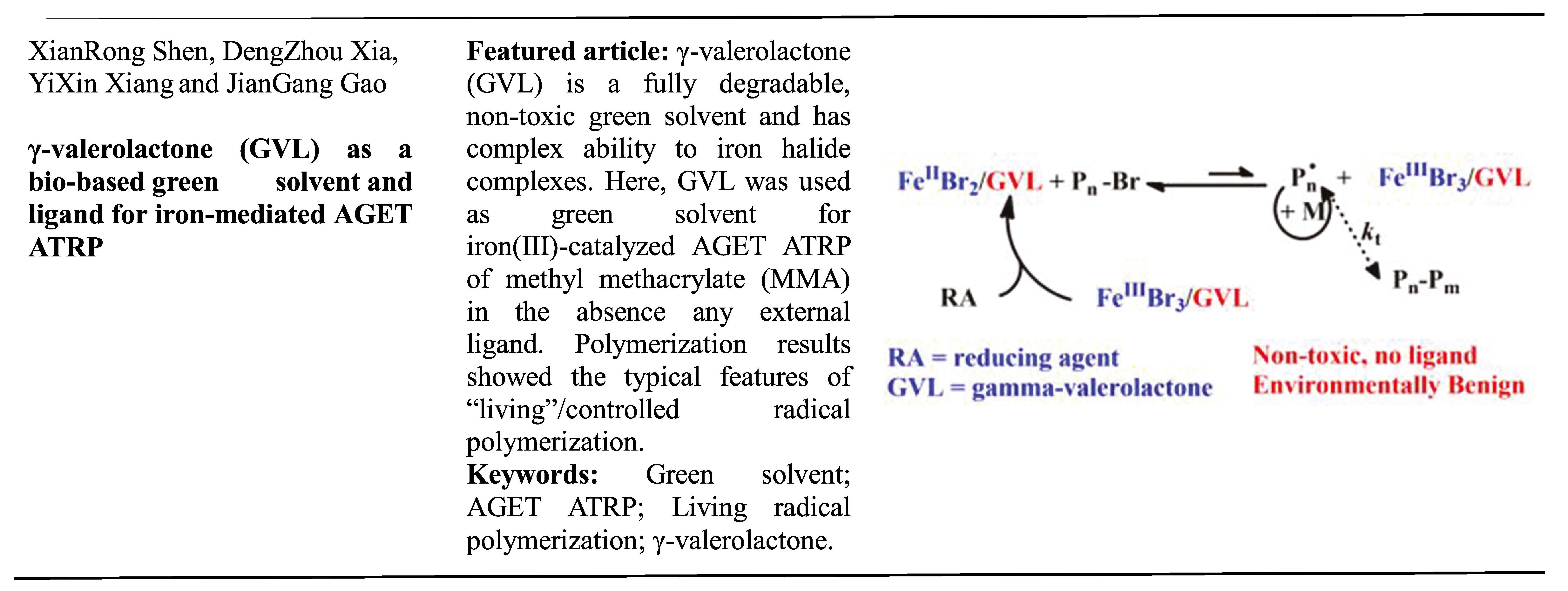
Abstract
In this paper, γ-valerolactone (GVL), a bio-based polar solvent, was applied as green solvent for iron(III)-catalyzed AGET ATRP without any external ligand. GVL is a fully degradable, non-toxic green solvent and has complex ability to iron halide complexes through –OCO- group. GVL as the solvent and the ligand for AGET ATRP of MMA in a controlled manner, as proved by kinetic study, the low PDI values and the increase in polymer molecular weight versus monomer conversion. Chain re-initiation experiments and 1HNMR characterization were conducted to further confirm the living feature.
Graphical Abstract
1 Introduction
Transition metal complex catalyzed ATRP (Atom Transfer Radical Polymerization) for the synthesis of well-defined polymers was reported over 20 years ago (1, 2, 3). For achieving a controlled/living polymerization process, it should maintain low numbers of growth free radicals and large excess of dormant centers in the ATRP reaction medium to suppress chain termination reactions, and should quickly establish a balance between the transition metal complexes in the low oxidation state and the higher oxidation state complexes which acts as the deactivator. Metal complexes play an important role on this equilibrium in regulating the catalytic cycle associated with the efficiency of the catalytic system. Various metal catalysis such as copper, ruthenium, iron, other transition metals have been investigated (4). Among these metals, copper-based ATRP catalysts are the most deeply investigated metal for the synthesis of well controlled polymers (5). However, the development of iron-based catalysts remains of great attention due to its unique advantages of low toxicity, abundance, low cost, biocompatibility and environmental friendliness (6).
