Abstract
C17H32BrNO, triclinic,
CCDC no.: 1983264
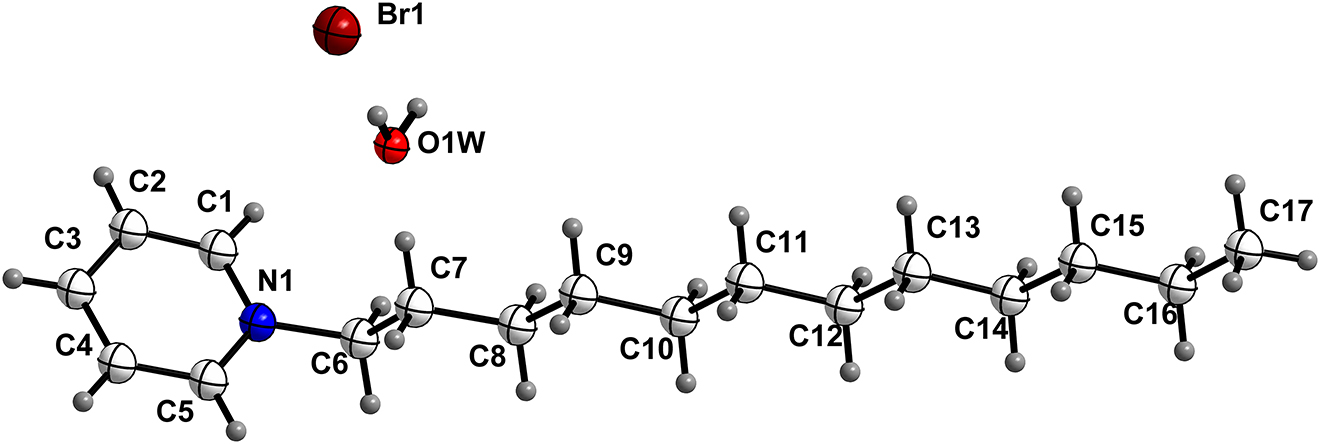
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.25 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.12 mm−1 |
| Diffractometer, scan mode: | Bruker, |
| θmax, completeness: | 25.0°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 4817, 3382, 0.031 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1876 |
| N(param)refined: | 182 |
| Programs: | Bruker [1], SHELX [2], [3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 0.46294 (11) | 0.82802 (8) | 0.12404 (3) | 0.0721 (3) |
| C1 | 0.3469 (11) | 0.3466 (7) | 0.0733 (2) | 0.0618 (15) |
| H1 | 0.5061 | 0.4157 | 0.0781 | 0.074* |
| C2 | 0.1999 (12) | 0.3378 (8) | 0.0239 (2) | 0.0715 (17) |
| H2 | 0.2503 | 0.4032 | −0.0048 | 0.086* |
| C3 | −0.0312 (12) | 0.2276 (8) | 0.0166 (3) | 0.0756 (17) |
| H3 | −0.1357 | 0.2129 | −0.0180 | 0.091* |
| C4 | −0.1043 (11) | 0.1404 (8) | 0.0613 (3) | 0.0706 (16) |
| H4 | −0.2623 | 0.0701 | 0.0574 | 0.085* |
| C5 | 0.0521 (11) | 0.1560 (7) | 0.1107 (2) | 0.0579 (14) |
| H5 | 0.0047 | 0.0946 | 0.1405 | 0.070* |
| C6 | 0.4453 (11) | 0.2851 (8) | 0.1721 (2) | 0.0719 (17) |
| H6A | 0.4472 | 0.1734 | 0.1859 | 0.086* |
| H6B | 0.6178 | 0.3296 | 0.1656 | 0.086* |
| C7 | 0.3634 (11) | 0.4049 (7) | 0.21697 (19) | 0.0633 (15) |
| H7A | 0.1918 | 0.3605 | 0.2241 | 0.076* |
| H7B | 0.3619 | 0.5172 | 0.2036 | 0.076* |
| C8 | 0.5425 (11) | 0.4241 (8) | 0.2723 (2) | 0.0740 (17) |
| H8A | 0.5423 | 0.3110 | 0.2851 | 0.089* |
| H8B | 0.7139 | 0.4655 | 0.2644 | 0.089* |
| C9 | 0.4765 (12) | 0.5454 (7) | 0.3204 (2) | 0.0686 (16) |
| H9A | 0.3040 | 0.5064 | 0.3281 | 0.082* |
| H9B | 0.4827 | 0.6601 | 0.3085 | 0.082* |
| C10 | 0.6566 (12) | 0.5549 (9) | 0.3746 (2) | 0.0807 (19) |
| H10A | 0.8295 | 0.5897 | 0.3663 | 0.097* |
| H10B | 0.6460 | 0.4406 | 0.3870 | 0.097* |
| C11 | 0.5988 (12) | 0.6840 (8) | 0.4244 (2) | 0.0759 (18) |
| H11A | 0.6103 | 0.7986 | 0.4122 | 0.091* |
| H11B | 0.4258 | 0.6496 | 0.4327 | 0.091* |
| C12 | 0.7776 (12) | 0.6916 (8) | 0.4777 (2) | 0.0774 (18) |
| H12A | 0.9503 | 0.7262 | 0.4692 | 0.093* |
| H12B | 0.7667 | 0.5766 | 0.4896 | 0.093* |
| C13 | 0.7221 (11) | 0.8207 (8) | 0.5288 (2) | 0.0752 (18) |
| H13A | 0.7338 | 0.9359 | 0.5171 | 0.090* |
| H13B | 0.5496 | 0.7863 | 0.5374 | 0.090* |
| C14 | 0.9011 (12) | 0.8258 (8) | 0.5812 (2) | 0.0774 (18) |
| H14A | 1.0735 | 0.8593 | 0.5724 | 0.093* |
| H14B | 0.8888 | 0.7105 | 0.5928 | 0.093* |
