.25% | 4 | H2O2 oxidative-extraction | [78] |
| DES:oil = 1:2, H2O2/S = 4, 60°C, 30 min | ||||||
| p-TsOH | TBAC (TBA chloride) | Actual diesel | 95.90% | N/A | H2O2 oxidative-extraction | |
| DES:oil = 1:2, H2O2/S = 4, 60°C, 30 min | ||||||
| p-TsOH | TEAC (TEA chloride) | Catalytic diesel | 93.50% | N/A | H2O2 oxidative-extraction | |
| DES:oil = 1:1, H2O2/S = 4, 50°C, 60 min | ||||||
| PEG | ChCl (choline chloride) | Untreated commercial diesel | 82% | N/A | Catalyst: [MoO2Cl2(DMB)2] (Complex 1) | [90] |
| Oxidant: 30 wt% H2O2, 70°C, 2 h, DES/Oil Ratio: 1:1 (v/v) |
Balali et al. employ the quantitative structure–property relationship method to systematically investigate the influence of HBD molecular structures in DESs on the thiophene distribution coefficient β 2 between the DES phase and hydrocarbon phase within ternary liquid systems. The research is based on 12 datasets comprising a total of 504 data points. These datasets encompass DESs utilizing ChCl as the HBA, combined with various hydrocarbon types, six categories of HBDs, two distinct temperatures, and two HBD-to-HBA molar ratios [79].
5.3.2 Mass transfer enhancement
In the work of Khan and Srivastava, the TMAC:2EG (ethylene glycol) DES system was used, and a five-stage extraction process was employed, improving the extraction rate from the initial 47% to 72%. When compared with the CHCl3:2EG system, the distribution coefficients (β) for the five-stage extraction were calculated. The results showed that the distribution coefficient (β) for TMAC:2EG (2.72) was higher than that of the latter (1.32), indicating that the TMAC system has a higher extraction efficiency [73]. In the work of Islam et al., a DES system consisting of betaine ethylene glycol was used for the extraction of thiophene from model oil, and a ternary component liquid–liquid equilibrium study was conducted. After four extraction stages, the extraction rate was improved from 20% to 57.2% [80]. In 2017, Ahmed Rahma et al. explored DESs composed of TBAB salt and two different lightweight PEGs [81]. After three extraction cycles, the extraction efficiency for DBT and thiophene reached 100% and 95.15%, respectively.
In the work of Mahdavi et al., the EDS performance of model diesel was studied using a microchannel contactor, providing new ideas and methods for the development of efficient desulfurization technologies. The microchannel setup used is shown in Figure 5, with ChCl and other compounds as hydrogen acceptors, and PEG 200 and others as hydrogen donors. Eight different DESs were used for the EDS study of model fuel. With a microchannel reaction time only of 25 s, the TEA/Pr system exhibited the highest desulfurization rate, reaching 78.3% [83]. Mahdavi et al. investigated the influence of micro-channel geometric parameters on the DES desulfurization rate and mass transfer. As the diameter of the microchannel increases, the desulfurization rate slightly decreases, while the mass transfer coefficient is higher for a 0.6 mm microchannel. As the length of the microchannel increases, the desulfurization rate improves. The desulfurization effects for microchannels with lengths of 20–30 cm are similar, with the final choice being a 0.6 mm diameter and 20 cm length microchannel. The maximum desulfurization rate can reach over 70% [85]. Ahmadet et al. optimized the DES EDS process in microchannels using response surface methodology simulations, revealing the influence mechanisms of operational parameters and channel geometries on sulfur removal. Multiphysics simulations analyzing the capillary, Weber (We = 6 × 10⁻⁵–0.83), and Reynolds numbers confirmed the slug flow regime (interfacial tension-dominated) as optimal. Model predictions and experimental validation demonstrated 73.2% sulfur removal within 2.5 s under optimized RF/D ratio conditions, highlighting the high efficiency of microchannel-DES technology for practical fuel processing [86].