In a typical iron-mediated ATRP catalytic system, ligands should be added to complex with iron halide to form iron-based catalysts and to modulate the redox potential of metal centers to maintain proper reactivity and kinetics of atom transfer during ATRP. Phosphorus-based ligands and simple amines ligands have been widely tested in the iron mediated ATRP systems (7). However, due to the expensive or toxic problems, these ligands have been limited in some areas such as biological materials. For this reason, some cheap and relatively less toxic ‘‘green’’ organic acids ligands, such as, iminodiacetic acid (8), pyromellitic acid (9), isophthalic acid (10) and succinic acid (11), also have been considered in iron-mediated ATRP. Nowdays, biocatalysis has become a research hot topic in polymer chemistry field. Some proteins, such as hemoglobin (12), have the complex ability to iron species, these compounds have also been applied as ligands for iron-mediated ATRP of vinyl monomers. Interestingly, where iron-mediated ATRP conducted in some traditional polar solvent, such as DMF, MeCN, DMSO and NMP, use of external ligands becomes unnecessary; Polar solvent works as the dual functions of ligand and solvent (13,14). However, these traditional organic solvent are harmful to the environment for its hazardous, toxic. To avoid these unfavorable effects, it is urgent to select more environmentally friendly and inexpensive solvents to extend the application of iron catalysts. For this reason, Some ‘green’ solvents, such as water (15,16), poly (ethylene glycol) (17), crown ether (18) and ionic liquids (19) have been selected as reaction media for ATRP reaction, and some of these also could be worked both as solvent and ligand for iron-catalyzed ATRP (17, 18, 19, 20).
Recently, utilization of renewable resources derived organic solvents has attracted more attention around the chemical world. Some bio-based green solvents now have been successfully employed in several chemical reactions such as glycerol and ethyl lactate (21, 22, 23). Among these agricultural biomass derived solvents, γ-valerolactone (GVL) is a room temperature stable liquid and has very low toxicity (LD50 oral rats = 8,800 mg/kg) (24). Since Dumesic reported an industrially acceptable process to produce GVL from biomass lignocellulosic, it makes suitable for the replacements of petroleum-derived solvents on a large scale (25). GVL has many excellent properties making it an ideal choice to traditional polar solvent alternatives. The polarity of GVL and other common polar solvents is very similar; For example, the measured GVL dielectric constant is 36.47 at 25°C, while the dielectric constants of DMF, NMP, CH3CN, and DMA are 36.7, 32.0, 37.5, and 37.8, respectively (26). Although many work has been reported to utilize GVL instead of common polar solvents in chemical processes. However, to date, there has never been a report of the application of γ-valerolactone to ATRP catalytic system (27,28).
It is well known that ROP of ε-caprolactone catalyzed by metal Lewis acid catalyst through coordination-insertion mechanism, and ε-caprolactone monomer has complex ability to metal chlorite through –OCO- group (Scheme 1) (29,30). These results mentioned us that ε-caprolactone and its derivatives which have lactones group could be act as solvent and ligand for iron-mediated ATRP. However, the ring of ε-caprolactone can be opened by a lewis acid catalyst; it is unsuitable candidate as solvent and ligand for ATRP reaction (31,32). The valerolactone ring has a lower tension and difficult to open polymerization, and may be applied as a solvent and ligand to iron-mediated ATRP (33).
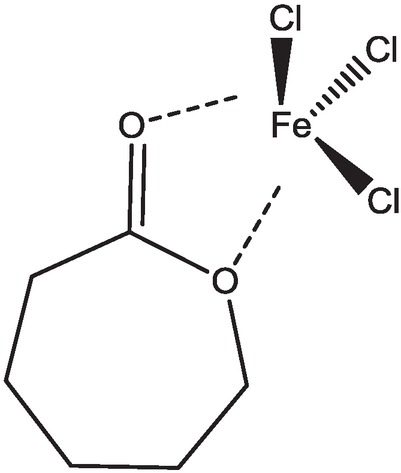
Coordination between ε-caprolactone and FeCl3.
