Abstract
C14H16O5, triclinic, P
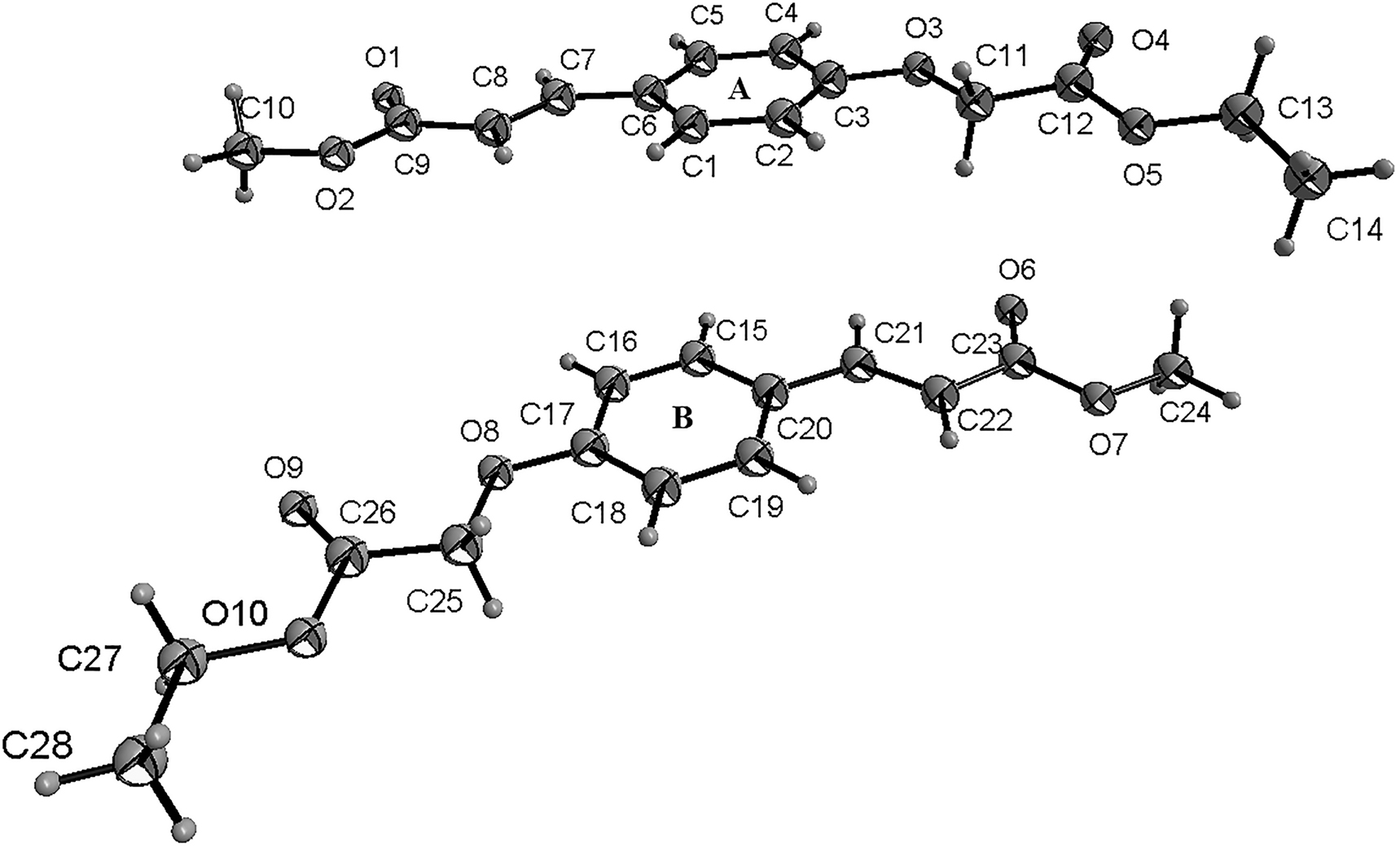
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.17 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.5°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 10,485, 5000, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3803 |
| N(param)refined: | 348 |
| Programs: | Bruker [1], SHELX [2], [, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.5279 (2) | −0.03036 (15) | 0.83500 (13) | 0.0583 (5) |
| H1 | 0.5195 | −0.0209 | 0.9009 | 0.070* |
| C2 | 0.4461 (2) | 0.04737 (15) | 0.78830 (13) | 0.0591 (5) |
| H2 | 0.3840 | 0.1086 | 0.8223 | 0.071* |
| C3 | 0.4571 (2) | 0.03348 (14) | 0.69001 (12) | 0.0488 (4) |
| C4 | 0.5508 (2) | −0.05755 (14) | 0.63956 (12) | 0.0524 (4) |
| H4 | 0.5586 | −0.0670 | 0.5737 | 0.063* |
| C5 | 0.6323 (2) | −0.13374 (14) | 0.68744 (12) | 0.0523 (4) |
| H5 | 0.6954 | −0.1943 | 0.6530 | 0.063* |
| C6 | 0.6226 (2) | −0.12251 (14) | 0.78623 (12) | 0.0498 (4) |
| C7 | 0.7077 (2) | −0.20735 (15) | 0.83439 (13) | 0.0545 (4) |
| H7 | 0.7680 | −0.2651 | 0.7946 | 0.065* |
| C8 | 0.7106 (2) | −0.21326 (16) | 0.92638 (14) | 0.0587 (5) |
| H8 | 0.6561 | −0.1561 | 0.9690 | 0.070* |
| C9 | 0.7981 (2) | −0.30808 (15) | 0.96279 (13) | 0.0544 (4) |
| C10 | 0.8606 (3) | −0.39440 (18) | 1.10074 (14) | 0.0696 (5) |
| H10A | 0.9752 | −0.3823 | 1.0833 | 0.104* |
| H10B | 0.8408 | −0.4679 | 1.0767 | 0.104* |
| H10C | 0.8202 | −0.3933 | 1.1703 | 0.104* |
| C11 | 0.2865 (2) | 0.19980 (14) | 0.68563 (13) | 0.0568 (4) |
| H11A | 0.3556 | 0.2497 | 0.7099 | 0.068* |
| H11B | 0.2027 | 0.1727 | 0.7406 | 0.068* |
| C12 | 0.2107 (2) | 0.26569 (14) | 0.61603 (12) | 0.0512 (4) |
| C13 | 0.0553 (3) | 0.43844 (16) | 0.60416 (15) | 0.0674 (5) |
| H13A | −0.0353 | 0.3993 | 0.5914 | 0.081* |
| H13B | 0.1279 | 0.4600 | 0.5423 | 0.081* |
| C14 | −0.0044 (3) | 0.54338 (17) | 0.66396 (16) | 0.0737 (6) |
| H14A | 0.0864 | 0.5834 | 0.6736 | 0.111* |
| H14B | −0.0726 | 0.5208 | 0.7260 | 0.111* |
| H14C | −0.0657 | 0.5936 | 0.6309 | 0.111* |
| C15 | 0.8044 (2) | 0.18059 (16) | 0.86747 (13) | 0.0598 (5) |
| H15 | 0.8318 | 0.1430 | 0.8079 | 0.072* |
| C16 | 0.8569 (2) | 0.13283 (15) | 0.94355 (13) | 0.0583 (5) |
| H16 | 0.9198 | 0.0642 | 0.9351 | 0.070* |