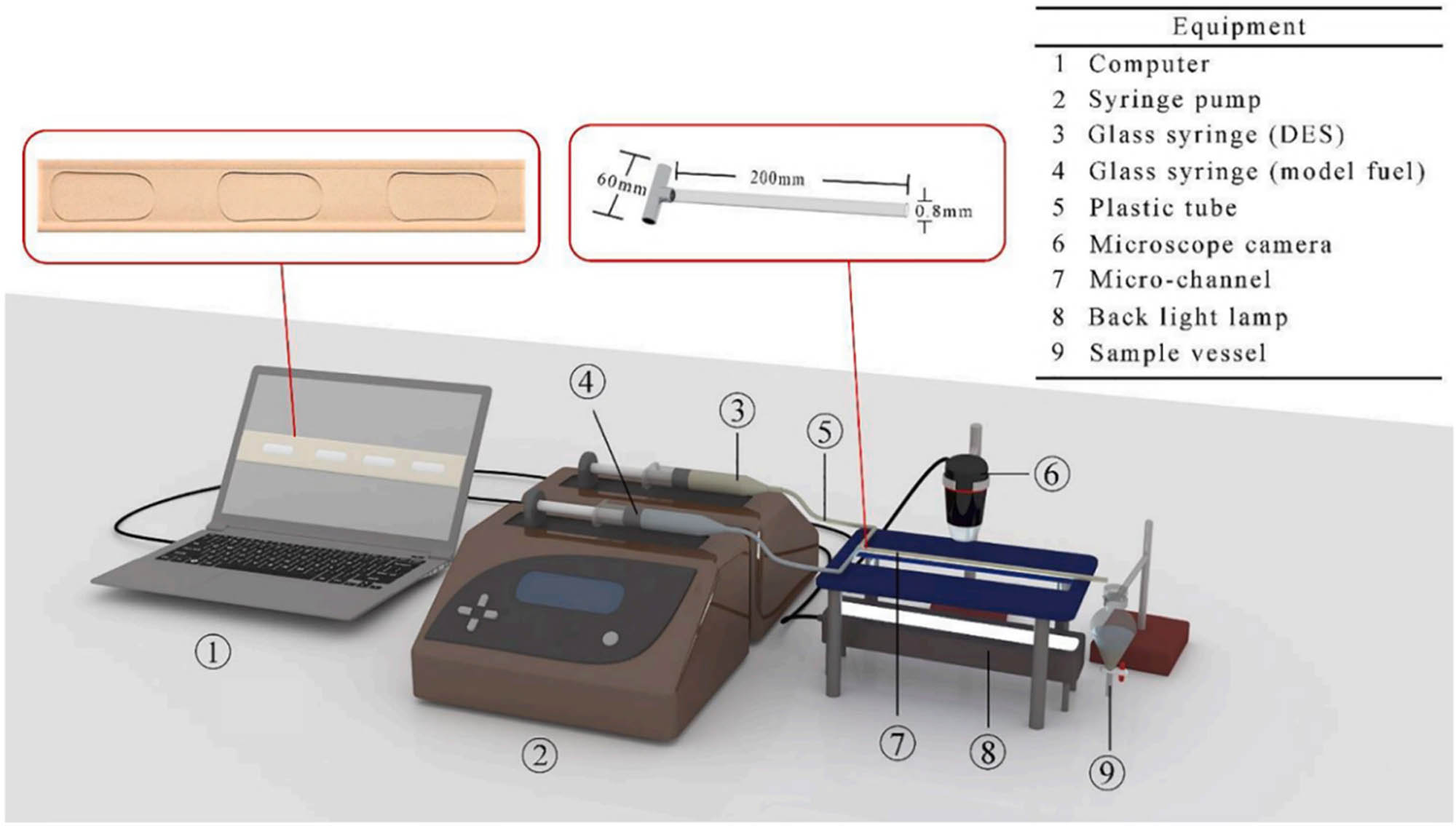
The micro-channel set up of extractive desulfurization.
In the work of Khan and Srivastava, three DESs were prepared: TPAB/EG, TBAB/EG, and TPAB/G. These were used for desulfurization extraction, with process intensification achieved through ultrasound technology. The performance, thermal stability, mass transfer coefficients, and the effect of ultrasound on the extraction process were studied. The results showed that ultrasound significantly improved the extraction efficiency, reaching a maximum of 82% [84].
In addition, adding oxidants such as H2O2 during the extraction process to perform ODS in combination with DESs can significantly enhance the desulfurization efficiency [78]. Under the influence of DES, H2O2 decomposes to generate reactive oxygen species (such as ·OH), which oxidize stubborn aromatic sulfur compounds (such as DBT) into more polar sulfone or sulfoxide products. These products are then efficiently captured by the DES, improving the extraction rate to over 90% [55,87]. For example, by combining oxidative extraction with desulfurization, adding a certain amount of H2O2 during the extraction process can increase the extraction efficiency to over 90% [88]. Jiang et al. prepared a series of ternary DES, such as [C4DMEA]Cl/H3BO3/EG, and conducted desulfurization in the presence of H2O2. The removal rate of DBT in the model oil reached over 95% [91].
5.3.3 Functionalized DES combined with carriers
In recent years, researchers have significantly enhanced the plasticity and desulfurization efficiency of DESs by incorporating them with carriers such as porous materials, nanoparticles, or polymers to create functionalized loading systems. This strategy effectively overcomes the limitations of traditional DES, such as high viscosity and mass transfer restrictions, through physical confinement, interface synergy, or chemical bonding interactions. At the same time, it introduces multiple desulfurization mechanisms, further improving the overall performance of the DES-based systems.
In the work of Eskandari et al., a solid–liquid host–guest of melamine foam impregnated with NMP/Benz/H2O DES was synthesized as MF-Im-DES. After three stages of extraction, it can achieve a desulfurization rate of 100% for the model oil [92]. Chen et al. prepared a supramolecular deep eutectic solvent (SUPRADES) for fuel desulfurization using β-cyclodextrin and lactic acid, in combination with carbon nanotubes (CNTs). The results showed that the DES β-CD/lactic acid, after being loaded with CNTs, significantly enhanced the desulfurization efficiency. Moreover, the properties of the desulfurized HL-β-CD/CNT system remained stable [93]. In addition, flexible metal frameworks, reduced graphene oxide, and cyclodextrin-based quantum dots were also fabricated into composite materials with DESs for simulated oil desulfurization, all of which exhibited promising sulfur removal efficiency [94–96].
Moreover, ultrasound-assisted oxidative desulfurization technology using DESs in microchannel reactors faces significant challenges in energy costs and industrial scale-up. On the one hand, microchannel systems incur substantial pumping energy consumption due to high-viscosity DES (e.g., ZC/PEG at 517 cP), and while achieving 78% desulfurization efficiency within 25 s is possible, this short-duration high efficiency requires severe pumping conditions. Simultaneously, ultrasound-assisted desulfurization demands continuous electrical energy to sustain the cavitation effect, leading to dramatically increased power consumption in scaled applications. On the other hand, during industrial scaling, the batch manufacturing and anti-clogging maintenance of precision microchannel structures (e.g., Ø0.6 mm channels) become prohibitively costly, and ensuring stability in large-scale flow patterns (droplet/annular flows) remains challenging. Furthermore, DES recovery requires additional regeneration steps (such as organic solvent washing or distillation), further increasing both energy consumption and operational complexity. Consequently, the high efficiency demonstrated at the laboratory scale has yet to be bridged with industrial feasibility due to these cost and complexity bottlenecks.