As mentioned above, lactones which have a seven-membered ring with high tension like ε-caprolactone can catalyzed by metal Lewis acid via ring open polymerization. For this reason, in this paper, we choose ‘non-polymerizable’ lactones with only five-membered ring like γ-valerolactone as solvent and ligand for Fe-mediated ATRP. Moreover, γ-butyrolactone, γ-caprolactone, γ-octanolactone which all have low tension five-membered ring also have been discussed as solvent and ligand to further confirm that bio-based γ-valerolactone was a high efficient solvent for this catalysis system.
2 Materials and methods
2.1 Materials
Methyl methacrylate (MMA, Aladdin Industrial Corporation (China)) (AR) was passed through a basic alumina column to remove the radical inhibitor in monomer before use. γ-valerolactone (GVL) (CP, Alfa Aesar), γ-butyrolactone (CP, Alfa Aesar), γ-caprolactone (CP, Alfa Aesar), γ-octanolactone (CP, Alfa Aesar), sodium ascorbate (Sinopharm Chemical Reagent Co. Ltd., SCRC, AsAc-Na), FeBr3 (Alfa Aesar), and ethyl bromophenylacetate (98%, Alfa Aesar, EBPA) were used as received. All other solvents and reagents were used as received except as noted.
2.2 Typical procedure for the AGET ATRP of MMA in γ-valerolactone
In a typical ATRP experiment, FeBr3 (21 mg, 0.07 mmol) was dissolved in 1.5 mL γ-valerolactone in a clean glass tube. Then, 28 mg AsAc-Na (0.14 mmol) and 3 mL MMA (28.2 mmol) were added, and the mixture was bubbled with nitrogen for 15 min and then sealed with a rubber septum. EBPA (25 μL, 0.14 mmol) was subsequently introduced via a syringe, then immersed in a thermostated oil bath at the designated temperature. After an expected time, the tube was opened to stop the reaction. The product PMMA was obtained after precipitation in large amounts of methanol and water mixture, filtered, and dried in vacuo to constant weight. The conversion of the monomer was determined gravimetrically.
2.3 Chain extension experiment
Chain extension was performed employing the above mentioned AGET ATRP technique in γ-valerolactone. In a polymerization tube, 0.18 g (0.0475 mmol, Mn = 3800 g/mol) of PMMA macroinitiator was dissolved in 3 mL (28.2 mmol) MMA under stirring and nitrogen atmosphere. Next, 14 mg (0.0475 mmol) FeBr3 and 18.7 mg (0.095 mmol) AsAc-Na were placed in 1.5 mL γ-valerolactone in another tube under stirring. Both solutions were then mixed, bubbled with nitrogen for 15 min, and sealed with a rubber septum. After an expected time, the tube was opened to stop the reaction. The chain-extended PMMA was obtained after precipitation in large amounts of methanol, filtered, and dried in vacuo to constant weight.
2.4 Characterization
Monomer conversion was determined by gravimetry and number average (Mn), and molecular weight distributions were determined by gel permeation chromatograph (GPC) on a PL GPC220 equipped with two PLgel 5-μm MIXED-C columns using a series of PMMA standards for calibrations. THF was used as the eluent at a flow rate of 1.0 mL/min at 40°C. The 1H NMR spectrum of the obtained samples were recorded on a Bruker Avance III 400 MHz spectrometer using CDCl3 as the solvent with TMS as an internal standard.
3 Result and discussion
3.1 Iron-mediated AGET ATRP in γ-valerolactone
In the previous experiment, we found that no polymer was formed with only γ-valerolactone, FeBr3 deactivator and sodium ascorbate (i.e., in absence of MMA) after 72 h reaction time. The result indicated that γ-valerolactone cannot be polymerized under the AGET ATRP reaction condition. Subsbsequently, iron-mediated ATRP of MMA was conducted in γ-valerolactone and its derivatives, such as γ-butyrolactone, γ-caprolactone, γ-octanolactone (Table 1). The typical polymerization was performed at 75°C under the ratio [MMA]:[EBPA]:[FeBr3]:[AsAc-Na]= 200:1:0.5:1 (EBPA:ethyl 2-bromo-2-phenylacetate, AsAc-Na: sodium ascorbate), VMMA/Vsolvent = 3 mL/1.5 mL, in the absence of any external ligand. After 5 h reaction time, the monomer conversions had reached 70.1%, 66.1%, 59.8%, 61.2% in γ-valerolactone, γ-butyrolactone, γ-caprolactone, γ-octanolactone, respectively. All these catalytic system provided PMMA with low PDI values (Mw/Mn < 1.3) and controlled molecular weight. The structure of PMMA samples made using these solvent and γ-valerolactone itself were also characterized by 1H NMR spectroscopy (supplementary material, Figures S1-S5). The calculated molecular weights were close to the values obtained by GPC analysis. The clear disappearance of GVL signals was further confirmed that the successful synthesis of PMMA polymer without any trace of ROP of the γ-valerolactone. The experiments results obviously indicated that the GVL and its derivatives could play a dual function both as green solvent and efficient ligand to mediate these iron catalysis system.