| C15 | 0.8496 (12) | 0.9539 (9) | 0.6323 (2) | 0.0819 (19) |
| H15A | 0.8593 | 1.0685 | 0.6203 | 0.098* |
| H15B | 0.6774 | 0.9193 | 0.6411 | 0.098* |
| C16 | 1.0293 (14) | 0.9634 (9) | 0.6856 (2) | 0.091 (2) |
| H16A | 1.2019 | 0.9964 | 0.6769 | 0.109* |
| H16B | 1.0175 | 0.8494 | 0.6983 | 0.109* |
| C17 | 0.9764 (16) | 1.0943 (12) | 0.7354 (3) | 0.129 (3) |
| H17A | 0.9759 | 1.2058 | 0.7225 | 0.193* |
| H17B | 1.1069 | 1.1026 | 0.7671 | 0.193* |
| H17C | 0.8136 | 1.0551 | 0.7475 | 0.193* |
| N1 | 0.2765 (8) | 0.2612 (5) | 0.11630 (17) | 0.0530 (11) |
| O1W | 0.9057 (7) | 0.5792 (5) | 0.12690 (19) | 0.0942 (14) |
| H1WA | 0.7905 | 0.6431 | 0.1261 | 0.113* |
| H1WB | 1.0483 | 0.6423 | 0.1261 | 0.113* |
Source of material
The title compound was synthesized by heating 1-bromododecane in pyridine at reflux for 3 h. Excess pyridine was removed under reduced pressure and the resulting solid dissolved in a minimum of CHCl3. Pouring this liquid slowly into stirring ethyl acetate resulted in the formation of a white solid, which was subsequently filtered and recrystallized from methanol. The solid was dissolved in water and left to slowly evaporate, affording colourless needles of the title compound.
Experimental details
The structure was solved by Direct Methods with SHELX [2]. All H-atoms were positioned geometrically and refined using a riding model with d(C–H) = 0.93 Å, Uiso = 1.2Ueq (C) for aromatic, 0.97 Å, Uiso = 1.2Ueq (C) for CH2, 0.96 Å, Uiso = 1.5Ueq (C) for CH3 hydrogen atoms and d(O–H) = 0.85 Å, Uiso = 1.2Ueq (O) for H2O. Restraints were applied to the displacement parameters of C2, C3, C6, C7, C8, C9, C16, C17 to approximate typical behaviour.
Comment
In recent years, all kinds of ionic liquids, especially imidazolium and pyridinium ionic liquids have attracted extensive attention and research interest, because these long-chain amphiphilic salts not only are surface-active, but also can form a new class of liquid crystals materials in suitable solvents, which combine the characteristics of ionic liquids and thermotropic liquid crystals [5]. They can be used as ionic conducting material, organic reaction solvent, functional nanomaterial template and ordered membrane [6], [7], [8], [9]. The characteristics of their crystal structures are very important for the application of these materials and many crystal structures have been reported. However, there is no report of the crystal structure of the 1-dodecylpyridinium bromide monohydrate.
