Abstract
A DOPO (9,10-dihydro-9-oxa-10-phosphaphen-anthrene-10-oxide)-based halogen-free flame retardant (ODOPM-CYC) was synthesized and incorporated in rigid polyurethane foam (RPUF). The structure of ODOPM-CYC was characterized by Fourier transform infrared spectra (FTIR), 1H NMR and 31P NMR. The effects of ODOPM-CYC on the flame resistance, mechanical performances, thermal properties and cell structure of RPUF were also investigated. The results showed that the incorporation of ODOPM-CYC strikingly enhanced flame retardant properties of RPUF. The flame retarded RPUF acquired a limiting oxygen index (LOI) value of 26% and achieved UL-94 V-0 rating with the phosphorus content of 3 wt%. The smoke production rate (SPR) also showed an obvious decrease and total smoke release (TSR) was 39.8% lower than that of neat RPUF. Besides, the results demonstrated that the incorporation of ODOPM-CYC provided RPUF better thermal stability but did not show any obvious influence on its thermal conductivity.
Graphical Abstract

1 Introduction
As an excellent polymeric material of great versatility, RPUF has been widely used in many fields, including cushions, sealants, aeronautics, and astronautics. Notably, taking the advantage of the ultra-low thermal conductivity (0.018-0.028 W m-1 K-1) and high mechanical properties, RPUFs are commonly used as the insulation materials in buildings (1).
However, the high flammability of RPUFs is a real threat to human life security, which greatly restricts its practical application. Once on fire, the produced toxic smokes may result in the great loss of economic risks and human lives. Therefore, the flame retardancy of RPUF, of necessity, needs to be improved to meet the strict standard of some established regulations for construction or transportation safety such as Final Draft prEN 13501-1 (2,3).
In recent years, a variety of flame retardants have been developed to improve the flame resistance of RPUF, including halogen-based materials, inorganic hydroxyl systems and phosphorus (P) or nitrogen (N) containing compounds, etc. (4, 5, 6). Among all classes of the retardants, halogen-based retardants, although efficient, have been restricted in certain applications due to the toxicity of the released smokes or gases towards surrounding environment and human health. On the basis of this point, various new-style flame retardants with satisfactory efficiency have been developed (7, 8, 9). For example, Jin et al. have synthesized a lignosulfonate/ammonium polyphosphate-based RPUF, which endowed a great thermal stability and ultrahigh char yield (10). Wen and co-workers have reported a one–pot RPUF with expandable graphite and aluminum hypophosphite as the flame retardant, which exhibited an excellent synergistic effect on flame retardancy (11). However, the additional amount for the reported retardant is usually high and the overall materials’ mechanical properties are always sacrificed. Therefore, it is of great significance for the preparation of efficient, less-doped and environmentally-friendly flame retardants.
DOPO (9,10-dihydro-9-oxa-10-phosphaphenanth-rene-10-oxide) is a new type of phosphorus-containing flame retardant intermediate with limited toxicity, from which numerous functional derivatives can be obtained (12, 13, 14, 15). Owing to the appealing inherent thermal and chemical stability caused by their main aromatic structures, DOPO and its derivatives have attracted large quantities of attentions for the application of efficient flame retardants in many inflammable materials (epoxy resins (16, 17, 18, 19), polyester (20,21), and flexible polyurethane foams (22, 23, 24), etc.) (25). For example, König et al. reported a new phosphorus flame retardant (methyl-DOPO), which showed an efficient flame retarding working mechanism in the gas phase when applied into flexible polyurethane foams (26). In addition, Cai and his co-workers synthesized a DOPO-based oligomer and incorporated it in epoxy resin, which achieved the LOI value of 35.3% and V-0 rating in VL-94 test with the addition amount of only 7 wt% (27). Recent reports have showed that DOPO and its derivatives suppress fire by releasing some low molecular weight phosphorous containing species, which are able to scavenge the ·H and ·OH radicals in fire (28). Besides, it has also been reported that the flame retardant efficiency would be significantly enhanced by the co-incorporation of both phosphorus and nitrogen (29, 30, 31). However, there are only limited reports on N, P-co-incorporated DOPO derivatives and its usage as flame retardants in RPUF.