AGET ATRP of MMA in GVL and its derivatives.
| Solvents | Conversion % | Mn,GPC | Mn,theo | PDI |
|---|---|---|---|---|
| γ-valerolactone | 70.1 | 15900 | 14300 | 1.27 |
| γ-butyrolactone | 66.1 | 15200 | 13500 | 1.25 |
| γ-caprolactone | 59.8 | 13500 | 12300 | 1.25 |
| γ-octanolactone | 61.2 | 13800 | 12500 | 1.26 |
Experiment conditions: [MMA]0/[EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/0.5/1 at 75°C, VMMA = 3 mL, Vsolvent = 1.5 mL. Reaction time = 5 h.
To expand the applicability of this catalytic system, a series of methacrylate monomer such as ethyl methacrylate, butyl methacrylate, benzyl methacrylate were also polymerized using FeBr3/GVL as a catalyst. The results are shown in Table 2, ethyl methacrylate was well initiated and reached up to 60% conversion, yielded polymers with low dispersity (Mw/Mn = 1.24) and controlled molecular weight. The same polymerization behaviors were also observed in the butylmethacrylate, benzylmethacrylate were systems, indicating the well controllability.
Polymerization for different monomers.
| Monomor | Conversion % | Mn,GPC | Mn,theo | PDI |
|---|---|---|---|---|
| Methyl methacrylate | 70.1 | 15900 | 14300 | 1.27 |
| Ethyl methacrylate | 60.1 | 16200 | 14000 | 1.24 |
| Butyl methacrylate | 50.6 | 16500 | 14600 | 1.25 |
| Benzyl methacrylate | 57.4 | 23800 | 20500 | 1.32 |
Experiment conditions: [Monomer]0/[EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/0.5/1 at 75°C, Vsolvent = 1.5 mL. Reaction time = 5 h.
3.2 Effect of concentration of FeBr3 on AGET ATRP of MMA
It is desirable to conduct ATRP with small amounts of FeBr3. However, sufficient catalysis dosage should be added to maintain a suitable control ability where using some polar solvent as solvent and ligand. Here, the polymerizations of MMA with different concentration of FeBr3 catalyst (5000 ppm to 5 ppm) were investigated. The results (Table 3) indicated that the polymerizations were well controlled when FeBr3 usage low to 50 ppm, which is reflected in the low molecular weight distribution and the designed molecular weight value. If the catalyst concentration is further lowered to 5 ppm, the polymerization reaction is out of control, giving PMMA a broad molecular weight distribution (PDI = 2.35). Anyway, FeBr3/GVL here is a highly active catalyst system and can be successfully conducted at low catalysis concentration.
AGET ATRP of MMA with different amounts of FeBr3.
| [MMA]0/[EBPA] 0/ [FeBr3]0/[VC-Na]0 | Fe (ppm) | Conversion % | Mn,GPC | Mn,theo | PDI |
|---|---|---|---|---|---|
| 200:1:1:1 | 5000 | 63.9 | 11900 | 13000 | 1.15 |
| 200:1:0.5:1 | 2500 | 70.1 | 15900 | 14300 | 1.27 |
| 200:1:0.1:1 | 500 | 66.9 | 15200 | 13600 | 1.30 |
| 200:1:0.01:1 | 50 | 59.5 | 14600 | 12200 | 1.39 |
| 200:1:0.001:0.1 | 5 | 42.6 | 19800 | 8900 | 2.35 |
Experiment conditions: [MMA]0/[EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/x/y at 75°C, VMMA = 3 mL, Vsolvent = 1.5 mL. Reaction time = 5 h.