6 Conclusion
DESs, as a new generation of green solvents, have demonstrated remarkable advantages in fuel desulfurization, combining environmental friendliness with high efficiency. Compared to traditional HDS and ILs, DESs enable targeted regulation of solvent polarity and sulfide affinity through flexible combinations of hydrogen bond acceptors (HBAs) and donors (HBDs) (e.g., TBAB/PEG400 and TEAC/OA systems), achieving over 90% extraction efficiency for aromatic sulfides such as thiophene and DBT under mild conditions. Their mechanisms involve hydrogen bonding interactions (verified by FT-IR/NMR), π–π stacking, and metal coordination synergies (e.g., FeCl3-based metal-doped DESs enhancing sulfur capture via Lewis acid interactions). Furthermore, an oxidative-extraction dual strategy coupled with H2O2 elevates desulfurization rates to above 95%. Process intensification technologies like ultrasonication-assisted extraction and microchannel reactors reduce extraction time to seconds, while DESs can be regenerated and reused for six to eight cycles with >85% efficiency retention. Biodegradable components (e.g., choline derivatives) align with green chemistry principles.
However, DES industrial application still faces core challenges such as high viscosity, limited sulfur capacity, and high regeneration energy consumption. Future research should focus on three key directions:
Functionalized solvent design: To address mass transfer limitations caused by high viscosity, develop low-viscosity ternary systems or design dendritic hydrogen-bond donors; introduce π-electron-enriched components to enhance the synergistic π–π and CH–π interactions with thiophenic sulfides, improving selectivity.
Process intensification integration: Construct oxidation–extraction coupled processes utilizing the enhanced polarity of oxidized sulfur for deep desulfurization; develop microwave/ultrasound-assisted dynamic extraction devices to increase desulfurization efficiency beyond 95%.
Sustainability optimization: Design photo-responsive solvents to reduce regeneration energy consumption by 40%; adopt bio-based components to lower environmental footprints.
The critical bottleneck requiring immediate breakthrough is the long-term stability of high-sulfur-capacity solvents (>0.5 g S/g solvent). This can be mitigated through metal coordination center encapsulation to suppress active component leaching. Concurrently, machine learning should be leveraged to predict structure–activity relationships of hydrogen-bond acceptor/donor combinations, accelerating the development of desulfurizers compliant with aviation fuel standards.
-
Funding information: The financial support from the Foundation of Tianjin Educational Committee (No. 2021KJ080).
-
Author contributions: Yue Wang: writing – original draft, writing – review & editing, methodology; Liang Yu: writing – original draft, formal analysis, visualization; Ying Chen: visualization, project administration; Jinxi Li: resources.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Song X, Wu D, Su Y, Li Y, Li Q. Review of health effects driven by aerosol acidity: Occurrence and implications for air pollution control. Sci Total Environ. 2024;955:176839. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.scitotenv.2024.176839.10.1016/j.scitotenv.2024.176839Suche in Google Scholar PubMed
[2] Zhang Q, Wang S, Zhen L. Evaluating eco-economic benefits of anchoring and drifting under government sulfur emission policies. Transp Res Part D: Transp Environ. 2024;136:104442. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.trd.2024.104442.10.1016/j.trd.2024.104442Suche in Google Scholar
[3] Kapitány S, Nagy D, Posta J, Béni Á. Determination of atmospheric sulphur dioxide and sulphuric acid traces by indirect flame atomic absorption method. Microchem J. 2020;157:104853. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.microc.2020.104853.10.1016/j.microc.2020.104853Suche in Google Scholar
[4] Gössling S, Meyer-Habighorst C, Humpe A. A global review of marine air pollution policies, their scope and effectiveness. Ocean Coast Manag. 2021;212:105824. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.ocecoaman.2021.105824.10.1016/j.ocecoaman.2021.105824Suche in Google Scholar
[5] Jion MMMF, Jannat JN, Mia MY, Ali MA, Islam MS, Ibrahim SM, et al. A critical review and prospect of NO2 and SO2 pollution over Asia: Hotspots, trends, and sources. Sci Total Environ. 2023;876:162851. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.scitotenv.2023.162851.10.1016/j.scitotenv.2023.162851Suche in Google Scholar PubMed
[6] Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022;31:210112.10.1183/16000617.0112-2021Suche in Google Scholar PubMed PubMed Central
[7] Yang L, Yang Y, Zhou Y, Shi X. Research the synergistic carbon reduction effects of sulfur dioxide emissions trading policy. J Clean Prod. 2024;447:141483. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jclepro.2024.141483.10.1016/j.jclepro.2024.141483Suche in Google Scholar
