Abstract
Polyimide (PI)/mica hybrid films were successfully prepared by in situ condensation polymerization method, in which the mica particles were modified by coupling agent γ-aminopropyltriethoxy silane (APTS) to strengthen the interaction between the mica particles and PI matrix. The morphology, structure, thermal and mechanical properties as well as dielectric properties of PI films were systematically studied via Scanning electron microscope (SEM), Fourier transform infrared spectrometer (FT-IR spectrometer), Thermal gravimetric analysis (TGA), tensile experiments, Thermal mechanical analyzer (TMA), impedance analyzer, etc. The results indicated that the mica particles were dispersed homogeneously in PI matrix, leading to an improvement of the mechanical property, thermal stability and hydrophobicity. It was novel to notice that hybrid films exhibited low coefficient of thermal expansion (CTE) and low dielectric constant simultaneously. The CTE and dielectric constant of hybrid film dropped to 25.36 ppm/k and 2.42 respectively, in the presence of 10 wt% mica into polyimide matrix.
1 Introduction
Polyimide (PI) has been well-known as a high-performance material that shows wide application in aerospace, electronics, petrochemical, precision machinery and other fields. Its unique structure endows PI with excellent thermal stability, low water-absorption, outstanding mechanical behavior and low dielectric constant (1, 2, 3, 4, 5). One of the important applications of PI film is to be used in manufacturing flexible printed circuit boards (FPCBs). In general, a FPCB is etched from a flexible copper clad laminate (FCCL) (6). The FCCL can be classified into two types, including a three-layer flexible copper clad laminate (3L FCCL) and a two-layer flexible copper clad laminate (2L FCCL) (7). The 3L FCCL is formed by bonding a PI film and a copper foil with an adhesive. The 2L FCCL is manufactured through depositing polyimide glue on a copper foil then heat pressing (8). In general, the PI film and the cooper foil should be bonded tightly in these two types. However, when the temperature is high, the FCCL may become warped or fractured because of the existence of thermal stress, resulting from the mismatch of the CTE between the PI film and the copper foil (9,10). Usually, the CTE of copper is 18 ppm/k, but the common industrial PI film (such as Dupont’s Kapton® film) has a higher CTE at about 40 ppm/k (11). Therefore, it is necessary to decrease the CTE of PI film in order to match the copper foil. Meanwhile, in the microelectronics industries, materials with a low dielectric constant have attracted wide attention, because it is beneficial to improve the electron transfer rate of integrated circuits (12,14).
In recent years, increasing attention has been paid to the polyimide organic–inorganic hybrid materials, and it has been displayed that the mechanical behavior, thermal stability, dielectric property and dimensional stability of PI hybrid films can be improved by incorporation of fillers such as alumina, montmorillonite (MMT) and silica (15,19). Mica, as one type of layered silicates, with one octahedral Al2O3 sheet between two tetrahedral SiO2, has very low CTE value and extraordinary insulating property (20,21). Therefore, when mica is added into polyimide matrix as filler, it is profound to reduce the CTE of polyimide/mica hybrid films. Although a large number of literatures aboutthe preparation and properties of PI/layered silicate hybrid films have been reported (22), properties of PI/mica hybrid films have not yet received sufficient attention. It refers that the dielectric properties of PI/mica hybrid films have not been fully studied, and no study has been reported on the coefficient of thermal expansion and thermal properties of this kind of hybrid films (23). Furthermore, dispersing inorganic particles evenly in PI matrix is a key factor that affects the properties of hybrid film, and the surface modification of inorganic particles can improve the compatibility between the inorganic phase and the organic phase (24).
In this paper, PI/mica hybrid films with various mica contents were prepared by in situ condensation polymerization method followed by solution casting and thermal imidization. The mica particles were modified by the γ-aminopropyltriethoxy silane (APTS) coupling agent, which could be used to improve the compatibility of mica particles and the polyimide matrix. The morphology, mechanical behavior, thermal properties, dielectric properties and thermal expansion of hybrid films were investigated. Finally, we acquired a kind of polyimide with low coefficient of thermal expansion (CTE) and low dielectric constant at the same time.
