Abstract
C13H16N2O3S, triclinic,
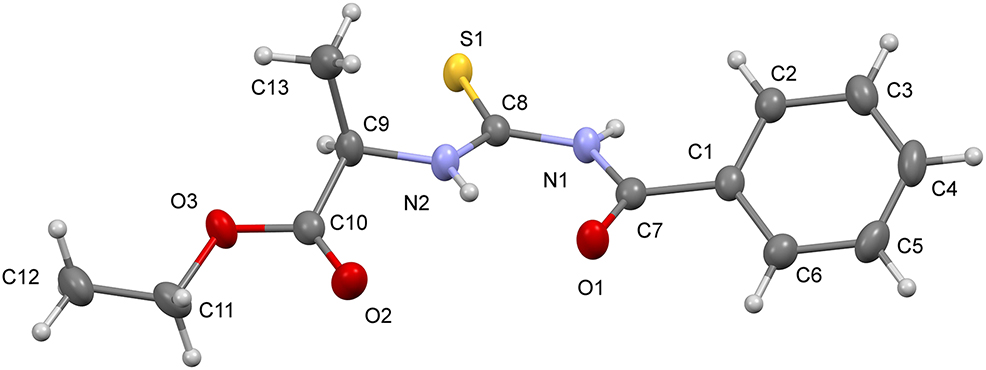
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.22 × 0.14 × 0.03 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 2.14 mm−1 |
| Diffractometer, scan mode: | Oxford Diffraction Gemini, ω |
| θmax, completeness: | 71.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13964, 2644, 0.026 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2445 |
| N(param)refined: | 182 |
| Programs: | CrysAlisPRO [1], SHELX [2], [, 3], PLATON [4], Olex2 [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.42897 (5) | 0.74998 (4) | 0.95514 (4) | 0.02776 (13) |
| O1 | 0.58278 (15) | 0.84892 (12) | 0.61333 (11) | 0.0312 (3) |
| O2 | 0.24347 (17) | 0.45286 (13) | 0.42096 (12) | 0.0384 (3) |
| O3 | 0.09157 (14) | 0.28051 (12) | 0.48094 (11) | 0.0313 (3) |
| N1 | 0.58871 (16) | 0.90186 (14) | 0.83662 (13) | 0.0241 (3) |
| N2 | 0.43456 (17) | 0.64057 (14) | 0.69922 (13) | 0.0259 (3) |
| C1 | 0.78337 (19) | 1.08993 (16) | 0.79635 (16) | 0.0240 (3) |
| C2 | 0.9107 (2) | 1.15302 (17) | 0.93946 (17) | 0.0274 (3) |
| H2 | 0.902510 | 1.104922 | 1.003690 | 0.033* |
| C3 | 1.0495 (2) | 1.28723 (18) | 0.98609 (19) | 0.0341 (4) |
| H3 | 1.135636 | 1.328195 | 1.081110 | 0.041* |
| C4 | 1.0596 (2) | 1.36000 (18) | 0.8910 (2) | 0.0369 (4) |
| H4 | 1.153061 | 1.449758 | 0.922111 | 0.044* |
| C5 | 0.9312 (2) | 1.29990 (19) | 0.7497 (2) | 0.0371 (4) |
| H5 | 0.937126 | 1.350950 | 0.686857 | 0.045* |
| C6 | 0.7936 (2) | 1.16379 (18) | 0.70121 (17) | 0.0301 (3) |
| H6 | 0.708961 | 1.122355 | 0.605722 | 0.036* |
| C7 | 0.64214 (19) | 0.93740 (16) | 0.73796 (16) | 0.0240 (3) |
| C8 | 0.48330 (18) | 0.75989 (16) | 0.82029 (15) | 0.0231 (3) |
| C9 | 0.3328 (2) | 0.48246 (16) | 0.67026 (16) | 0.0267 (3) |
| H9 | 0.251469 | 0.491004 | 0.718485 | 0.032* |
| C10 | 0.21925 (19) | 0.40686 (16) | 0.50950 (16) | 0.0268 (3) |
| C11 | −0.0242 (2) | 0.19127 (19) | 0.32983 (17) | 0.0341 (4) |
| H11A | 0.048876 | 0.157055 | 0.279343 | 0.041* |
| H11B | −0.093019 | 0.256178 | 0.285852 | 0.041* |
| C12 | −0.1500 (2) | 0.0509 (2) | 0.3227 (2) | 0.0421 (4) |
| H12A | −0.229608 | −0.010463 | 0.224172 | 0.063* |
| H12B | −0.220676 | 0.086364 | 0.373689 | 0.063* |
| H12C | −0.080314 | −0.012834 | 0.365584 | 0.063* |
| C13 | 0.4585 (2) | 0.38273 (18) | 0.72445 (18) | 0.0373 (4) |
| H13A | 0.387471 | 0.278342 | 0.700561 | 0.056* |
| H13B | 0.525739 | 0.430600 | 0.826769 | 0.056* |
| H13C | 0.541315 | 0.376581 | 0.680090 | 0.056* |
| H1A | 0.607 (2) | 0.9762 (19) | 0.9107 (17) | 0.031 (5)* |
| H2A | 0.463 (3) | 0.657 (2) | 0.6367 (19) | 0.046 (6)* |
Source of material
Benzoyl chloride, ammonium thiocyanate, l-alanine, lanthanum(III) chloride and solvents were purchased and used without further purification.