In this work, a halogen-free phosphorus-nitrogen containing flame retardant (denoted as ODOPM-CYC) was successfully synthesized and used as the flame retardant in RPUFs. Flame retarded RPUFs with different contents of ODOPM-CYC were prepared and the fire behaviors were investigated via the LOI, vertical burning test (UL-94), and cone calorimeter testing. The thermal properties were determined by thermogravimetric analysis (TGA). Meanwhile, the effects of addition on the overall mechanical performances, morphological properties and thermal conductivities of foams were investigated by mechanical property measurement, scanning electron microscopic (SEM) and thermal conductivity analyzer, respectively.
2 Experimental
2.1 Materials
Polymethylene polyphenylene isocyanate (WANNATE PM-200, 30.5-32.0 NCO%) and polyether polyol (Wanefoam RB2*, hydroxyl value 350 ± 30 mg KOH g-1) were supplied by Wanhua Chemical Group Co., Ltd. DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide, 97%) was provided by Meryer (Shanghai) Chemical Technology Co., Ltd. Paraformaldehyde (AR) and cyanuric chloride (99%) were obtained from Aladdin Reagents (Shanghai) Co., Ltd. Xylene, 1,4-dioxane, triethylamine were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd.
2.2 Synthesis of ODOPM
The ODOPM was prepared according to the previous report (32), which was shown in Scheme 1.

Synthesis of ODOPM.
A three-necked round bottom flask (500 mL) equipped with a reflux condenser was charged with DOPO (21.6 g, 0.10 mol) and xylene (120 mL). Then the mixture was heated to 90°C under nitrogen atmosphere and stirred until DOPO was dissolved completely. After that, paraformaldehyde (3.0 g, 0.10 mol) was added in batches for over 2 h. The reaction mixture was heated to 135°C after the completion of paraformaldehyde addition and refluxed for another 6 h. After cooled to the room temperature, the white precipitation was filtered and washed three times by xylene. The powdery product was dried at 60°C under vacuum to a constant weight. The yield of ODOPM was 97.6%.
2.3 Synthesis of ODOPM-CYC
At first, ODOPM (36.9 g, 0.15 mol, dissolved in 200 mL 1,4-dioxane) was added in a 500 mL three-necked round bottom flask equipped with a nitrogen inlet and a reflux condenser. Then cyanuric chloride (9.225 g, 0.05 mol, dissolved in 50 mL of 1,4-dioxane) was added into the above-mentioned solution and stirred for over 2 h. After that, triethylamine (15.18 g, 0.15 mol) was added dropwise in three portions. After the addition of the first two portions, the reaction mixture was stirred at room temperature and 50°C for 3 h, respectively. When the last addition completed, the mixture was further heated at reflux temperature for another 10 h. Afterwards, the mixture was filtered while hot and the filtrate was distilled through rotary evaporator to remove 1,4-dioxane before dried at 80°C to a constant weight. The reaction process was illustrated in Scheme 2.

Synthesis of ODOPM-CYC.
2.4 Foam preparation
The RPUF samples were prepared by one-pot free-rise method. PM-200 and polyether polyol were dried at 70°C for 12 h before using. The phosphorus content of the RPUF was fixed to 1 wt% and 4 wt%, respectively.
The flame retardant was first mixed and dispersed in polyether polyol. Subsequently, PM-200 was addedand stirred vigorously at 1800 rpm for 10 s. Then the mixture was quickly poured into an open mold (150 × 150 × 100 mm3) to obtain a free-rise foam. Finally, the foam was placed in an oven for 24 h at 80°C to complete the polymerization reaction.
2.5 Characterization
The chemical structure of ODOPM and ODOPM-CYC were characterized by Fourier transform infrared (FTIR) spectra along with 1H and 31P nuclear magnetic resonance (1H NMR, 31P NMR) spectroscopy. FTIR spectra were obtained using an EQUNOX55 spectrometer with the range of wavenumber from 500 cm-1 to 4000 cm-1 at room temperature using KBr pellets. The 1H NMR and 31P NMR analyses were performed on an AVANCE III NMR spectrometer at 300 MHz and the samples were dissolved in DMSO-d6.