2 Experimental
2.1 Materials
Mica particles were obtained from Shanghai Botong Chemical Co., Ltd, and were dried at 100°C for 12 h prior to use. 4,4’-oxybisbenzenamine (99%, ODA), pyromellitic dianhydride (99%, PMDA) and dimethylacetamide (99%, DMAc) were purchased from Sigma-Aldrich Company. PMDA was dried at 150°C for 6 h, and ODA was dried at 80°C for 5 h prior to use. γ-Aminopropyltriethoxy silane (99%, APTS) was purchased from Shanghai Aladdin Biological Technology Co., Ltd.
2.2 Preparation of mica/DMAc suspension
The prescribed amount of mica particles and DMAc was added to the flask and the calculated coupling agent APTS was added to the solution. The mixture was stirred under ultrasonic for 3 h and then the ultrasonic was removed. Sequentially, the mixture was stirred for 12 h until the suspension became homogeneous. The recipes for preparation of pure PI and PI/mica hybrid films were shown in Table 1.
Recipes for preparation of pure PI and PI/mica hybrid films.
| Mica (wt%) | mica (g) | PMDA (g) | ODA (g) | DMAc (ml) | APTS (g) |
|---|---|---|---|---|---|
| 0 | 0 | 4.365 | 3.929 | 60 | 0 |
| 1 | 0.0829 | 4.365 | 3.929 | 60 | 0.00829 |
| 3 | 0.2490 | 4.365 | 3.929 | 60 | 0.02490 |
| 5 | 0.4130 | 4.365 | 3.929 | 60 | 0.04130 |
| 10 | 0.8290 | 4.365 | 3.929 | 60 | 0.08290 |
2.3 Preparation of PAA and PAA/mica solution
The calculated amount of ODA and DMAc were added into a 250 ml three-neck round-bottom flask equipped with a mechanical stirrer, and the mixture was stirred at 25°C for 1 h until ODA was dissolved completely. PMDA and the rest of DMAc were subsequently added through three times at 12°C every once in 1 h. Afterwards, the mixture was thoroughly stirred for 12 h at 8°C. Finally, the high viscosity PAA solution was obtained with a 15 wt% of solid content.
Based on PAA solution, PAA/mica solution was obtained by mixing with mica/ DMAc suspension. The mass fraction of mica was set to 0%, 1%, 3%, 5% and 10% respectively. The mixture was stirred for 24 h with a mechanical stirrer to obtain an even dispersion.
2.4 Preparation of PI and PI/mica hybrid films
The PAA and PAA/mica solution was coated on clean and dry glass substrate, and thermally treated at 130°C for 3 h under vacuum condition to release the solvent. The obtained semidried films were then dried and imidized at 180/1 h, 200/1 h, 230/1 h, 260/1 h, 290/1 h, 310/1 h, and 330°C/1 h, sequentially. Finally, a series of pure PI films and hybrid films were obtained with the thickness of 25-28 μm. The preparation procedure of PI/mica hybrid films is shown in Figure 1.

The flowchart of the preparation of PI/mica hybrid films.
3 Characterization
Fourier transform infrared spectroscopy (FTIR) was chosen to examine the structure of PI films and IR spectra was recorded on a Fourier Transform infrared spectrometer (SHIMADZU FTIR-8400s) from 400 to 4000 cm-1. The morphology of the mica particles and the samples were measured by a scanning electronmicroscope (Hitachi TM-1000). Thermal gravimetric analysis (TGA) was performed with the analyzer (DTG-60) produced by Shimadzu Corporation under N2 (flowing rate of 20 mL/min) at a heating rate of 20°C/min from 30 to 800°C. Tensile test was performed of pure PI and PI/mica hybrid films cut from 25μm thick sheet and was tested using universal tester (CMT-4254) according to testing standard of ASTMD882. The specimen size was 10 mm (L) × 1.25 mm (W). The coefficient of thermal expansion (CTE) of films were examined from room temperature to 200°C with a heating rate of 10°C/min via a thermal mechanical analyzer (TMA Q400) produced by TA Instruments. The dielectric properties of samples were characterized using a precision impedance analyzer (Agilent 4294A) on room temperature at the frequency from 5 KHz to 1 MHz.