| C17 | 0.8157 (2) | 0.18734 (14) | 1.03318 (12) | 0.0507 (4) |
| C18 | 0.7214 (2) | 0.28931 (15) | 1.04514 (13) | 0.0574 (5) |
| H18 | 0.6922 | 0.3258 | 1.1052 | 0.069* |
| C19 | 0.6711 (2) | 0.33635 (16) | 0.96771 (13) | 0.0587 (5) |
| H19 | 0.6086 | 0.4051 | 0.9763 | 0.070* |
| C20 | 0.7111 (2) | 0.28407 (15) | 0.87698 (12) | 0.0529 (4) |
| C21 | 0.6633 (2) | 0.33272 (16) | 0.79310 (13) | 0.0590 (5) |
| H21 | 0.6886 | 0.2865 | 0.7380 | 0.071* |
| C22 | 0.5891 (2) | 0.43337 (16) | 0.78423 (13) | 0.0601 (5) |
| H22 | 0.5563 | 0.4818 | 0.8378 | 0.072* |
| C23 | 0.5575 (2) | 0.46999 (16) | 0.69200 (14) | 0.0585 (5) |
| C24 | 0.4518 (3) | 0.61708 (17) | 0.60725 (15) | 0.0704 (5) |
| H24A | 0.5530 | 0.6258 | 0.5592 | 0.106* |
| H24B | 0.3943 | 0.6908 | 0.6187 | 0.106* |
| H24C | 0.3874 | 0.5635 | 0.5843 | 0.106* |
| C25 | 0.8304 (2) | 0.18562 (15) | 1.19728 (12) | 0.0534 (4) |
| H25A | 0.7132 | 0.1962 | 1.2191 | 0.064* |
| H25B | 0.8775 | 0.2610 | 1.1938 | 0.064* |
| C26 | 0.8909 (2) | 0.10903 (15) | 1.26773 (12) | 0.0516 (4) |
| C27 | 0.8913 (3) | 0.09761 (17) | 1.43497 (13) | 0.0647 (5) |
| H27A | 1.0080 | 0.0890 | 1.4249 | 0.078* |
| H27B | 0.8469 | 0.0212 | 1.4408 | 0.078* |
| C28 | 0.8182 (3) | 0.16530 (18) | 1.52402 (15) | 0.0772 (6) |
| H28A | 0.8626 | 0.2408 | 1.5172 | 0.116* |
| H28B | 0.8420 | 0.1263 | 1.5792 | 0.116* |
| H28C | 0.7027 | 0.1727 | 1.5334 | 0.116* |
| O1 | 0.8814 (2) | −0.38141 (13) | 0.91315 (11) | 0.0909 (5) |
| O2 | 0.77875 (17) | −0.30365 (12) | 1.05856 (9) | 0.0685 (4) |
| O3 | 0.37976 (15) | 0.10434 (10) | 0.63651 (8) | 0.0579 (3) |
| O4 | 0.21016 (19) | 0.23713 (12) | 0.53647 (10) | 0.0748 (4) |
| O5 | 0.14039 (17) | 0.36314 (11) | 0.65826 (9) | 0.0675 (4) |
| O6 | 0.5950 (3) | 0.41540 (14) | 0.61845 (11) | 0.1085 (7) |
| O7 | 0.48292 (18) | 0.57381 (11) | 0.69658 (9) | 0.0696 (4) |
| O8 | 0.87424 (16) | 0.13399 (10) | 1.10454 (8) | 0.0593 (3) |
| O9 | 0.95911 (18) | 0.01679 (11) | 1.25174 (9) | 0.0709 (4) |
| O10 | 0.85264 (17) | 0.16062 (10) | 1.35406 (9) | 0.0624 (3) |
Source of material