[8] Tamjidi S, Esmaeili H. A review on biodesulfurization of crude oil using different microorganisms: Reaction mechanisms, effective factors, and removal efficiency of organic sulfur compounds. Process Biochem. 2025;150:288–305. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.procbio.2025.01.018.10.1016/j.procbio.2025.01.018Suche in Google Scholar
[9] Lum MMX, Ng KH, Lai SY, Mohamed AR, Alsultan AG, Taufiq-Yap YH, et al. Sulfur dioxide catalytic reduction for environmental sustainability and circular economy: A review. Process Saf Environ Prot. 2023;176:580–604. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.psep.2023.06.035.10.1016/j.psep.2023.06.035Suche in Google Scholar
[10] Uyar M, Aydın H. Production of low sulfur diesel-like fuel from crude oil wastes by pyrolytic distillation and its usage in a diesel engine. Energy. 2022;244:122683. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.energy.2021.122683.10.1016/j.energy.2021.122683Suche in Google Scholar
[11] Wu L, Wang Y, Zheng L, Han X. Stepwise optimization of hydrogen network integrated sulfur compound removal kinetics and a fluid catalytic cracker. Chem Eng Res Des. 2019;151:168–78. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cherd.2019.09.012.10.1016/j.cherd.2019.09.012Suche in Google Scholar
[12] Jha D, Gomey AK, Kumari G, Maheshwari P, Haider MB, Kumar R, et al. A review on the role of nanocomposites for desulfurization of liquid transportation fuels. J Environ Manag. 2025;375:124255. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jenvman.2025.124255.10.1016/j.jenvman.2025.124255Suche in Google Scholar PubMed
[13] Said S, Mikhail S, Riad M. Recent trends for clean fuel using environmental protecting oxidative desulfurization process. Clean Chem Eng. 2025;11:100140. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.clce.2024.100140.10.1016/j.clce.2024.100140Suche in Google Scholar
[14] Mohammad AA, AlJimaz AS, AlKhaldi KHAE, Alenzi AF, AlTuwaim MS. Evaluation of two imidazolium dicyanamide ionic solvents for extractive desulfurization of model fuels. J Ion Liq. 2024;4:100121. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jil.2024.100121.10.1016/j.jil.2024.100121Suche in Google Scholar
[15] Tahir S, Qazi UY, Naseem Z, Tahir N, Zahid M, Javaid R, et al. Deep eutectic solvents as alternative green solvents for the efficient desulfurization of liquid fuel: A comprehensive review. Fuel. 2021;305:16. 10.1016/j.fuel.2021.121502.Suche in Google Scholar
[16] Tian F, Wang W, Liu B, Pan Y, Dong B, Li Y, et al. Synergistic effect between CoSx and MoS2 at the micrometer scale: Considerable promotion of the hydrodesulfurization of DBT. Chem Eng J. 2024;484:149579. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cej.2024.149579.10.1016/j.cej.2024.149579Suche in Google Scholar
[17] Liang J, Fan M, Wu M, Hua J, Cai W, Huang T, et al. In situ synthesis of MoS2 nanoflakes within a 3D mesoporous carbon framework for hydrodesulfurization of DBT. J Catal. 2022;415:153–64. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jcat.2022.10.006.10.1016/j.jcat.2022.10.006Suche in Google Scholar
[18] Zou Y, Xiao C, Kong X, Qiao L, Wang W, Wang C, et al. Influence of grain size of acidic NiMo/TS-1 on its catalytic performance for hydrodesulfurization of dibenzothiophenes. Carbon Resour Convers. 2025;8:100299. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.crcon.2024.100299.10.1016/j.crcon.2024.100299Suche in Google Scholar
[19] Pu N, Hu A, Yang Q, Zheng A, Chen L, Wang Z, et al. Atomic decoration of MoS2 using Fe, Co or Ni for highly efficient and selective hydrodesulfurization. Chem Eng J. 2024;499:156100. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cej.2024.156100.10.1016/j.cej.2024.156100Suche in Google Scholar
[20] Liu B, Lv H, Tian F, Xue W, Cui X, Pan Y, et al. Fabrication of a Single-Atom Mo catalyst for significantly enhanced hydrodesulfurization activity of dibenzothiophene. Chem Eng J. 2025;505:159747. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cej.2025.159747.10.1016/j.cej.2025.159747Suche in Google Scholar
[21] Feng Y, Deng J, Lang W, Chen D, Zhu Z, Yang Z, et al. Efficient hydrotreatment of waste tire pyrolysis oil using N-doped biochar loaded with cobalt-molybdenum carbide. J Hazard Mater. 2024;480:136238. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jhazmat.2024.136238.10.1016/j.jhazmat.2024.136238Suche in Google Scholar PubMed
[22] Keivanimehr F, Habibzadeh S, Mokhtarian M. Enhanced product quality through hydrodesulfurization of pyrolysis gasoline over a mixed metal oxide catalyst: An experimental and DFT study. Fuel. 2022;317:123458. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2022.123458.10.1016/j.fuel.2022.123458Suche in Google Scholar
[23] Al-zaqri N, Alsalme A, Adil SF, Alsaleh A, Alshammari SG, Alresayes SI, et al. Comparative catalytic evaluation of nickel and cobalt substituted phosphomolybdic acid catalyst supported on silica for hydrodesulfurization of thiophene. J Saudi Chem Soc. 2017;21:965–73. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jscs.2017.05.004.10.1016/j.jscs.2017.05.004Suche in Google Scholar