4 Results and discussion
4.1 FTIR of PI/mica hybrid films
The FTIR spectra of pure PI and PI/mica hybrid films are investigated in Figure 2. It can be seen for all samples that the characteristic absorption peaks of the imide groups were observed at 1782 cm-1 and 1722 cm-1 due to the asymmetrical and symmetrical carbonyl stretching vibrations (C=O). Meanwhile, the absorption peaks appeared around at 1376 cm-1 belong to the C-N stretching vibration and the absorption peaks appeared at 725 cm-1 belong to the bending vibration of C=O. Moreover, there were no obvious absorption peaks around at 1660 cm-1 and 1560 cm-1, indicating that the PAA precursors were converted into polyimides completely. Differing from pure PI, the characteristic absorption peaks of hybrid films were observed at 1028 cm-1 and 750 cm-1 due to the stretching vibrations of Si-O-Si groups and Al-O-Al groups. Besides, the absorption peak located at 3620 cm-1 for the stretching vibration of Si-OH groups. With the increase of mica in hybrid films, the intensity peak of Si-OH decreased and the intensity peak of Si-O-Si increased. Because the Si-OH groups of mica reacted with the Si-OH groups of APTS. What’s more, the shifting of absorption peak at 1376 cm-1 (C-N stretching) indicated that mica particles had an interaction with polymer chains and the existence of this interaction may bring about good dispersion of mica in polyimide matrix.

FTIR spectra of pure PI and PI/mica hybrid films.
4.2 Morphology of PI/mica hybrid films
Figure 3 illustrates the SEM images of mica particles and hybrid films with various mica contents. As shown in Figure 3a, the mica particles exhibited cuboid shape with a length of about 1 μm and a thickness of about 200 nm, which could agglomerate with other mica particles and became bigger blocky structure. Figure 3b shows the morphology of pure PI film. The surface of PI matrix, which was not added with mica, was glossy. As shown in Figures 3c and 3d, mica particles were well wrapped by PI matrix with 1 wt% and 3 wt% mica content. This phenomenon benefited from the coupling agent APTS, which makes the mica amino and grafted into polymer chains. Although a slight agglomeration appeared with 5 wt% and 10 wt% mica content, the consistency between mica and polymer matrix was still at a good level. Mica particles were diffused homogeneously into PI matrix and there were no distinct phase separation for PI/mica hybrids, which is shown in Figures 3e and 3f. However, the existence of agglomeration reduced the mechanical and thermal stability properties of the PI/ mica films when the mica content was 5 wt% and 10 wt%.

SEM images of mica particles (a) and PI/mica hybrid films with different mica content: pure PI (b), 1 wt% (c), 3 wt% (d), 5 wt% (e), and 10 wt% (f).
4.3 Thermal stability of PI/mica hybrid films
The TGA curves of pure PI films and PI/mica hybrid films are shown in Figure 4. The temperature at a mass loss of 5% was defined as T5, and temperature at a mass loss of 10% was defined as T10. The residual rate at 800°C was defined as R800 in this study. The samples of T5, T10 and R800 are listed in Table 2. As can be seen, the thermal stability of hybrid films was inferior to pure PI. The T5 of pure PI was 572.2°C. With the mica contents increasing, the value decreased. When the mica content increased to 1 wt%, 3 wt% and 5 wt%, the T5 of hybrid film was 547.2°C, 510.2°C and 504.7°C respectively. When the mass fraction of mica reached 10 wt%, this value declined to 486.1°C. The T10 of all samples were above 538°C, and the R800 values of all samples were over 50%. The addition of mica destroyed the regularity of macromolecular chains and weakened the stability of polyimide. In general, the PI/mica hybrid films had good thermal stability, and they were expected to be used as high-temperature materials.

TGA curves of pure PI and PI/mica hybrid films.