LOI was obtained at room temperature by an oxygen index instrument (HC-2, Jiangning Analysis Instrument Company) with samples measurement of 100 × 10 × 10 mm3. UL-94 burning tests were performed on a vertical testing apparatus (CZF-3, Jiangning Analysis Instrument Company). The specimens for measurement were cut to a size of 100 × 25 × 13 mm3.
Cone calorimetry (CONE) was performed using a commercial cone calorimeter (FTT2000, FTT company, UK) with specimens exposed to a heat flux of 35 kW m-2. The size of the specimen was 100 × 100 × 20 mm3.
The thermal stability of the foams was carried out by a thermal analyzer (STD Q600) and the temperature was scanned from room temperature to 800°C with a heating rate of 10°C min-1 under nitrogen atmosphere.
The compression strength and flexural strength were measured by a universal mechanical tester (SANS7 CMT-5105). The compression test was carried out with compression rate of 2 mm min-1 and specimens size of 20 × 20 × 20 mm3. All samples were run in quintuplicate and the average value was reported. The size of flexural strength tests specimen was 120 × 25 × 20 mm3 and the crosshead speed was 10 mm min-1. All samples were run in triplicate and the average value was reported.
The morphology of the foams were observed by scanning electron microscopy (SEM, Nova Nano SEM 450) at an accelerating voltage of 10 kV. The specimens were gold-coated with a conductive layer before test.
Thermal conductivities of the foams were measured using a thermal conductivity analyzer (DHR-II, Xiangtan Xiangyi Instrument Company). The size of the specimen was 100 × 100 × 20 mm3.
3 Results and discussion
3.1 Characterization of ODOPM and ODOPM-CYC
The chemical structure of ODOPM-CYC was characterized by FTIR, 1H NMR and 31P NMR (presented in Figure 1).
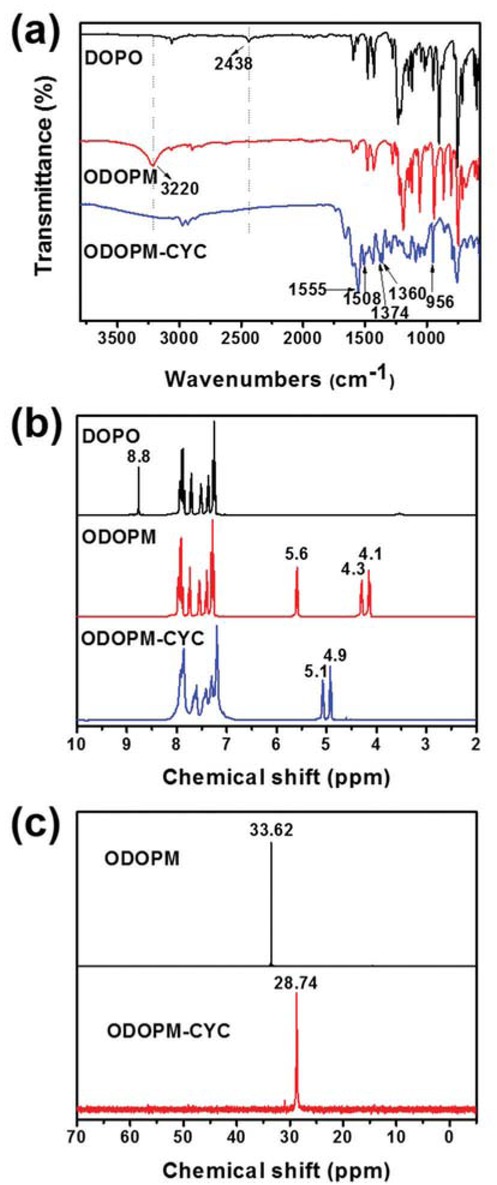
FTIR spectra (a) and 1H NMR (b) of DOPO, ODOPM and ODOPM-CYC; (c) 31P NMR spectra of ODOPM and ODOPM-CYC.
As seen from the FTIR spectra of ODOPM-CYC (Figure 1a), the absorption peaks at 1555, 1508, 1374 and 1360 cm-1 were attributed to the stretching vibration absorption of triazine ring skeleton (33,34). The absorption peaks at 1280 and 956 cm-1 belonged to the stretching vibration of P=O and P-O-Ph, respectively. In contrast to DOPO, the characteristic band for P-H stretching vibration of OPOPM at 2438 cm-1 disappeared completely, while a new absorption peak at 3220 cm-1 for -OH appeared, suggesting that ODOPM was successfully produced during this feasible process. In addition, the absorption peaks at 3220 cm-1 disappeared in ODOPM-CYC, which might be ascribed to the successful reaction of OPOPM with cyanuric chloride.