The title compound (I) is an ester analogue of 2-(3-benzoylthioureido)propionic acid (II) [6]. In the first step, II was prepared as described by Ngah et al. [6]. Next, II was refluxed with LaCl3 in ethanol for 17 h to yield the title compound. After cooling to room temperature, the solvent was reduced under atmospheric pressure. The crude product was extracted with water and dichloromethane to remove the catalyst (LaCl3). The organic layer was dried over anhydrous MgSO4, filtered and the solvent was removed in vacuo. It was recrystallised from an ethanol solution at room temperature to give colourless crystals suitable for single crystal X-ray diffraction. Elem. Anal.: Calc. for C13H16N2O3S: C = 55.70; H = 5.75; N = 9.99; S = 11.44%. Found: C = 55.54; H = 5.05; N = 9.52; S = 12.36%. IR (KBr, cm−1): 3234 [ν(N–H)], 1732 [ν(C=Ocarboxylate)], 1672 [ν(C=Oamide)], 913 [ν(C=S)]. 1H NMR (CDCl3, 400 MHz) δ (ppm): 11.20 (s, 1H), 9.05 (s, 1H), 7.86 (d, J = 7.3 Hz, 2H), 7.63 (t, J = 7.4 Hz, 1H), 7.53 (t, J = 7.3 Hz, 2H), 5.00–5.06 (m, 1H), 4.27 (q, J = 7.1 Hz, 2H), 1.62 (d, J = 7.1 Hz, 3H), 1.32 (t, J = 7.1 Hz, 3H). 13C NMR (CDCl3, 400 MHz) δ (ppm): 179.6, 171.7, 166.8, 133.7, 131.8, 129.2, 127.6, 61.9, 54.2, 17.6, 14.2.
Experimental details
All H atoms, except amine H atoms, were positioned geometrically and refined in a riding model approximation (C–H = 0.93–0.98 Å) with Uiso(H) = 1.2–1.5Ueq(C). The hydrogen atoms on N1 and N2, namely H1A and H2A, respectively, were located in a difference Fourier map and refined with a distance restraint of N–H = 0.86 ± 0.02 Å.
Comment
Benzoylthiourea derivatives represent a well-known category of organic compounds due to the diverse biological applications including insecticidal [7], antibacterial [8], anticancer [9], [, 10] and antifungal [11]. In view of the interesting properties of this class of compounds, we synthesised a derivative linked with an amino acid congener. The adjunct amino acid derivative on the benzoylthiourea core is a significant chemical constituent found in biological systems. These class of compounds were reported to exhibit enhanced biological properties [12], [, 13]. Herein, the synthesis and crystal structure determination of the title compound (I) is described.
The compound adopts a cis-trans conformation with respect to the ethyl propanoate and benzoyl moieties, relative to the S atom across the C8–N2 and C8–N1 bonds, respectively. This conformation is commonly observed in other benzoylthiourea derivatives [14], [15], [16]. The C8–S1, N1–C8 and N2–C8 bond lengths of the thiourea core are almost equivalent to the corresponding bond lengths of related structures [17], [, 18]. The aforementioned benzoyl and ethyl propanoate fragments are inclined to the thiourea least-square plane (S1/N1/N2/C8) with dihedral angles of 33.61(5) and 45.70(5)°, respectively. Additionally, the benzoyl moiety and ethyl propanoate fragment subtend a dihedral angle of 12.11(6)°.