Properties of pure PI and PI/mica hybrid films.
| Samples | pure PI | PI/1 wt% | PI/3 wt% | PI/5 wt% | PI/10 wt% |
|---|---|---|---|---|---|
| Tensile strength (MPa) | 97.9 ± 2.3 | 103.7 ± 1.7 | 109.4 ± 1.5 | 93.3 ± 2.6 | 90.6 ± 2.4 |
| Elongation at break (%) | 19.8 ± 0.9 | 24.1 ± 1.3 | 25.5 ± 1.8 | 18.1 ± 2.2 | 16.4 ± 1.4 |
| Dielectric constant | 3.43 ± 0.05 | 3.02 ± 0.09 | 2.87 ± 0.11 | 2.79 ± 0.07 | 2.42 ± 0.06 |
| Dielectric loss | 0.062 ± 0.002 | 0.023 ± 0.005 | 0.054 ± 0.001 | 0.028 ± 0.006 | 0.037 ± 0.003 |
| CTE (ppm/k) | 37.87 ± 0.4 | 33.15 ± 0.2 | 30.17 ± 0.5 | 28.56 ± 0.1 | 25.63 ± 0.3 |
| T5% (°C) | 572.2 | 547.2 | 510.2 | 504.7 | 486.1 |
| T10% (°C) | 593.0 | 584.3 | 568.6 | 551.9 | 538.7 |
| R800% (%) | 52.8 | 52.6 | 52.3 | 51.8 | 53.8 |
4.4 Mechanical properties of PI/mica hybrid films
The tensile strength and the elongation at the break of the PI/mica hybrid films are summarized in Figure 5a and Table 2. For pure PI film, the tensile strength and elongation at break were only 97.9 MPa and 19.8%. When fraction of mica increased from 1 wt% to 3 wt%, the tensile strength was enhanced by 6% and 12% and the elongation at break was improved by 22% and 28% respectively. However, with continuous increasing fraction of mica (5 wt% and 10 wt%), the tensile strength dramatically decreased by 5% and 7%, and the elongation at break was brought down by 4% and 18% respectively. The improvement of mechanical property with low mass fraction of mica (1 wt% and 3 wt%) may be caused by the strong adhesive between mica and PI matrix, which was brought by the coupling agent APTS. When the mica content raised, the mica particles did not disperse homogeneously in the PI matrix. Moreover, agglomeration appeared in PI/mica hybrids and the compatibility between mica and PI matrix became worse. Therefore, the tensile strength and the elongation at break reduced. Even so, the mechanical properties of pure PI and PI/mica film were still excellent and they could meet the industrial requirements.

(a) The tensile strength and elongation at break of the hybrid films, (b) effects of APTS on the tensile strength of the hybrid films.
By contrast, PI/mica films without surface modification of APTS were also prepared in this study and the tensile strength of those two kinds of hybrid films were studied. The effects of APTS on the tensile strength of the two kinds of hybrid films are shown in Figure 5b. It is clear that the tensile strength of PI/mica films with surface modification by APTS were better, compared with the PI/mica film without any surface modification. When the mica content was 10 wt%, the tensile strength of hybrid film without modification was only 40.0 MPa. But the value of the hybrid film reached as high as 90.6 MPa with surface modification of APTS, which increased by 126.5% than the former one. It means that the coupling agent APTS strengthened the interaction between mica and the PI matrix so that enhanced mechanical properties.
4.5 The TMA and CTEs of pure PI and PI/mica hybrid films
Figure 6a presents the TMA curves of the prepared films and the effect of mica content on the calculated CTE values is described in Figure 6b. In addition, the CTE values of samples are shown in Table 2. The CTE of the prepared films declined obviously as the mica content increased. The CTE was 37.87 ppm/k for pure PI film and dropped to 33.15 ppm/ k for the PI/mica hybrid film (1 wt%). When the mica content gradually increased to 3 wt% and 5 wt%, the CTE value of PI/mica hybrid film linearly decreased. When the fraction of mica was 10 wt%, the CTE value of PI/mica film reached 25.63 ppm/k, which was close to the CTE value of aluminum foil (23 ppm/k) or copper foil (18 ppm/k). The match of CTE of polyimide and copper can prevent the delamination in layered PI-Cu composites for microelectronic applications. For inorganic/polymer hybrid materials, the CTE values depended on this value of inorganic fillers, polymers and interface layers. As we all know, the CTE value of mica is extremely low. Meanwhile, the coupling agent APTS provides strong interaction between mica and PI matrix, and the mica particles are bonded into the polymer chains. At the same time, the expansion of polymer chains are held back by mica particles through heating, thus the CTE values decline. The mechanism was also suitable for silica and PI hybrid films. In that paper, the CTE values also decreased evidently with the mass fraction of silica increasing (25).