[24] Jiang W, Dong L, Liu W, Guo T, Li H, Yin S, et al. Biodegradable choline-like deep eutectic solvents for extractive desulfurization of fuel. Chem Eng Process: Process Intensif. 2017;115:34–8. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cep.2017.02.004.10.1016/j.cep.2017.02.004Suche in Google Scholar
[25] Aghaei A, Sobati MA. Extraction of sulfur compounds from middle distillate fuels using ionic liquids and deep eutectic solvents: A critical review. Fuel. 2022;310:122279. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2021.122279.10.1016/j.fuel.2021.122279Suche in Google Scholar
[26] Juliao D, Gomes AC, Cunha-Silva L, Pillinger M, Gonçalves IS, Balula SS. Dichloro and dimethyl dioxomolybdenum(VI)-bipyridine complexes as catalysts for oxidative desulfurization of dibenzothiophene derivatives under extractive conditions. J Organomet Chem. 2022;967:10. 10.1016/j.jorganchem.2022.122336.Suche in Google Scholar
[27] Balaraman H, Rathnasamy S. Synergetic ultrasound assisted catalytic oxidative extractive desulfurization of tire pyrolysis oil employing sustainable protic deep eutectic solvents. Fuel. 2023;351:129031. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2023.129031.10.1016/j.fuel.2023.129031Suche in Google Scholar
[28] Li Z, Fu YH, Zhou AN, Yang C. Desulfurization of model FCC gasoline by extraction with ionic liquid and conventional extraction solvents. Pet Sci Technol. 2017;35:1699–705. 10.1080/10916466.2017.1358280.Suche in Google Scholar
[29] Katasonova ON, Savonina EY, Maryutina TA. Extraction methods for removing sulfur and its compounds from crude oil and petroleum products. Russ J Appl Chem. 2021;94:411–36. 10.1134/s1070427221040017.Suche in Google Scholar
[30] Mohammed MY, Ali AM, Albayati TM. Choline chloride-based deep eutectic solvents for ultrasonic-assisted oxidative desulfurization of actual heavy crude oil. Chem Eng Res Des. 2022;182:659–66. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cherd.2022.03.047.10.1016/j.cherd.2022.03.047Suche in Google Scholar
[31] Almashjary KH, Khalid M, Dharaskar S, Jagadish P, Walvekar R, Gupta TCSM. Optimisation of extractive desulfurization using Choline Chloride-based deep eutectic solvents. Fuel. 2018;234:1388–400. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2018.08.005.10.1016/j.fuel.2018.08.005Suche in Google Scholar
[32] Wu PW, Deng C, Liu F, Zhu HN, Chen LL, Liu RY, et al. Magnetically recyclable high-entropy metal oxide catalyst for aerobic catalytic oxidative desulfurization. Chin J Catal. 2023;54:238–49. 10.1016/s1872-2067(23)64541-3.Suche in Google Scholar
[33] Ahmed B, Ahmad Z, Naz S, Ihsan A, Khan B. Oxidative desulfurization of liquid fuels using deep eutectic solvents as a catalyst and extractant: A review. Chem Eng Res Des. 2024;211:253–68. 10.1016/j.cherd.2024.10.006.Suche in Google Scholar
[34] Mushtaq A, Nadeem M, Shaaban IA, Assiri MA, Sajid M, Khan MA, et al. Catalytic oxidative desulfurization of thio-compounds by employing? -Anderson-type polyoxometalates-porphyrin covalent organic framework (COF). Tetrahedron. 2023;144:13. 10.1016/j.tet.2023.133575.Suche in Google Scholar
[35] Yue JS, Li M, Ding N, Cheng SD, Gao CY. Effect of oxalic acid and sodium hydroxide on the desulfurization of coal using UV-H2O2 oxidation system. J Sulfur Chem. 2022;43:37–52. 10.1080/17415993.2021.1970166.Suche in Google Scholar
[36] Ebrahimi AA, Bandehali S. A review paper: Recent advance catalysts for desulfurization of fuel oil by ODS technology. J Ind Eng Chem. 2025;147:85–103. 10.1016/j.jiec.2024.12.054.Suche in Google Scholar
[37] Armandsefat F, Hamzehzadeh S, Azizi N, Hosseini S. Deep eutectic solvents as sustainable and extraordinary all-in-one systems for oxidative desulfurization. J Mol Liq. 2024;410:8. 10.1016/j.molliq.2024.125560.Suche in Google Scholar
[38] Sun XX, Zhou NR, Liu MM. Adsorption desulfurization over porous carbons derived from ZIF-67 and AC. J Solid State Chem. 2023;322:10. 10.1016/j.jssc.2023.123985.Suche in Google Scholar
[39] Li L, Sun J, Ling H, Ju F. Reactive adsorption desulfurization of FCC gasoline over self-sulfidation adsorbent. Sep Purif Technol. 2023;318:10. 10.1016/j.seppur.2023.123989.Suche in Google Scholar
[40] Chen M, Zhang D, Wang Z, Zhang Y, Liu Y. Investigation on the adsorption desulfurization effect of carboxyl and phosphotungstic acid modified UiO-66. Inorg Chim Acta. 2022;542:121135. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.ica.2022.121135.10.1016/j.ica.2022.121135Suche in Google Scholar
[41] Sun Y, Li L, Ju F, Ling H. Evolution of nickel species in reactive adsorption desulfurization of benzothiophene. Sep Purif Technol. 2022;283:120204. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.seppur.2021.120204.10.1016/j.seppur.2021.120204Suche in Google Scholar
[42] Chen Z, Yang G, Mu T, Yang M, Samak NA, Peh S, et al. Rate-based model for predicting and evaluating H2S absorption in the haloalkaliphilic biological desulfurization process. J Ind Eng Chem. 2022;110:479–90. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jiec.2022.03.020.10.1016/j.jiec.2022.03.020Suche in Google Scholar