3.3 Effect of temperature on AGET ATRP of MMA
Figure 1a depicts the kinetic results of the AGET ATRP of MMA in GVL at different temperature (60°C, 75°C, 90°C respectively). As shown in Figure 1a, when polymerization performed at low temperature (60°C, 75°C), approximately first-order kinetics have been observed, these means a constant numbers of growth radicals during the whole reaction processes. Meantime, an induction period appeared in the cases of lower reaction temperatures (1h at 75°C, 2 h at 60°C). These may be caused by slow generation rate of FeBr2 species in the initial stage of polymerization reaction, and needed enough time to build a dynamic equilibrium between the active species (iron(II)) and deactive species (iron(III)). Polymerization rate increased as the temperature increased from 75°C to 90°C, and the induction period was disappearance. The ln([M]0/[M]) plots versus reaction time is linear up until the reaction time was about 1 h (45.6% conversion) and a platform was observed. This means that the polymerizations is consistent with first order kinetics and indicates that the growth radical numbers was constant during the initial stage time of the reaction. At longer reaction period, the polymerizations slowed. The possible reason is that higher temperature can accelerate the reduction rates resulted in a higher concentration of iron(II) species and then increased the propagating radicals concentration, causing an increase proportion of termination reaction.
![Figure 1 (a) Kinetic plots of ln([M]0/[M]) versus time and (b) Mn,GPC and Mw/Mn versus conversion for the AGET ATRP of MMA in GVL at different polymerization temperatures without any external ligands. Experiment conditions: [MMA]0/[EBPA]0/[FeBr3]0/[ AsAc-Na]0 = 200/1/0.5/1, VMMA = 3 mL, Vsolvent = 1.5 mL.](/document/doi/10.1515/epoly-2019-0033/asset/graphic/j_epoly-2019-0033_fig_002.jpg)
(a) Kinetic plots of ln([M]0/[M]) versus time and (b) Mn,GPC and Mw/Mn versus conversion for the AGET ATRP of MMA in GVL at different polymerization temperatures without any external ligands. Experiment conditions: [MMA]0/[EBPA]0/[FeBr3]0/[ AsAc-Na]0 = 200/1/0.5/1, VMMA = 3 mL, Vsolvent = 1.5 mL.
Figure 1b depicted the dependence of Mn and Mw/Mn versus the monomer conversion. It can be concluded that measured Mn,GPC values of the obtained PMMA increased linearly with monomer conversion while maintaining relatively low PDI values (Mw/Mn < 1.35), however, these values were slightly higher than the calculated Mn,theo values. Gel permeation chromatography (GPC) traces of polymers obtained at low reaction temperature shifted clearly and completely to higher Mw with conversion (Figure 2). These results all strongly suggest that the polymerization of MMA was well-controlled in GVL solvent at low polymerization temperature.
![Figure 2 GPC traces for the polymerization of MMA in GVL at 60°C. Experiment conditions: [MMA]0/[EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/0.5/1, VMMA = 3 mL, Vsolvent = 1.5 mL.](/document/doi/10.1515/epoly-2019-0033/asset/graphic/j_epoly-2019-0033_fig_003.jpg)
GPC traces for the polymerization of MMA in GVL at 60°C. Experiment conditions: [MMA]0/[EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/0.5/1, VMMA = 3 mL, Vsolvent = 1.5 mL.
3.4 Analysis of chain end and chain extension
1H NMR spectrum of the PMMA (Mn,GPC = 3800 g mol−1, Mw/Mn = 1.21) was measured for analyzing chain end structure (Figure 3) and calculating Mn,NMR valure.
![Figure 3 1H NMR spectrum of PMMA (Mn,GPC = 3.8 ×103 g/mol, Mw/ Mn = 1.21) with CDCl3 as the solvent. Experiment conditions: [MMA]0/ [EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/0.5/1 at 60°C, VMMA = 3 mL, Vsolvent = 1.5 mL. Reaction time = 3.5 h. Conversion = 17.1%.](/document/doi/10.1515/epoly-2019-0033/asset/graphic/j_epoly-2019-0033_fig_004.jpg)
1H NMR spectrum of PMMA (Mn,GPC = 3.8 ×103 g/mol, Mw/ Mn = 1.21) with CDCl3 as the solvent. Experiment conditions: [MMA]0/ [EBPA]0/[FeBr3]0/[AsAc-Na]0 = 200/1/0.5/1 at 60°C, VMMA = 3 mL, Vsolvent = 1.5 mL. Reaction time = 3.5 h. Conversion = 17.1%.