(a) Thermal expansion of polyimide/mica hybrid films with various mica contents, (b) the effect of mica content on the coefficient of thermal expansion (CTE).
4.6 Dielectric properties of PI/mica hybrid films
Figure 7a displays the trend of the dielectric constants of pure PI and PI/ mica hybrid films at room temperature with the frequencies varying from 1 KHz to 1 MHz. It indicated that with the frequency increasing, the dielectric constants of samples tend to decrease. The dielectric constant was related to polarization. At higher frequencies, some dipolar polarizable units could not keep up with the speed of the applied alternating current electric field, so the dynamic polarization was low. As a result, the dielectric constant decreased. To summarize, the dielectric constant changed slightly at a higher frequency. The dielectric constants and the dielectric losses of samples at 1MHz are summarized in Table 2 and Figure 7b. The dielectric constant of PI/mica hybrid films were lower than that of pure PI film. Moreover, with the mica content increasing, the dielectric constant dramatically declined. When the mica content reached 10 wt%, the dielectric constant of PI/mica film was only 2.42, which was 29.4% lower than that of pure PI film (3.43) at 1MHz. When the mass fraction of mica was 1 wt%, 3 wt% and 5 wt%, the dielectric constant of hybrid films dropped sharply, valued at 3.02, 2.87 and 2.79 respectively at 1 MHz. Although the dielectric constant of mica material used in this study was higher than that value of polyimide material, the dielectric constant of PI/mica hybrid films decreased as the mica content increased. The abnormal phenomenon may be attributed to a complex polarization which was caused by the specific microscopic structure of the PI/mica hybrid films prepared in this study. It is well known that the dielectric constant of materials were associated with polarization. The microscopic structure of the PI/mica hybrid films may have an effect on the polarization behavior. For the mica particles used in this study had a multi-layer structure, and the multilayer mica would disturb the polarization of the hybrid. So the dielectric constant of PI/mica hybrid films decreased with increasing fraction of mica particle. Furthermore, the dielectric losses of PI/mica hybrid films were in the range of 0.023-0.054 at 1 MHz, which were also extremely low. The addition of mica particles interdicted the dipole polarization between molecular chains. As a result, the dielectric loss decreased.
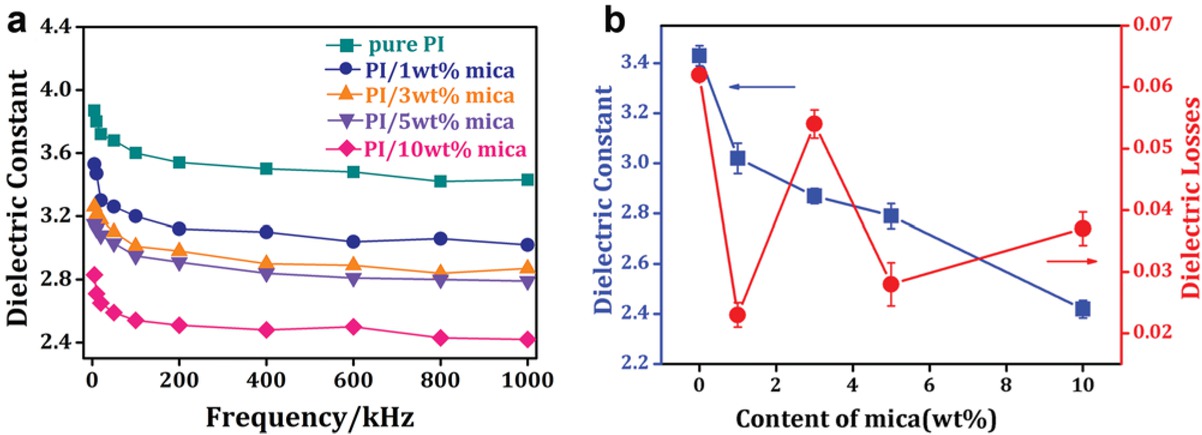
(a) Dielectric constants of the pure PI film and PI/mica hybrid films at room temperature (from 1KHz to 1MHz), (b) dielectric properties of pure PI film and PI/mica hybrid films (at 1 MHz).