The crystal structure features an intramolecular N–H···O [N(2)–H(2A)···O(1): H(2A)···O(1) = 0.83(2) Å, N(2)···O(1) = 2.6696(18) Å at an angle of 135.2(17)°] hydrogen bond generating a S(6) ring according to graph-set notation [19]. This characteristic is also featured in the solid-state structure of archetypal benzoylthiourea compounds [11], [20], [21]. In the molecular packing, the only directional interaction observed is the N–H···S [N(1)–H(1A)···S(1): H(1A)···S(1) = 0.836(17) Å, N(1)···S(1) = 3.3923(14) Å at an angle of 152.7(15)°; symmetry operation of (i) 1 − x, 2 − y, 2 − z] contact, which leads to the formation of dimeric aggregates. The dimers form a stacking column along the a-axis which is stabilised by C‒H⋯π interactions but no other significant directional interactions.
To probe the supramolecular aggregation further, Hirshfeld surface analysis in conjunction with two-dimensional fingerprint (FP) plots were obtained by utilizing Crystal Explorer 17 [22] with reference to previous methods [23], [, 24]. The FP plot delineated H···S/S···H contacts into distinct complementary long sharp spikes corresponding to the intermolecular N–H···S hydrogen bonds. H···S/S···H contacts contribute 11.9% of all contacts to the Hirshfeld surface. Noteworthy, the H···H contacts contribute 45.8% of all contacts to the surface. Other significant contributions to the surface are from H···C/C···H [18.1%] and H···O/O···H [18.0%] contacts.
Funding source: Centre for Research and Instrumentation Management (UKM)
Funding source: Ministry of Higher Education (MOHE) Malaysia
Award Identifier / Grant number: FRGS/1/2018/STG01/UKM/01/3
Acknowledgements
The authors thank the Department of Chemical Sciences, Faculty of Science and Technology (UKM) for the provision of experimental facilities and the Department of Chemistry, Faculty of Science (UPM) for the X-ray analysis.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The authors thank the Centre for Research and Instrumentation Management (UKM) for a postdoctoral fellowship to YYC and the Ministry of Higher Education (MOHE) Malaysia for FRGS/1/2018/STG01/UKM/01/3 research grant.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO; Agilent Technologies: Santa Clara, CA, USA, 2012.Suche in Google Scholar
2. Sheldrick, G. M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Suche in Google Scholar
5. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
6. Ngah, N., Kassim, M. B., Yamin, B. M. 2-(3-Benzoylthioureido)propionic acid. Acta Crystallogr. 2006, E62, o381–o382; https://doi.org/10.1107/s1600536805039607.Suche in Google Scholar
7. Xu, X., Qian, X., Li, Z., Huang, Q., Chen, G. Synthesis and insecticidal activity of new substituted N-aryl-N′-benzoylthiourea compounds. J. Fluor. Chem. 2003, 121, 51–54; https://doi.org/10.1016/s0022-1139(02)00330-5.Suche in Google Scholar
8. Marzi, M., Pourshamsian, K., Hatamjafari, F., Shiroudi, A., Oliaey, A. R. Synthesis of new N-benzoyl-N′-triazine thiourea derivatives and their antibacterial activity. Russ. J. Bioorg. Chem. 2019, 45, 391–397; https://doi.org/10.1134/s106816201905008x.Suche in Google Scholar
9. Ruswanto, Miftaha, A. M., Tjahjono, D. H., Siswandono Synthesis and in vitro cytotoxicity of 1-benzoyl-3-methylthiourea derivatives. Procedia Chem. 2015, 17, 157–161; https://doi.org/10.1016/j.proche.2015.12.105.Suche in Google Scholar
10. Kesuma, D., Siswandono, Purwanto, B. T., Rudyanto, M. Synthesis and anticancer evaluation of N-benzoyl-N′-phenyltiourea derivatives against human breast cancer cells (T47D). J. Chin. Pharmaceut. Sci. 2020, 29, 123–129.10.5246/jcps.2020.02.010Suche in Google Scholar