The structures of ODOPM and ODOPM-CYC were further confirmed by 1H NMR and 31P NMR. As shown in Figure 1b, the peaks at 7.0-8.2 ppm were attributed to the Ar-H protons. Compared to the pure DOPO, the peak at 8.8 ppm, which was attributed to the proton of P-H, disappeared in ODOPM. The brand-new signals at 4.1 and 4.3 ppm might be assigned to -CH2- protons. Meanwhile, the shifting value of 5.6 ppm for ODOPM was attributed to the proton of -OH (31,35). And the single peak at 5.6 ppm disappeared in the spectrum of ODOPM-CYC, together with the signals of -CH2- shifted to 4.9 and 5.1 ppm, indicating the reaction between ODOPM and cyanuric chloride. Correspondingly, the 31P NMR spectra (Figure 1c) showed that the single peak in ODOPM at 33.6 ppm shifted to 28.7 ppm in ODOPM-CYC. These results indicated that ODOPM-CYC was successfully synthesized.
3.2 Characterization of flame retarded RPUFs
3.2.1 Fire behavior
In order to evaluate the flame retardance of ODOPM-CYC in RPUF, the LOI and UL-94 test results of RPUFs with different P mass ratios were listed in Table 1. For neat RPUF, the LOI value was only 18.5% and it failed to pass the UL-94 test. However, the LOI values showed continuous increase upon the increasing P content with the incorporation of ODOPM-CYC and the final RPUF containing ODOPM-CYC satisfied the V-0 rating with a low P content of only 3.5 wt%. Therefore, ODOPM-CYC was able to be recognized as an efficient flame retardant for RPUF. The remarkable increase of flame retardancy was mainly related to the existence of the inbuilt melamine structural unit in ODOPM-CYC. And the incorporation of nitrogen-containing group in DOPO compounds might achieved synergistic effect of phosphorus and nitrogen groups (30).
Formula and density, thermal conductivity, LOI, UL-94 results of RPUFs.
| Sample | P content (wt%) | LOI (%) | UL-94 rating | Density (kg m-3) | Thermal conductivity (W m-1 K-1) |
|---|---|---|---|---|---|
| FBlank | 0 | 18.5 | Fail | 34.5 | 0.0238 |
| F1% | 1 | 21.5 | Fail | 35 | 0.0238 |
| F1.5% | 1.5 | 22.5 | V-2 | 35 | 0.0237 |
| F2% | 2 | 23 | V-1 | 36 | 0.0238 |
| F2.5% | 2.5 | 24 | V-1 | 35 | 0.0239 |
| F3% | 3 | 25 | V-1 | 36 | 0.0238 |
| F3.5% | 3.5 | 26 | V-0 | 37 | 0.0240 |
| F4% | 4 | 26.5 | V-0 | 37 | 0.0241 |
CONE has been widely used to evaluate the burning performance of materials in real fires and the testing results were summarized in Table 2. It can be found that the time to ignition (TTI) values of RPUFs containing ODOPM-CYC increased obviously, indicating that the presence of ODOPM-CYC delayed the ignition of foam and hindered the combustion process. The heat release rate (HRR) is commonly used for predicting the scale of flame propagation. The peak heat release rate (PHRR) usually represents the point where heat is likely to propagate or ignite adjacent objects in a fire. For flame retarded RPUF, the PHRR was 151.19 and 145.52 kW m-2 with 3 and 3.5 wt% P, which was reduced by 31.2 and 33.8% than that of neat RPUF, respectively. This promising results were mainly due to the formation of intumescent char in flame retarded RPUF which performed as a barrier and obstructed the heat and mass transportation (36). Figure 2 displayed the digital photos of the residual neat RPUF and flame retarded RPUFs after CONE tests. It could be seen that obvious intumescent char was formed in the samples containing ODOPM-CYC. The total heat release (THR) for neat and flame retarded RPUFs were shown in Figure 3a. The THR of flame retarded RPUF with 3 and 3.5 wt% P was observed to be decreased by 13.0 and 24.0%, respectively, indicating that the heat transfer and flame spread were hindered.