The mixtrue of methyl (E)-3-(4-hydroxyphenyl)acrylate (1.78 g, 0.01 mol), ethyl 2-bromoacetate (2.00 g, 0.012 mol), K2CO3 (2.76 g, 0.02 mol) and DMF (10 mL) was reacted at 80 °C for 2 h. After the reaction completed (monitored by TLC), the mixture was poured into 50 mL ice water and a large amount of white product was precipitated. The product was filtered and washed with water three times respectively. The yield was 86% (based on methyl (E)-3-(4-hydroxyphenyl)acrylate). Elemental Anal. Calcd. (%) for C14H16O5(264.27): C, 63.63; H, 6.10. Found (%): C, 61.53; H, 6.27. The crystals were obtained after one week of slow volatilisation at room temperature.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.93 Å with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all other H atoms.
Comment
The p-coumaric acid, (E)-3-(4-hydroxyphenyl) acrylic acid, is a natural phenolic acid of cinnamic acid core structure [5]. p-Coumaric acid is mainly found in fruits, vegetables, grains, and fungi, and is also abundant in Chinese herbal medicines [6], [7], [8], [9]. The pharmacological effects of p-coumaric acid has anti-oxidant, anti-inflammatory, antitumor effects, antiplatelet aggregation, and cardiovascular protection, while the anti-oxidant activities is the important basis of other pharmacological effects [10], [11], [12]. The synthesis and application of p-coumaric acid and its derivatives have attracted much attention [10], [11], [12], [13], [14], [15], [16]. We are committed to the detection and regulation of cosmetics. In order to establish a rapid and effective method for the determination of coumaric acid derivatives, a series of p-coumaric acid derivatives were synthesized.
There are two crystallographic independent molecules in the asymmetric unit (shown in the figure). In the molecules of the title structure bond lengths and angles are very similar to those given in the literature for p-coumaric acid derivatives [17], [, 18]. In the title structure, the parts of methyl p-coumaric acid of molecule A and B were approximately planar. The dihedral angles of molecule A formed by the C1–C6 plane, the carboxylate group O1–C9–O2 plane and the carboxylate group O4–C12–O5 plane were 5.0°, 10.1° and 14.6, respectively, while the dihedral angles of molecule B formed by the C15–C20 plane, the carboxylate group O6–C23–O7 plane and the carboxylate group O9–C26–O10 plane were 6.5°, 6.1° and 1.1°, respectively.