The chemical shift at 3.8 ppm (c’ in Figure 3) was corresponded to the methyl ester group at the chain end, as mentioned by Sawamoto et al. (34), the signal at 3.60 ppm (c in Figure 3) attributed to other methyl ester groups in main PMMA chains. The chemical shifts at 3.9–4.2 ppm (d in Figure 3), 3.4 ppm (e in Figure 3), and 7.1–7.4 ppm (f in Figure 3) belonged to the protons of methylene, methane, and phenyl derived from the initiator EBPA respectively. The molecular weight (Mn,NMR) of the PMMA can be calculated from the integrals in the 1H NMR spectrum, according to equation:
The calculated molecular weights (Mn,NMR = 4100 g mol−1) was in full agreement with the values obtained by GPC analysis (Mn,GPC = 3800 g mol−1), indicating that the PMMA obtained was end capped by the EBPA moieties with high fidelity. Additionally, according to the mechanism of AGET ATRP, the obtained PMMA with Br end groups could be applied as macroinitiators to operate chain re-initiation polymerization. Therefore, the same PMMA sample (Mn,GPC = 3800 g mol−1, Mw/Mn = 1.21, reaction condition shown in Figure 3) was applied as a macroinitiator in chain extension experiments to further confirm the mechanism of the AGET ATRP.
As shown in Figure 4, the GPC curves shown a peak shift from the original polymers to the chain extended PMMA with Mn,GPC = 16200 (Mw/Mn = 1.32). However, a slight increase in the value of Mw/Mn has been observed, which may be attributed to a slight polymer loss of its activity in the polymer. The successful re-initiation experiment further confirms that the AGET ATRP of MMA in GVL solvent catalyzed by FeBr3 was a high efficient ‘‘living’’ radical polymerization process.
![Figure 4 GPC traces of the PMMA before (right curve) and after the chain extension (left curve) experiment. Reaction conditions: [MMA]0/[PMMA-Br]0/[FeBr3]0/[ AsAc-Na]0 = 600/1/1/1, reaction temperature = 75°C.](/document/doi/10.1515/epoly-2019-0033/asset/graphic/j_epoly-2019-0033_fig_005.jpg)
GPC traces of the PMMA before (right curve) and after the chain extension (left curve) experiment. Reaction conditions: [MMA]0/[PMMA-Br]0/[FeBr3]0/[ AsAc-Na]0 = 600/1/1/1, reaction temperature = 75°C.
4 Conclusions
In summary, we here reported that bio-based polar solvent GVL can be applied as an efficient reaction medium for the AGET ATRP. Well controlled polymers could be synthesized by using a nontoxic and inexpensive iron catalyst, and the use of external ligands become unnecessary. The GVL can play the dual function both of solvent and ligand. Well polymerization behavior also observed in other ‘non-polymerizable’ lactones like γ-butyrolactone, γ-caprolactone, γ-octanolactone. These results indicated that GVL is an excellent green solvent and we believe that its application in lager scale is very promising. Further investigations are ongoing to extend the scope of its use as universal solvent to other polymerization condition.
Acknowledgments
This work was supported by the Natural Science Foundation of the Anhui Higher Education Institutions of China (KJ2016A059), the Key Program in the Youth Elite Support Plan in Universities of Anhui Province (gxyqZD2018051) and Pre-Research Project for National Natural Science Foundation supported by Anhui Polytechnic University (KZ00417052).