The digital photos for the residues of (a) FBlank, (b) F3% and (c) F3.5% after CONE test.

(a) The heat release rate curves for RPUFs; The SPR (b) and TSR (c) curves for RPUFs.
Cone calorimetry testing data for RPUFs.
| Sample | TTI (s) | PHRR (kW m-2) | THR (MJ m-2) | TSR (m2 m-2) | A-MLR (g s-1) | CO/CO 2 (kg kg-1) | Char residue yield (%) |
|---|---|---|---|---|---|---|---|
| FBlank | 3 | 219.68 | 17.08 | 328.44 | 0.052 | 0.051 | 18.96 |
| F3% | 6 | 151.19 | 14.86 | 197.57 | 0.039 | 0.028 | 26.80 |
| F3.5% | 7 | 145.52 | 12.98 | 197.89 | 0.034 | 0.021 | 31.51 |
As we know, suffocation due to the inhalation of excess smokes is a major cause of casualties in fire accidents. Therefore, the reducing of SPR and TSR is of great necessity. As presented in Figure 3b, compared with neat RPUF, the two peaks of SPR were greatly suppressed in RPUFs containing ODOPM-CYC and the first peak of SPR was postponed from 10 s to 20 s with P content of 3.5 wt%. Correspondingly, the peak of SPR values for flame retarded RPUF with 3 and 3.5 wt% P decreased from 0.069 to 0.040 and 0.034 m2 s-1.
The TSR values (Figure 3c) were 197.57 and 197.89 m2 m-2, respectively, which were significantly lower than that of neat RPUF (328.44 m2 m-2). The smoke suppression was mainly due to the successful formation of the char layer, which prevented inner matrix from further degradation and fewer smokes were released (37). The char residue value (shown in Table 2) increased from 18.96% (neat RPUF)
to 31.51% (flame retarded RPUF with 3.5 wt% P), which might be attributed to the transformation towards polyphosphoric acid for P groups in ODOPM-CYC.
A-MLR represents the thermal cracking, volatilization and combustion of materials under a certain fire intensity. According to Table 2, the A-MLR value reduced from 0.052 to 0.034 g s-1, indicating that much more char residues were formed in flame retarded RPUFs (confirmed by Figure 2). What is more, it can be found that the CO/CO2 ratios for flame retarded RPUF with 3 and 3.5 wt% P were 0.028 and 0.021, which were significantly lower than that of neat RPUF (0.051), further revealing the effective smoke suppression of ODOPM-CYC for RPUF.
3.2.2 Thermal stability
To investigate the effect of ODOPM-CYC on the thermal stability of RPUF, TGA and differential thermogravimetry (DTG) were carried out and the results were presented in Figure 4. As shown in Table 3, the onset degradation temperature (Tonset) of flame retarded RPUF with 3 wt% P was observed to show a slight increase in comparison with neat RPUF, which might be attributed to the high thermal stability of aromatic structure in ODOPM-CYC. Figure 4 showed the temperature range of significant weight loss narrowed when ODOPM-CYC was incorporated and the maximum decomposition temperature (Tmax) alsoincreased from 305°C to 319°C in comparison with neat RPUF. Some radical species such as PO, PO2 and POCH3 were reported to be released with the decomposition of DOPO and its derivatives, which could react with ·H and ·OH radicals to lower the heat production amount and improve the thermal stability (28). In addition, flame retarded RPUF with 3 wt% P exhibited higher residues (24.33%) than neat RPUF (13.18%) at 800°C, indicating

TGA (a) and DTG (b) curves of RPUFs.
The TGA data for RPUFs.
| Sample | P content (%) | Tonset (°C) | Tmax (°C) | Residues at 800°C (%) |
|---|---|---|---|---|
| FBlank | 0 | 247 | 305 | 13.18 |
| F3% | 3 | 256 | 319 | 24.33 |
that ODOPM-CYC might act as a charring agent during the thermal treatment.