5 Conclusions
PI/mica hybrid films were successfully synthesized by in situ condensation polymerization method, solution casting and thermal imidization. The coupling agent APTS was used to enhance the consistency between mica and polyimide matrix. The results indicated the mass fraction of mica significantly influenced the morphology, structure, thermal and mechanical properties as well as dielectric properties of PI/mica films. With the mica content increasing, the CTE and dielectric constant of hybrid films kept decreasing. It was found that hybrid films exhibited low coefficient of thermal expansion (CTE) and low dielectric constant simultaneously. The CTE and dielectric constant of hybrid film dropped to 25.36 ppm/k and 2.42 respectively, in the presence of 10 wt% mica into polyimide matrix.
Acknowledgements
The authors are grateful to the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
1 Su X., Xu Y., Li L.S., Song C.R., Characterization and thermal degradation kinetics of thermoplastic polyimide based on BAPP. High Perform Polym, 2017, DOI:10.1177/0954008317729741.10.1177/0954008317729741Search in Google Scholar
2 Zhang Q.Q., Xu Y., Yang Y., Li L.C., Song C.L., Su X., Conductive mechanism of carbon black/polyimide composite films. J Polym Eng, 2017, 38, 147-156.10.1515/polyeng-2016-0273Search in Google Scholar
3 Xu Y., Zhao A.L, Wang X.L., Xue H., Liu F.L., Influence of curing accelerators on the imidization of polyamic acids and properties of polyimide films. J Wuhan Univ Techno Mater Sci Ed, 2016, 31, 1137-1143.10.1007/s11595-016-1502-9Search in Google Scholar
4 Li L.S., Xu Y., Che J.F., Su X., Song C.R., Ma X.P., Transparent fluorinated poly(imide siloxane) copolymers with good adhesivity. Macromol Res, 2017, 25, 1076-1084.10.1007/s13233-017-5146-1Search in Google Scholar
5 Lei Y.L., Shu Y.J., Peng J.H., Tang YJ., Huo J.C., Synthesis and properties of low coefficient of thermal expansion copolyimides derived from biphenyltetracarboxylic dianhydride with p-phenylenediamine and 4,4’-oxydialinine. e-Polymers, 2016, 16, 295-302.10.1515/epoly-2015-0267Search in Google Scholar
6 Bang S.H., Improvement of NiMoNb to polyimide adhesion by inductively coupled nitrogen plasma treatment. Appl Surf Sci, 2016, 360, 553-558.10.1016/j.apsusc.2015.10.202Search in Google Scholar
7 Li K., Tong L., Jia K., Liu X., Design of flexible copper clad laminate with outstanding adhesion strength induced by chemical bonding. J Mater Sci-Mater El, 2014, 25, 5446-51.10.1007/s10854-014-2327-ySearch in Google Scholar
8 Xue L., Liang Q., Lu Y., Electroless copper plating on 1,2-ethylenediamine grafted poly(ethyleneterepthalate) for the fabrication of flexible copper clad laminate. J Mater Sci-Mater El, 2013, 24, 2211-2217.10.1007/s10854-013-1081-xSearch in Google Scholar
9 Jin X.Z., Ishii H., Positive-type Photosensitive Polyimide with Low Modulus based on Soluble Block Copoly(imide-siloxane). J Photopolym Sci Tec, 2004, 17, 201-206.10.2494/photopolymer.17.201Search in Google Scholar
10 Ebisawa S., Ishii J., Sato M., Vladimirov L., Hasegawa M., Spontaneous molecular orientation of polyimides induced by thermal imidization (5). Effect of ordered structure formation in polyimide precursors on CTE. Eur Polym J, 2010, 46, 283-297.10.1016/j.eurpolymj.2009.10.015Search in Google Scholar
11 Hassanzadeh-Aghdam M.K., Ansari R., Darvizeh A., Micromechanical modeling of thermal expansion coefficients for unidirectional glass fiber-reinforced polyimide composites containing silica nanoparticles. Compos Part A-Appl S, 2017, 96, 110-121.10.1016/j.compositesa.2017.02.015Search in Google Scholar