References
1 Wang J.S., Matyjaszewski K., Controlled/”living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc., 1995, 117(20), 5614-5615.10.1021/ja00125a035Suche in Google Scholar
2 Percec V., Barboiu B., “Living” radical polymerization of styrene initiated by arenesulfonyl chlorides and CuI (bpy)nCl. Macromolecules, 1995, 28(23), 7970-7972.10.1021/ma00127a057Suche in Google Scholar
3 Kato M., Kamigaito M., Sawamoto M., Higashimura T., Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium (II)/methyl-aluminum bis (2, 6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules, 1995, 28(5), 1721-1723.10.1021/ma00109a056Suche in Google Scholar
4 Matyjaszewski K., Atom transfer radical polymerization (ATRP): current status and future perspectives. Macromolecules, 2012, 45(10), 4015-4039.10.1021/ma3001719Suche in Google Scholar
5 Ribelli T.G., Fantin M., Daran J.C., Augustine K.F., Poli R., Matyjaszewski K., Synthesis and Characterization of the Most Active Copper ATRP Catalyst Based on Tris [(4-dimethylami-nopyridyl) methyl] amine. J. Am. Chem. Soc., 2018, 140(4), 1525-1534.10.1021/jacs.7b12180Suche in Google Scholar PubMed
6 Xue Z., He D., Xie X., Iron-catalyzed atom transfer radical polymerization. Polym. Chem., 2015, 6(10), 1660-1687.10.1039/C4PY01457JSuche in Google Scholar
7 Wang Y., Kwak Y., Matyjaszewski K., Enhanced activity of ATRP Fe catalysts with phosphines containing electron donating groups. Macromolecules, 2012, 45(15), 5911-5915.10.1021/ma3010795Suche in Google Scholar
8 Zhang L., Cheng Z., Shi S., Li Q., Zhu X., AGET ATRP of methyl methacrylate catalyzed by FeCl3iminodiacetic acid in the presence of air. Polymer, 2008, 49(13-14), 3054-3059.10.1016/j.polymer.2008.04.057Suche in Google Scholar
9 Wang G., Zhu X., Cheng Z., Zhu J., Reverse atom transfer radical polymerization of methyl methacrylate with FeCl3pyromellitic acid. Eur. Polym. J., 2003, 39(11), 2161-2165.10.1016/S0014-3057(03)00137-XSuche in Google Scholar
10 Chen H., Yang L., Liang Y., Hao Z., Lu Z., ARGET ATRP of acrylonitrile catalyzed by FeCl3isophthalic acid in the presence of air. J. Polym. Sci. Pol. Chem., 2009, 47(12), 3202-3207.10.1002/pola.23406Suche in Google Scholar
11 Hou C., Qu R., Ji C., Wang C., Sun C., ATRP of acrylonitrile catalyzed by FeCl2succinic acid under microwave irradiation. J. Appl. Polym. Sci., 2006, 101(3), 1598-1601.10.1002/app.23560Suche in Google Scholar
12 Silva T.B., Spulber M., Kocik M.K., Seidi F., Charan H., Rother M., et al., Hemoglobin and red blood cells catalyze atom transfer radical polymerization. Biomacromolecules, 2013, 14(8), 2703-2712.10.1021/bm400556xSuche in Google Scholar PubMed
13 Wang Y., Matyjaszewski K., ATRP of MMA in polar solvents catalyzed by FeBr2 without additional ligand. Macromolecules, 2010, 43(9), 4003-4005.10.1021/ma1002276Suche in Google Scholar
14 Yang D., He D., Liao Y., Xue Z., Zhou X., Xie X., Iron‐mediated AGET ATRP of methyl methacrylate in the presence of polar solvents as ligands. J. Polym. Sci. Pol. Chem., 2014, 52(7), 1020-1027.10.1002/pola.27083Suche in Google Scholar
15 He W., Zhang L., Miao J., Cheng Z., Zhu X., Facile iron‐mediated AGET ATRP for water‐soluble Poly (ethylene glycol) monomethyl ether methacrylate in water. Macromol. Rapid. Comm., 2012, 33(12), 1067-1073.10.1002/marc.201100892Suche in Google Scholar PubMed
16 Cao J., Zhang L., Jiang X., Tian C., Zhao X., Ke Q., et al., Facile iron‐mediated dispersant‐free suspension polymerization of methyl methacrylate via reverse ATRP in water. Macromol. Rapid Comm., 2013, 34(22), 1747-1754.10.1002/marc.201300513Suche in Google Scholar PubMed
17 Ding M., Jiang X., Peng J., Zhang L., Cheng Z., Zhu X., An atom transfer radical polymerization system: catalyzed by an iron catalyst in PEG-400. Green Chem., 2015, 17(1), 271-278.10.1039/C4GC01169DSuche in Google Scholar