3.2.3 Physical and mechanical properties
The mechanical property is an important parameter of RPUF, which greatly depends on its cell structure and density (collected in Table 1). The cell structures of foams were observed by SEM (shown in Figure 6). The foam density showed a slight increase with the increasing addition of ODOPM-CYC, which was probably due to the decrease of cell size and the embedding of flame retardant particles into cell wall.

The compressive (a) and flexural (b) strength of RPUFs.

SEM images of RPUFs: (a) FBlank, (b) F1%, (c) F2%, (d) F3% and (e) F4%. The magnification of all images, 100 ×.
As seen from Figure 5, the compressive strength increased with rising P content at first and reached the peak value at 0.26 MPa with the addition of 2 wt% P, which was nearly double than that of neat RPUF (0.15 MPa). The flexural strength also increased from 0.187 to 0.269 MPa under the same addition content. The improvement of mechanical properties might be attributed to the increase of foam density and negligible cell collapse or collision (shown in Table 1 and Figure 6). The results indicated that ODOPM-CYC possessed good miscibility with RPUF. However, when the addition of P was beyond 3 wt%, it can be seen that the integrity of the cell was undermined and cell size distribution became wider, thus the mechanical properties decreased correspondingly.
3.2.4 Thermal conductivity
For insulation materials, a low thermal conductivity is of great necessity. According to the test results listed in Table 1, there is no significant increase in thermal conductivity values after the incorporation of ODOPM-CYC. The slight increase might be attributed to the decrease of closed cell content and the formation of airflow passage caused by the collapse of cells (shown in Figure 6).
4 Conclusions
In this study, an intumescent halogen-free flame retardant ODOPM-CYC was successfully prepared and applied to RPUFs. It has been confirmed that ODOPM-CYC displayed excellent flame retardant properties in RPUF. The flame retarded RPUF acquired a LOI value of 26% and achieved UL-94 V-0 rating with the P content of 3 wt%. Meanwhile, a suppression effect on smoke and heat was also observed. Compared with neat RPUF, the PHRR, THR, SPR and TSR of foams containing ODOPM-CYC all decreased, which is due to the barrier effect of the formed intumescent char. Furthermore, when P content was less than 3 wt%, the foam cell showed slight collapse or collision in the presence of ODOPM-CYC, thus resulting in the promotion of physical mechanical. Besides, the incorporation of ODOPM-CYC improved the thermal stability of RPUF along with negligible change on its thermal conductivity. This study implies that ODOPM-CYC is a promising flame retardant for RPUF.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. U1205114).
References
1 Ciecierska E., Jurczyk-Kowalska M., Bazarnik P., Kowalski M., Krauze S., Lewandowska M., The influence of carbon fillers on the thermal properties of polyurethane foam. J Therm Anal Calorin, 2016, 123, 283-291.10.1007/s10973-015-4940-2Search in Google Scholar
2 Ding H., Xia C., Wang J., Wang C., Chu F., Inherently flame-retardant flexible bio-based polyurethane sealant with phosphorus and nitrogen-containing polyurethane prepolymer. J Mater Sci, 2016, 51, 5008-5018.10.1007/s10853-016-9805-ySearch in Google Scholar
3 Zhao Q., Chen C., Fan R., Yuan Y., Xing Y., Ma X., Halogen-free flame-retardant rigid polyurethane foam with a nitrogen–phosphorus flame retardant. J Fire Sci, 2017, 35, 99-117.10.1177/0734904116684363Search in Google Scholar
4 Li A., Yang D.D., Li H.N., Jiang C.L., Liang J.Z., Flame-retardant and mechanical properties of rigid polyurethane foam/MRP/Mg (OH)2GF/HGB composites. Appl Polym Sci, 2018, 135, 46551.10.1002/app.46551Search in Google Scholar