11. Limban, C., Chifiriuc, M. C., Caproiu, M. T., Dumitrascu, F., Ferbinteanu, M., Pintilie, L., Stefaniu, A., Vlad, I. M., Bleotu, C., Marutescu, L. G., Nuta, D. C. New substituted benzoylthiourea derivatives: from design to antimicrobial applications. Molecules 2020, 25, 1478–1497; https://doi.org/10.3390/molecules25071478.Suche in Google Scholar PubMed PubMed Central
12. Chohan, Z. H., Arif, M., Akhtar, M. A., Supuran, C. T. Metal-based antibacterial and antifungal agents: synthesis, characterization, and in vitro biological evaluation of Co(II), Cu(II), Ni(II), and Zn(II) complexes with amino acid-derived compounds. Bioinorgan. Chem. Appl. 2006, 2006, 1–13; https://doi.org/10.1155/bca/2006/83131.Suche in Google Scholar PubMed PubMed Central
13. Kadir, M. A., Ramli, R., Yusof, M. S. M., Ismail, N., Ngah, N. Synthesis, spectroscopic studies and antibacterial activity of new lauroyl thiourea amino acid derivatives. Asian J. Chem. 2016, 28, 596–600; https://doi.org/10.14233/ajchem.2016.19430.Suche in Google Scholar
14. Chong, Y. Y., Tahir, M. I. M., Kassim, M. B. 2-(3-Benzoylthioureido)-3-phenylpropanoic acid. IUCrData 2016, 1, x161091; https://doi.org/10.1107/s2414314616010919.Suche in Google Scholar
15. Mark-Lee, W. F., Nasir, M. F. M., Kassim, M. B. Structural and optical properties investigation on H-bonded 1D helical self-assembly of 1,1-dibenzyl-3-(2-bromobenzoyl)thiourea molecules for nonlinear optical application. Sains Malays. 2018, 47, 741–747; https://doi.org/10.17576/jsm-2018-4704-12.Suche in Google Scholar
16. Abosadiya, H. M., Anouar, E. H., Yamin, B. M. Synthesis, X-ray, spectroscopic characterization (FT-IR, NMR, UV-Vis) and quantum chemical calculations of some substituted benzoylthiourea derivatives. J. Mol. Struct. 2019, 1194, 48–56; https://doi.org/10.1016/j.molstruc.2019.05.060.Suche in Google Scholar
17. Tan, S. L., Azizan, A. H. S., Jotani, M. M., Tiekink, E. R. T. 3,3-Bis(2-hydroxyethyl)-1-(4-methylbenzoyl)thiourea: crystal structure, Hirshfeld surface analysis and computational study. Acta Crystallogr. 2019, E75, 1472–1478; https://doi.org/10.1107/s2056989019012581.Suche in Google Scholar PubMed PubMed Central
18. Abosadiya, H. M. Synthesis, crystal structure and antioxidant evaluation of N-(4-formylpiperazine-1-carbonothioyl)benzamide. Eur. J. Chem. 2020, 11, 156–159; https://doi.org/10.5155/eurjchem.11.2.156-159.1981.Suche in Google Scholar
19. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Suche in Google Scholar
20. Hassan, I. N., Yamin, B. M., Kassim, M. B. Methyl 2-(3-benzoylthioureido)acetate. Acta Crystallogr. 2009, E65, o3078; https://doi.org/10.1107/s1600536809046169.Suche in Google Scholar PubMed PubMed Central
21. Hassan, I. N., Chong, Y. Y., Kassim, M. B. Methyl 3-(3-benzoylthioureido)propanoate. Acta Crystallogr. 2011, E67, o780; https://doi.org/10.1107/s160053681100568x.Suche in Google Scholar
22. Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D., Spackman, M. A. Crystal Explorer v17; The University of Western Australia: Australia, 2017.Suche in Google Scholar
23. Mark-Lee, W. F., Chong, Y. Y., Kassim, M. B. Supramolecular structures of rhenium(I) complexes mediated by ligand planarity via the interplay of substituents. Acta Crystallogr. 2018, C74, 997–1006; https://doi.org/10.1107/s2053229618010586.Suche in Google Scholar PubMed
24. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Suche in Google Scholar
© 2020 Yan Yi Chong et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10