Funding source: Jiangxi key R & D project
Award Identifier / Grant number: 20203BBGL73212
Funding source: Key Research Foundation of Educational Department of Jiangxi Province of China
Award Identifier / Grant number: GJJ200386
Award Identifier / Grant number: GJJ160382
Funding source: Reform of Higher Education Foundation of Jiangxi Province
Award Identifier / Grant number: JXJG-17-3-18
Acknowledgements
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: Jiangxi key R & D project (20203BBGL73212), the Key Research Foundation of Educational Department of Jiangxi Province of China (GJJ200386, GJJ160382) and the Reform of Higher Education Foundation of Jiangxi Province (No. JXJG-17-3-18).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Wang, D., Miao, X. Y., Guo, X. D., Zhu, J. Preparation of coumaric acid amide derivatives and their application in cosmetics. Chin. J. Chem. 2020, 61, 305–311.Search in Google Scholar
6. Taofiq, O., González-Paramás, A. M., Barreiro, M. F., Ferreira, I. C. F. R. Hydroxycinnamic acids and their derivatives: cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 1–24; https://doi.org/10.3390/molecules22020281.Search in Google Scholar
7. Pei, K., Ou, J., Huang, J., Ou, S. Y. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962; https://doi.org/10.1002/jsfa.7578.Search in Google Scholar
8. Clifford, M. N. Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372.10.1002/(SICI)1097-0010(19990301)79:3<362::AID-JSFA256>3.0.CO;2-DSearch in Google Scholar
9. Kim, J. S. Investigation of phenolic, flavonoid, and vitamin contents in different parts of Korean ginseng (Panax ginseng C. A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 263–270; https://doi.org/10.3746/pnf.2016.21.3.263.Search in Google Scholar
10. Chung, I. M., Lim, J. J., Ahn, M. S., Jeong, H. N., An, T. J. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016, 40, 68–75; https://doi.org/10.1016/j.jgr.2015.05.006.Search in Google Scholar
11. Pereira, J. A., Oliveira, I., Sousa, A., Valentāo, P., Andrade, P. B., Ferreira, I. C. F. R., Ferreres, F., Bento, A., Seabra, R., Estevinho, L. Walnut (Juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295; https://doi.org/10.1016/j.fct.2007.06.004.Search in Google Scholar
12. Cheng, J., Dai, F., Zhou, B., Yang, L., Liu, Z. L. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: mechanism and structure-activity relationship. Food Chem. 2007, 104, 132–139; https://doi.org/10.1016/j.foodchem.2006.11.012.Search in Google Scholar
13. Camarero, S., Canas, A. I., Nousiainen, P., Record, E., Lomascolo, A., MartÍnez, M. J., MartÍnez, Á. T. p-Hydroxycinnamic acids as natural mediators for laccase oxidation of recalcitrant compounds. Environ. Sci. Technol. 2008, 42, 6703–6709; https://doi.org/10.1021/es8008979.Search in Google Scholar
14. Wang, J. R., Ma, L., Li, W. F., Tang, X. H., Zhao, G., Peng, L. X., Zhao, J. L. Effect of trace elements on the flavonoids and phenolic acids in tartary Buckwheat Sprouts. Acta Agric. Univ. Jiangxiensis 2017, 39, 55–63.Search in Google Scholar
15. Li, X., Zhao, J., Liu, J. X., Li, G., Zhao, Y., Zeng, X. Systematic analysis of absorbed anti-inflammatory constituents and metabolites of Sarcandra glabra in rat plasma using ultra-high-pressure liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry. PLoS One 2016, 11, e150063; https://doi.org/10.1371/journal.pone.0150063.Search in Google Scholar PubMed PubMed Central
16. Shailasree, S., Venkataramana, M., Niranjana, S. R., Prakash, H. S. Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol. Neurobiol. 2015, 51, 119–130; https://doi.org/10.1007/s12035-014-8700-2.Search in Google Scholar PubMed
17. Faini, F., Gonzalez, F. S., Labbe, C., Rodilla, J. M., Torres, R., Rocha, P. M., Monache, F. D. Crystal structure of 9-trans-p- coumaroyloxy-α-terpineol, C19H24O4. Z. Kristallogr. NCS 2009, 224, 277–279; https://doi.org/10.1524/ncrs.2009.0122.Search in Google Scholar
18. Jing, L., Ma, H., Li, Q., He, L., Jia, Z. Crystal structure of (1S,4S,5S,8R)-8-nitrooxy-2,6-dioxabicyclo[3.3.0] octan-4-yl-3-(4-acetoxyphenyl)acrylate, C17H17NO9. Z. Kristallogr. NCS 2012, 227, 297–298; https://doi.org/10.1524/ncrs.2012.0138.Search in Google Scholar
© 2021 Guo Chun-Mei et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10