5 Titelman G.I., Gonen Y., Keidar Y., Bron S., Discolouration of polypropylene-based compounds containing magnesium hydroxide. Polym Degrad Stab, 2002, 77, 345-352.10.1016/S0141-3910(02)00064-2Search in Google Scholar
6 Lu S.Y., Hamerton I., Recent developments in the chemistry of halogen-free flame retardant polymers. Prog Polym Sci, 2002, 27, 1661-1712.10.1016/S0079-6700(02)00018-7Search in Google Scholar
7 Wang S.X., Zhao H.B., Rao W.H., Huang S.C., Wang T., Liao W., et al., Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer, 2018, 153, 616-626.10.1016/j.polymer.2018.08.068Search in Google Scholar
8 Rao W.H., Xu H.X., Xu Y.J., Qi M., Liao W., Xu S., et al., Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem Eng J, 2018, 343, 198-206.10.1016/j.cej.2018.03.013Search in Google Scholar
9 Guo W., Wang X., Zhang P., Liu J., Song L., Hu Y., Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr Polym, 2018, 195, 71-78.10.1016/j.carbpol.2018.04.063Search in Google Scholar PubMed
10 Lu W., Li Q., Zhang Y., Yu H., Hirose S., Hatakeyama H., Jin Z., Lignosulfonate/APP IFR and its flame retardancy in lignosulfonate-based rigid polyurethane foams. J Wood Sci, 2018, 64, 287-293.10.1007/s10086-018-1701-4Search in Google Scholar
11 Xu W.Z., Liu L., Wang S.Q., Hu Y., Synergistic effect of expandable graphite and aluminum hypophosphite on flame-retardant properties of rigid polyurethane foam. Appl Polym Sci, 2015, 132, 42842.10.1002/app.42842Search in Google Scholar
12 Salmeia K.A., Gaan S., An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym Degrad Stab, 2015, 113, 119-134.10.1016/j.polymdegradstab.2014.12.014Search in Google Scholar
13 Yang L., Xu L., Tao Z., Palladium-catalyzed arylation of 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) with Halogen-Substituted Phenols. Phosphorus Sulfur Silicon Relat Elem, 2009, 184, 3175-3181.10.1080/10426500802705420Search in Google Scholar
14 Vasiljević J., Jerman I., Jakša G., Alongi J., Malucelli G., Zorko M., et al., Functionalization of cellulose fibres with DOPO-polysil-sesquioxane flame retardant nanocoating. Cellulose, 2015, 22, 1893-1910.10.1007/s10570-015-0599-xSearch in Google Scholar
15 Xie C., Zeng B., Gao H., Xu Y., Luo W., Liu X., Dai L., Improving thermal and flame-retardant properties of epoxy resins by a novel reactive phosphorous-containing curing agent. Polym Eng Sci, 2014, 54, 1192–1200.10.1002/pen.23642Search in Google Scholar
16 Ciesielski M., Schäfer A., Döring M., Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym Adv Technol, 2010, 19, 507-515.10.1002/pat.1090Search in Google Scholar
17 Wang X., Hu Y., Song L., Yang H., Xing W. Lu H., Synthesis and characterization of a DOPO-substitued organophosphorus oligomer and its application in flame retardant epoxy resins. Prog Org Coat, 2011, 71, 72-82.10.1016/j.porgcoat.2010.12.013Search in Google Scholar
18 Huo S., Wang J., Yang S., Wang J., Zhang B., Zhang B., et al., Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphen-anthrene and its flame retardant effect on epoxy resin. Polym Degrad Stab, 2016, 131, 106-113.10.1016/j.polymdegradstab.2016.07.013Search in Google Scholar
19 Qian L., Qiu Y., Liu J., Xin F., Chen Y., The flame retardant group-synergistic-effect of a phosphaphenanthrene and triazine double-group compound in epoxy resin. J Appl Polym Sci, 2014, 131, 1082-1090.10.1002/app.39709Search in Google Scholar
20 Bai Z., Song L., Hu Y., Yuen R.K.K., Preparation, flame retardancy, and thermal degradation of unsaturated polyester resin modified with a novel phosphorus containing acrylate. Ind Eng Chem Res, 2013, 52, 12855-12864.10.1021/ie401662xSearch in Google Scholar
21 Zhang C., Liu S.M., Zhao J.Q., Huang J.Y., Synthesis and properties of a modified unsaturated polyester resin with phosphorus-containing pendant groups. Polym Bull, 2013, 70, 1097-1111.10.1007/s00289-012-0889-5Search in Google Scholar