[43] Chen Z, Hao X, Wen Q, Jia Y, Samak NA, Yang M, et al. Alleviating the thiols-induced inhibition of bio-sulfur particles and bio-oxidation in the biological desulfurization process under haloalkaline conditions. Chem Eng J. 2024;496:153683. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cej.2024.153683.10.1016/j.cej.2024.153683Suche in Google Scholar
[44] Cattaneo CR, Muñoz R, Korshin GV, Naddeo V, Belgiorno V, Zarra T. Biological desulfurization of biogas: A comprehensive review on sulfide microbial metabolism and treatment biotechnologies. Sci Total Environ. 2023;893:164689. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.scitotenv.2023.164689.10.1016/j.scitotenv.2023.164689Suche in Google Scholar PubMed
[45] Johnston KAKY, van Lankveld M, de Rink R, Mol AR, Keesman KJ, Buisman CJN. Influence of oxidation–reduction potential and pH on polysulfide concentrations and chain lengths in the biological desulfurization process under haloalkaline conditions. Water Res. 2024;259:121795. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.watres.2024.121795.10.1016/j.watres.2024.121795Suche in Google Scholar PubMed
[46] Mu T, Yang M, Xing J. Performance and characteristic of a haloalkaliphilic bio-desulfurizing system using Thioalkalivibrio verustus D301 for efficient removal of H2S. Biochem Eng J. 2021;165:107812. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.bej.2020.107812.10.1016/j.bej.2020.107812Suche in Google Scholar
[47] Jha D, Maheshwari P, Singh Y, Haider MB, Kumar R, Balathanigaimani MS. A comparative review of extractive desulfurization using designer solvents: Ionic liquids & deep eutectic solvents. J Energy Inst. 2023;110:101313. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.joei.2023.101313.10.1016/j.joei.2023.101313Suche in Google Scholar
[48] Jha D, Haider MB, Kumar R, Balathanigaimani MS. Extractive desulfurization of fuels using diglycol based deep eutectic solvents. J Environ Chem Eng. 2020;8:104182. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jece.2020.104182.10.1016/j.jece.2020.104182Suche in Google Scholar
[49] Li CP, Zhang JJ, Li Z, Yin JM, Cui YN, Liu Y, et al. Extraction desulfurization of fuels with ‘metal ions’ based deep eutectic solvents (MDESs). Green Chem. 2016;18:3789–95. 10.1039/c6gc00366d.Suche in Google Scholar
[50] Chandran D, Khalid M, Walvekar R, Mubarak NM, Dharaskar S, Wong WY, et al. Deep eutectic solvents for extraction-desulphurization: A review. J Mol Liq. 2019;275:312–22. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.molliq.2018.11.051.10.1016/j.molliq.2018.11.051Suche in Google Scholar
[51] Lima F, Branco LC, Silvestre AJD, Marrucho IM. Deep desulfurization of fuels: Are deep eutectic solvents the alternative for ionic liquids? Fuel. 2021;293:120297. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2021.120297.10.1016/j.fuel.2021.120297Suche in Google Scholar
[52] Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem Rev. 2014;114:11060–82. 10.1021/cr300162p.Suche in Google Scholar PubMed
[53] Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;1:70–1. 10.1039/B210714G.Suche in Google Scholar
[54] Liu X, Fang J, Zheng W, Tan Z, Zheng X, Di J. Study on desulfurization mechanism of ionic liquid extractant based on Gaussian quantitative calculation. Comput Theor Chem. 2021;1204:113353. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.comptc.2021.113353.10.1016/j.comptc.2021.113353Suche in Google Scholar
[55] Yu G, Jin D, Zhang F, Li Q, Zhou Z, Ren Z. Oxidation-extraction desulfurization of fuel with a novel green acidic deep eutectic solvent system. Fuel. 2022;329:125495. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2022.125495.10.1016/j.fuel.2022.125495Suche in Google Scholar
[56] Bösmann A, Datsevich L, Jess A, Lauter A, Schmitz C, Wasserscheid P. Deep desulfurization of diesel fuel by extraction with ionic liquids. Chemical Communications. 2001:2494-5. 10.1039/B108411A.Suche in Google Scholar PubMed
[57] Asumana C, Yu G, Li X, Zhao J, Liu G, Chen X. Extractive desulfurization of fuel oils with low-viscosity dicyanamide-based ionic liquids. Green Chem. 2010;12:2030–7. 10.1039/C0GC00118J.Suche in Google Scholar
[58] Ban L-L, Liu P, Ma C-H, Dai B. Deep extractive desulfurization of diesel fuels by FeCl3/ionic liquids. Chin Chem Lett. 2013;24:755–8. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cclet.2013.04.031.10.1016/j.cclet.2013.04.031Suche in Google Scholar
[59] Li Z, Li C, Chi Y, Wang A, Zhang Z, Li H, et al. Extraction process of dibenzothiophene with new distillable amine-based protic ionic liquids. Energy Fuels. 2012;26:3723–7.10.1021/ef3005064Suche in Google Scholar
[60] Lu X, Yue L, Hu M, Cao Q, Xu L, Guo Y, et al. Piperazinium-based ionic liquids with lactate anion for extractive desulfurization of fuels. Energy Fuels. 2014;28:1774–80.10.1021/ef402154jSuche in Google Scholar
[61] Gutierrez A, Atilhan M, Aparicio S. Theoretical study of oil desulfuration by ammonium-based deep eutectic solvents. Energy Fuels. 2018;32:7497–507.10.1021/acs.energyfuels.8b01403Suche in Google Scholar