18 Peng J., Ding M., Cheng Z., Zhang L., Zhu X., Iron-mediated AGET ATRP with crown ether as both ligand and solvent. RSC Adv., 2015, 5(127), 104733-104739.10.1039/C5RA20480ASuche in Google Scholar
19 Deng Z., Guo J., Qiu L., Zhou Y., Xia L., Yan F., Basic ionic liquids: a new type of ligand and catalyst for the AGET ATRP of methyl methacrylate. Polym. Chem., 2012, 3(9), 2436-2443.10.1039/c2py20262jSuche in Google Scholar
20 Wu J., Zhang L., Cheng Z., Zhu X., Photocatalyzed iron-based ATRP of methyl methacrylate using 1, 3-dimethyl-2-imidazolidinone as both solvent and ligand. RSC Adv., 2017, 7(7), 3888-3893.10.1039/C6RA27307FSuche in Google Scholar
21 Gu Y., Jérôme F., Bio-based solvents: an emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev., 2013, 42(24), 9550-9570.10.1039/c3cs60241aSuche in Google Scholar
22 Sheldon R.A., Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem., 2014, 16(3), 950-963.10.1039/C3GC41935ESuche in Google Scholar
23 Horvath I.T., Solvents from nature. Green Chem., 2008, 10(10), 1024-1028.10.1039/b812804aSuche in Google Scholar
24 Alonso D.M., Wettstein S.G., Dumesic J.A., Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem., 2013, 15(3), 584-595.10.1039/c3gc37065hSuche in Google Scholar
25 Wettstein S.G., Alonso D.M., Chong Y., Dumesic J.A., Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energ. Environ. Sci., 2012, 5(8), 8199-8203.10.1039/c2ee22111jSuche in Google Scholar
26 Aparicio S., Alcalde R., Characterization of two lactones in liquid phase: an experimental and computational approach. Phys. Chem. Chem. Phys., 2009, 11(30), 6455-6467.10.1039/b823507dSuche in Google Scholar
27 Ismalaj E., Strappaveccia G., Ballerini E., Elisei F., Piermatti O., Gelman D., et al., γ-Valerolactone as a renewable dipolar aprotic solvent deriving from biomass degradation for the Hiyama reaction. ACS Sustainable Chem. Eng., 2014, 2(10), 2461-2464.10.1021/sc5004727Suche in Google Scholar
28 Strappaveccia G., Luciani L., Bartollini E., Marrocchi A., Pizzo F., Vaccaro L., γ-Valerolactone as an alternative biomass-derived medium for the Sonogashira reaction. Green Chem., 2015, 17(2), 1071-1076.10.1039/C4GC01728ESuche in Google Scholar
29 Abraham G.A., Gallardo A., Lozano A.E., San Roman J., ε‐ Caprolactone/ZnCl2 complex formation: Characterization and ring‐opening polymerization mechanism. J. Polym. Sci. Pol. Chem., 2000, 38(8), 1355-1365.10.1002/(SICI)1099-0518(20000415)38:8<1355::AID-POLA20>3.0.CO;2-ZSuche in Google Scholar
30 Kricheldorf H.R., Mang T., Jonté J.M., Polylactones. 1. Copolymerizations of glycolide and iε-caprolactone. Macromolecules, 1984, 17(10), 2173-2181.10.1007/BF00271606Suche in Google Scholar
31 Hege C.S., Schiller S.M., Non-toxic catalysts for ring-opening polymerizations of biodegradable polymers at room temperature for biohybrid materials. Green Chem., 2014, 16(3), 1410-1416.10.1039/C3GC42044BSuche in Google Scholar
32 Gowda R.R., Chakraborty D., Environmentally benign process for bulk ring opening polymerization of lactones using iron and ruthenium chloride catalysts. J. Mol. Catal. A Chem., 2009, 301(1-2), 84-92.10.1016/j.molcata.2008.11.010Suche in Google Scholar
33 Houk K.N., Jabbari A., Hall H.K., Alemán C., Why δ-valerolactone polymerizes and γ-butyrolactone does not. J. Org. Chem., 2008, 73(7), 2674-2678.10.1021/jo702567vSuche in Google Scholar PubMed
34 Fujimura K., Ouchi M., Sawamoto M., Ferrocene cocatalysis for iron-catalyzed living radical polymerization: Active, robust, and sustainable system under concerted catalysis by two iron complexes. Macromolecules, 2015, 48(13), 4294-4300.10.1021/acs.macromol.5b00836Suche in Google Scholar
© 2019 Shen et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