22 Gaan S., Liang S., Mispreuve H., Perler H., Naescher R., Neisius M., Flame retardant flexible polyurethane foams from novel DOPO-phosphonamidate additives. Polym Degrad Stab, 2015, 113, 180-188.10.1016/j.polymdegradstab.2015.01.007Search in Google Scholar
23 Gaan S., Neisius M., Cuchere O., Liang S., Mispreuve H., Flame retardant polyurethanes based on novel phosphonamidate additives. Fire Safety Sci, 2014, 11, 821-831.10.3801/IAFSS.FSS.11-821Search in Google Scholar
24 König A., Kroke E., Flame retardancy working mechanism of methyl-DOPO and MPPP in flexible polyurethane foam. Fire Mater, 2012, 36, 1-15.10.1002/fam.1077Search in Google Scholar
25 Liu Y., He J., Yang R., The preparation and properties of flame-retardant polyisocyanurate-polyurethane foams based on two DOPO derivatives. J Fire Sci, 2016, 34, 431-444.10.1177/0734904116662667Search in Google Scholar
26 König A., Kroke E., Methyl-DOPO: a new flame retardant for flexible polyurethane foam. Polym Adv Technol, 2011, 22, 5-13.10.1002/pat.1728Search in Google Scholar
27 Wang P., Yang F., Li L., Cai Z., Flame retardancy and mechanical properties of epoxy thermosets modified with a novel DOPO-based oligomer. Polym Degrad Stab, 2016, 129, 156-167.10.1016/j.polymdegradstab.2016.04.005Search in Google Scholar
28 Rakotomalala M., Wagner S., Döring M., Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials, 2010, 3, 4300-4327.10.3390/ma3084300Search in Google Scholar PubMed PubMed Central
29 Li R.M., Deng C., Deng C.L., Dong L.P., Di H.W., Wang Y.Z., An efficient method to improve simultaneously the water resistance, flame retardancy and mechanical properties of POE intumescent flame-retardant systems. RSC Adv, 2015, 5, 16328-16339.10.1039/C4RA15971CSearch in Google Scholar
30 Deng C.L., Du S.L., Zhao J., Shen Z.Q., Deng C., Wang Y.Z., An intumescent flame retardant polypropylene system with simultaneously improved flame retardancy and water resistance. Polym Degrad Stab, 2014, 108, 97-107.10.1016/j.polymdegradstab.2014.06.008Search in Google Scholar
31 Guan Y.H., Huang J.Q., Yang J.C., Shao Z.B., Wang Y.Z., An effective way to flame-retard biocomposite with ethanolamine modified ammonium polyphosphate and its flame retardant mechanisms. Ind Eng Chem Res, 2015, 54, 3524-3531.10.1021/acs.iecr.5b00123Search in Google Scholar
32 Shieh J.Y., Wang C.S., Synthesis of novel flame retardant epoxy hardeners and properties of cured products. Polymer, 2001, 42, 7617-7625.10.1016/S0032-3861(01)00257-9Search in Google Scholar
33 Yang S., Wang J., Huo S., Wang M., Cheng L., Synthesis of a phosphorus/nitrogen-containing additive with multifunctional groups and its flame-retardant effect in epoxy resin. Ind Eng Chem Res, 2015, 54, 7777-7786.10.1021/acs.iecr.5b02026Search in Google Scholar
34 Chang S.C., Condon B., Graves E., Uchimiya M., Fortier C., Easson M., et al., Flame retardant properties of triazine phosphonates derivative with cotton fabric. Fiber Polym, 2011, 12, 334-339.10.1007/s12221-011-0334-7Search in Google Scholar
35 Xu D.M., Ding F., Hao J.W., Jian-Xin D.U., Preparation of modified expandable graphite and its flame retardant application in rigid polyurethane foam. Chem J Chinese U, 2013, 34, 2674-2680.Search in Google Scholar
36 Brehme S., Schartel B., Goebbels J., Fischer O., Pospiech D., Bykov Y., et al., Phosphorus polyester versus aluminium phosphinate in poly(butylene terephthalate) (PBT): Flame retardancy performance and mechanisms. Polym Degrad Stab, 2011, 96, 875-884.10.1016/j.polymdegradstab.2011.01.035Search in Google Scholar
37 Hoang D.Q., Synthesis and applications of biscyclic phosphorus flame retardants. Polym Degrad Stab, 2008, 93, 36-42.10.1016/j.polymdegradstab.2007.10.020Search in Google Scholar
© 2019 Liu and Lv, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Articles in the same Issue
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