[62] Li CP, Li D, Zou SS, Li Z, Yin JM, Wang AL, et al. Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents. Green Chem. 2013;15:2793–9. 10.1039/c3gc41067f.Suche in Google Scholar
[63] Xu LX, Luo YP, Liu H, Yin J, Li HP, Jiang W, et al. Extractive desulfurization of diesel fuel by amide-based type IV deep eutectic solvents. J Mol Liq. 2021;338:8. 10.1016/j.molliq.2021.116620.Suche in Google Scholar
[64] Saini N, Negi M, Yadav P, Singh R. Oxidative desulfurization of fuels using alcohol-based DESs. Environ Sci Pollut Res. 2024;330934. 10.1007/s11356-024-33093-4.Suche in Google Scholar PubMed
[65] Zarin L, Saien J, Jafari F, Ahmadi F. Simultaneous extraction of sulfur and nitrogen contents from fuel: Emphasizing the recent progress of the green solvents: A review. J Mol Liq. 2024;415:126276. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.molliq.2024.126276.10.1016/j.molliq.2024.126276Suche in Google Scholar
[66] Yan J, Luo F, Du Y, Yi Q, Hao X, Dong H, et al. Extraction desulfurization with mixed solvents of organic solvent + organic solvent or deep eutectic solvent as extractants: Liquid-liquid equilibrium experiments and molecular dynamics simulations. Fluid Phase Equilibria. 2023;565:113655. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fluid.2022.113655.10.1016/j.fluid.2022.113655Suche in Google Scholar
[67] Zhang WX, Xu P, Chen ZR, Liu QH, Li GX, Cui PZ. Carboxylic acid-based deep eutectic solvent for efficient desulfurization: Experimental and computational thermodynamics. Fuel. 2024;378:9. 10.1016/j.fuel.2024.132975.Suche in Google Scholar
[68] Gosu V, Kumar R, Ramalingam A, Kumar UKA, Kashyap AK, Subbaramaiah V. Desulfurization of gasoline using deep eutectic solvents based on tetrabutylammonium bromide. J Chem Eng Data. 2022;67:2486–94. 10.1021/acs.jced.2c00172.Suche in Google Scholar
[69] Shu C, Sun T. Extractive desulfurisation of gasoline with tetrabutyl ammonium chloride-based deep eutectic solvents. Sep Sci Technol. 2016;51:1336–43. 10.1080/01496395.2016.1155602.Suche in Google Scholar
[70] Kobotaeva NS, Savinykh YV, Skorokhodova TS. Synthesis of deep eutectic solvents for the removal of sulfur-containingcompounds from the diesel fraction. Izv Vyss Uchebnykh Zaved Khim Khimichesk Tekhnol. 2024;67:68–75. 10.6060/ivkkt.20246708.5t.Suche in Google Scholar
[71] Zhang WS, Li Y, Zhang X, Shen J, Wang YG, Niu YX, et al. Study on extraction desulfurization of road-paving asphalt by deep eutectic solvents. J Ind Eng Chem. 2025;144:310–22. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jiec.2024.09.025.10.1016/j.jiec.2024.09.025Suche in Google Scholar
[72] Li JJ, Xiao H, Tang XD, Zhou M. Green carboxylic acid-based deep eutectic solvents as solvents for extractive desulfurization. Energy Fuels. 2016;30:5411–8. 10.1021/acs.energyfuels.6b00471.Suche in Google Scholar
[73] Khan N, Srivastava VC. Engineering evaluation of extractive desulfurization using ethylene glycol-based deep eutectic solvents: Kinetics and mass transfer study. Energy Fuels. 2024;38:10072–86. 10.1021/acs.energyfuels.4c00781.Suche in Google Scholar
[74] Babu MKS, Mehra S, Kumar A, Kancharla S. Diesel purification through imidazole-based deep eutectic solvents: Desulfurization, dearomatization, and denitrogenation. Fuel. 2025;387:12. 10.1016/j.fuel.2025.134317.Suche in Google Scholar
[75] Khezeli T, Daneshfar A. Synthesis and application of magnetic deep eutectic solvents: Novel solvents for ultrasound assisted liquid-liquid microextraction of thiophene. Ultrason Sonochem. 2017;38:590–7. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.ultsonch.2016.08.023.10.1016/j.ultsonch.2016.08.023Suche in Google Scholar PubMed
[76] Asumana C, Yu G, Guan Y, Yang S, Zhou S, Chen X. Extractive denitrogenation of fuel oils with dicyanamide-based ionic liquids. Green Chem. 2011;13:3300–5. 10.1039/c1gc15747g.Suche in Google Scholar
[77] Khalid A, Tahir S, Khalid AR, Hanif MA, Abbas Q, Zahid M. Breaking new grounds: metal salts based-deep eutectic solvents and their applications- A comprehensive review. Green Chem. 2024;26:2421–53. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1039/d3gc04112c.10.1039/D3GC04112CSuche in Google Scholar
[78] Yin J, Wang J, Li Z, Li D, Yang G, Cui Y, et al. Deep desulfurization of fuels based on an oxidation/extraction process with acidic deep eutectic solvents. Green Chem. 2015;17:4552–9. 10.1039/C5GC00709G.Suche in Google Scholar
[79] Balali M, Sobati MA, Gorji AE. QSPR modeling of thiophene distribution between deep eutectic solvent (DES) and hydrocarbon phases: Effect of hydrogen bond donor (HBD) structure. J Mol Liq. 2021;342:117496. 10.1016/j.molliq.2021.117496.Suche in Google Scholar
[80] Islam S, Rubio C, Rafikova K, Mutelet F. Desulfurization and denitrogenation using betaine-based deep eutectic solvents. J Chem Eng Data. 2024;69:2244–54. 10.1021/acs.jced.4c00052.Suche in Google Scholar
[81] Ahmed Rahma WS, Mjalli FS, Al-Wahaibi T, Al-Hashmi AA. Polymeric-based deep eutectic solvents for effective extractive desulfurization of liquid fuel at ambient conditions. Chem Eng Res Des. 2017;120:271–83. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cherd.2017.02.025.10.1016/j.cherd.2017.02.025Suche in Google Scholar