12 Mehdipour-Ataei S., Aram E., New Polyimide and Nanoporous Structures with Low Dielectric Constant. Adv Polym Tech, 2014, 33, 1082-1088.10.1002/adv.21407Search in Google Scholar
13 Tapaswi P.K., Choi M., Jung Y.S., Cho H.J., Seo D.J., Ha C., Synthesis and characterization of fully aliphatic polyimides from an aliphatic dianhydride with piperazine spacer for enhanced solubility, transparency, and low dielectric constant. J Polym Sci Pol Chem, 2014, 52, 2316-2328.10.1002/pola.27242Search in Google Scholar
14 Miyagawa T., Fukushima T., Oyama T., Iijima T., Tomoi M., Photosensitive fluorinated polyimides with a low dielectric constant based on reaction development patterning. J Polym Sci Pol Chem, 2003, 41, 861-871.10.1002/pola.10638Search in Google Scholar
15 Kovalev M.K., Kalinina F., Androsov D.A., Cho C., Synthesis of transparent and thermally stable polyimide-aramid nanocomposites Prospective materials for high-temperature electronic manufacture applications. Polymer, 2013, 54, 127-133.10.1016/j.polymer.2012.11.051Search in Google Scholar
16 Köytepe S., Seçkin T., Kıvrılcım N., Adıgüzel H.İ., Synthesis and Dielectric Properties of Polyimide-Titania Hybrid Composites. J Inorg Organomet Polym, 2008, 18, 222-228.10.1007/s10904-007-9169-5Search in Google Scholar
17 Tseng I.H., Liao Y.F., Chiang J.C., Tsai M.H., Transparent polyimide/graphene oxide nanocomposite with improved moisture barrier property. Mater Chem Phys, 2012, 136, 247-253.10.1016/j.matchemphys.2012.06.061Search in Google Scholar
18 Hamciuc C., Hamciuc E., Olariu M., Ciobanu R., Thermal and electrical behaviour of some hybrid polyimide films containing barium and titanium oxides. Polym Int, 2010, 59, 668-675.10.1002/pi.2747Search in Google Scholar
19 Iroh J.O., Longun J., Viscoelastic Properties of Montmorillonite Clay/Polyimide Composite Membranes and Thin Films. J Inorg Organomet Polym, 2012, 22, 653-661.10.1007/s10904-011-9613-4Search in Google Scholar
20 Ju C.H., Kim J.C., Chang J.H., Synthesis and characterization of colorless polyimide nanocomposite films. J Appl Polym Sci, 2007, 106, 4192-4201.10.1002/app.26987Search in Google Scholar
21 Kaddami H., Beckerwillinger C., Schmid H., Monitoring morphology and properties of hybrid organicinorganic materials from in situ polymerization of tetraethoxysilane in polyimide polymer: 1. Effect of the coupling agent on the microstructure and interfacial interaction. e-Polymers, 2006, 6, 117-130.10.1515/epoly.2006.6.1.117Search in Google Scholar
22 Zhang Y.H., Dang Z.M., Xin J.H., Daoud W.A., Ji J.H., Liu Y., et al., Dielectric Properties of Polyimide Mica Hybrid Films. Macromol Rapid Comm, 2010, 26, 1473-1477.10.1002/marc.200500310Search in Google Scholar
23 Zhang Q., Li D., Lai D., You Y., Qu B., Preparation, microstructure, mechanical, and thermal properties of in situ polymerized polyimide/organically modified sericite mica composites. Polym Composite, 2016, 37, 2243-2251.10.1002/pc.23402Search in Google Scholar
24 Magaraphan R., Lilayuthalert W., Sirivat A., Schwank J.W., Preparation, structure, properties and thermal behavior of rigid-rod polyimide/montmorillonite nanocomposites. Compos Sci Technol, 2001, 61, 1253-1264.10.1016/S0266-3538(01)00026-4Search in Google Scholar
25 Huang Z., Zhao J., Yuan Y., Yan S., Liu S., Zan X., Preparation and characterization of polyimide/pure silica zeolite hybrid films. Polym Advan Technol, 2013, 24, 600-608.10.1002/pat.3127Search in Google Scholar
© 2019 Zhao et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Articles in the same Issue
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