The asymmetric unit of the title compound (see the Figure) comprises the desired alkyl pyridinium cation with a bromide counter anion and a water molecule. The hydrophobic C12 alkyl chain has a trans-planar arrangement. The C–C bond lengths found in the hydrocarbon chain are between 1.479(7)A-1.575(7) and the C–C–C bond angles are between 110.7(5) and 114.6(5)°. These values are close to those found in 1-dodecylpyridinium chloride monohydrate [10] and 1-tetradecylpyridinium bromide monohydrate [11]. The dihedral angle formed between the alkyl chain and the pyridinium ring is 78.29(20)°, slightly less than the 79.16° seen in 1-dodecylpyridinium chloride monohydrate [10], and different to the 52.73(7)° seen in 1-tetradecylpyridinium bromide monohydrate [11]. The torsion angles in the hydrocarbon chain show that the largest deviation from the trans-conformation is 179.8(5)° for N–C6–C7–C8 slightly greater than the 179.0(4)° seen in 1-dodecylpyridinium chloride monohydrate [10] and 177.00(18)° seen in 1-tetradecylpyridinium bromide monohydrate [11]. The compound has a lipid bilayered structure and water molecules participate in the formation of the structure which is consistent with similar structures. The bromide ion accepts a weak hydrogen bond [O1W···Br1 = 3.284(4) Å and O1W–H1WA···Br1 = 179.6°] from the water molecule and symmetry links it to another water molecule [O1···Br1i = 3.328(4) Å and <(O1W–H1WB···Br1i) = 179.6 (i = 1 + X, +Y, +Z)], forming an infinite O–H···Br hydrogen bonded chain in the (100) direction. In addition, there are four non-classical hydrogen bonds between C–H donors and the water molecule or bromide anion. Two of these interactions involve the methylene group attached directly to the pyridinium nitrogen whilst two involve pyridinium C–H groups which is different from 1-tetradecylpyridinium bromide monohydrate.
Funding source: Natural Science Foundation of Hebei Province
Award Identifier / Grant number: B2018101017
Funding source: Scientific and technological research project of institutions of higher education in Hebei Province
Award Identifier / Grant number: QN2019315
Award Identifier / Grant number: BJ2019205
Funding source: Hebei Normal University
Award Identifier / Grant number: QN2017001
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: Natural Science Foundation of Hebei Province (grant No. B2018101017); Scientific and technological research project of institutions of higher education in Hebei Province (QN2019315, BJ2019205) and the 2017 fund project of Hebei Normal University for Nationalities (grant No. QN2017001).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SADABS, SAINT and SMART; Bruker AXS Inc.: Madison, Wisconsin, USA, 2008.Search in Google Scholar
2. Sheldrick, G. M. Shelxs-97, Program for X-ray Crystal Structure Determination; University of Gottingen: Gottingen, Germany, 1997.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Goossens, K., Lava, K., Bielawski, C. W., Binnemans, K. Ionic liquid crystals: versatile materials. Chem. Rev. 2016, 116, 4643–4807; https://doi.org/10.1021/cr400334b.Search in Google Scholar PubMed
6. Yildirim, A., Szymoniak, P., Sentker, K., Butschies, M., Bühlmeyer, A., Huber, P., Schönhals, A. Dynamics and ionic conductivity of ionic liquid crystals forming a hexagonal columnar mesophase. Phys. Chem. Chem. Phys. 2019, 20, 5626–5635.10.1039/C7CP08186CSearch in Google Scholar
7. Lee, C. K., Huang, H. W., Lin, I. J. Simple amphiphilic liquid crystalline N-alkylimidazolium salts. a new solvent system providing a partially ordered environment. Chem. Commun. 2000, 19, 1911–1912; https://doi.org/10.1039/b004462h.Search in Google Scholar
8. Kang, X., Sun, X., Han, B. Synthesis of functional nanomaterials in ionic liquids. Adv. Mater. 2016, 28, 1011–1030; https://doi.org/10.1002/adma.201502924.Search in Google Scholar PubMed
9. Carmichael, A. J., Hardacre, C., Holbrey, J. D., Nieuwenhuyzen, M., Seddon, K. R. Molecular layering and local order in thin films of 1-alkyl-3-methylimidazolium ionic liquids using X-ray reflectivity. Mol. Phys. 2001, 99, 795–800; https://doi.org/10.1080/00268970010012301.Search in Google Scholar
10. Vongbupnimit, K., Noguchi, K., Okuyama, K. 1–Dodecylpyridinium chloride monohydrate. Acta Crystallogr. 1995, C51, 1940–1941; https://doi.org/10.1107/s0108270195004537.Search in Google Scholar
11. Jordan, D. K., Kitchen, J. A., Hegh, D. Y., Brooker, S., Tan, E. W. 1–Tetradecylpyridinium bromide monohydrate. Acta Crystallogr. 2008, E64, o2457; https://doi.org/10.1107/s1600536808039020.Search in Google Scholar
© 2021 Yanwen Sun et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6