[82] Deng H, Wang X, Chen J, Zhao J, Jiang Z, Tian Z, et al. Experimental and molecular dynamics study of fuel desulfurization process using deep eutectic solvent. J Environ Chem Eng. 2023;11:110277. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.jece.2023.110277.10.1016/j.jece.2023.110277Suche in Google Scholar
[83] Mahdavi AR, Sobati MA, Movahedirad S. Extractive desulfurization of model diesel fuel in a micro-channel contactor using deep eutectic solvents (DESs). Fuel. 2022;327:14. 10.1016/j.fuel.2022.125111.Suche in Google Scholar
[84] Khan N, Srivastava VC. Extractive desulfurization using ethylene glycol and glycerol-based deep eutectic solvents: engineering aspects and intensification using ultrasound. Chem Eng Process. 2022;180:14. 10.1016/j.cep.2022.108973.Suche in Google Scholar
[85] Mahdavi AR, Sobati MA, Movahedirad S. Intensification of extractive desulfurization in micro-channels using triethylamine/propionic acid as deep eutectic solvent. Chem Eng Process. 2023;191:14. 10.1016/j.cep.2023.109459.Suche in Google Scholar
[86] Mahdavi AR, Sobati MA, Movahedirad S. Extractive desulfurization in microchannel contactors using deep eutectic solvents: optimization using central composite design (CCD) and flow pattern mapping. Energy Fuels. 2023;37:13658–72. 10.1021/acs.energyfuels.3c01663.Suche in Google Scholar
[87] Wang Y, Luan H, Gong J, Hua M, Wu P, Cheng H, et al. Mechanochemical driven oxidative desulfurization of high-sulfur petroleum coke over [Bpy]PMoVn coupled with amide-based binary deep eutectic solvents. Chem Eng Sci. 2025;304:121021. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.ces.2024.121021.10.1016/j.ces.2024.121021Suche in Google Scholar
[88] Wang XY, Li ZH, Wang XH, Wu CC, Gates ID, Guo SH, et al. Intermolecular interactions induced desulfurization/denitrification of oil with deep eutectic solvents. J Mol Liq. 2022;366:10. 10.1016/j.molliq.2022.120159.Suche in Google Scholar
[89] Shang Z, Xu P, Chen Z, Zhang W, Li G. Efficient extraction of S-heterocyclic aromatics from fuel oil with oxalic acid-based deep eutectic solvent. J Mol Liq. 2023;386:122531.10.1016/j.molliq.2023.122531Suche in Google Scholar
[90] Julião D, Gomes AC, Pillinger M, Lopes AD, Valença R, Ribeiro JC, et al. Desulfurization of diesel by extraction coupled with Mo-catalyzed sulfoxidation in polyethylene glycol-based deep eutectic solvents. J Mol Liq. 2020;309:113093. 10.1016/j.molliq.2020.113093.Suche in Google Scholar
[91] Jiang W, Zhu K, Jia H, Zhu L, Wang C, Xu L, et al. Synthesis of task-specific ternary deep eutectic solvents for deep desulfurization via reactive extraction. Chem Eng Process. 2022;171:108754.10.1016/j.cep.2021.108754Suche in Google Scholar
[92] Eskandari H, Shahvelayati AS, Bide Y. Synthesis of solid–liquid host–guest of melamine foam impregnated with deep eutectic solvent for highly effective desulfurization of fuel oil. Fuel. 2024;374:132540. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.fuel.2024.132540.10.1016/j.fuel.2024.132540Suche in Google Scholar
[93] Chen M, Zou C, Tang W, Trivedi J. Supramolecular-based preparation of deep eutectic solvents synergized with carbon nanotubes for oxidative desulfurization of fuels. J Mol Liq. 2025;424:127077. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.molliq.2025.127077.10.1016/j.molliq.2025.127077Suche in Google Scholar
[94] Chu F, Wei X, Lu B, Zhao G, Li Y, Yang K, et al. Flexible encapsulation polyoxometalate with electrostatically interaction into metal−organic framework for ultra-deep aerobic oxidation desulfurization of diesel via a biomimetic approach in deep eutectic solvents. Chem Eng J. 2024;481:148549. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.cej.2024.148549.10.1016/j.cej.2024.148549Suche in Google Scholar
[95] Liu Y, Su X, Cui Y, Zhou X. One-step preparation of deep eutectic solvents/reduced graphene oxide composite materials for the removal of dibenzothiophene in fuel oil. Sci Rep. 2023;13:832.10.1038/s41598-023-28041-0Suche in Google Scholar PubMed PubMed Central
[96] Chen M, Zou C, Tang W, Cao Y. Stable hydrogen bonding interactions in supramolecular deep eutectic solvents based on carbon quantum dots: For extraction and oxidative desulfurization. Sep Purif Technol. 2023;323:124491. https://wgmktpgxscrjl-s.p.lib.tju.edu.cn/10.1016/j.seppur.2023.124491.10.1016/j.seppur.2023.124491Suche in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Artikel in diesem Heft
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Optimizing ultrasound-assisted extraction process of anti-inflammatory ingredients from Launaea sarmentosa: A novel approach
- Eggshell membranes as green carriers for Burkholderia cepacia lipase: A biocatalytic strategy for sustainable wastewater bioremediation
- Research progress of deep eutectic solvents in fuel desulfurization
- Enhanced electrochemical synthesis of Ni–Fe/brass foil alloy with subsequent combustion for high-performance photoelectrode and hydrogen production applications
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

