Abstract
C46H36Cu2O10, monoclinic, C2/c (no. 15), a = 32.663(7) Å, b = 7.4214(15) Å, c = 21.785(4) Å, β = 131.68(3)°, V = 3944.0(19) Å3, Z = 4, Rgt(F) = 0.0478, wRref(F2) = 0.1583, T = 295 K.
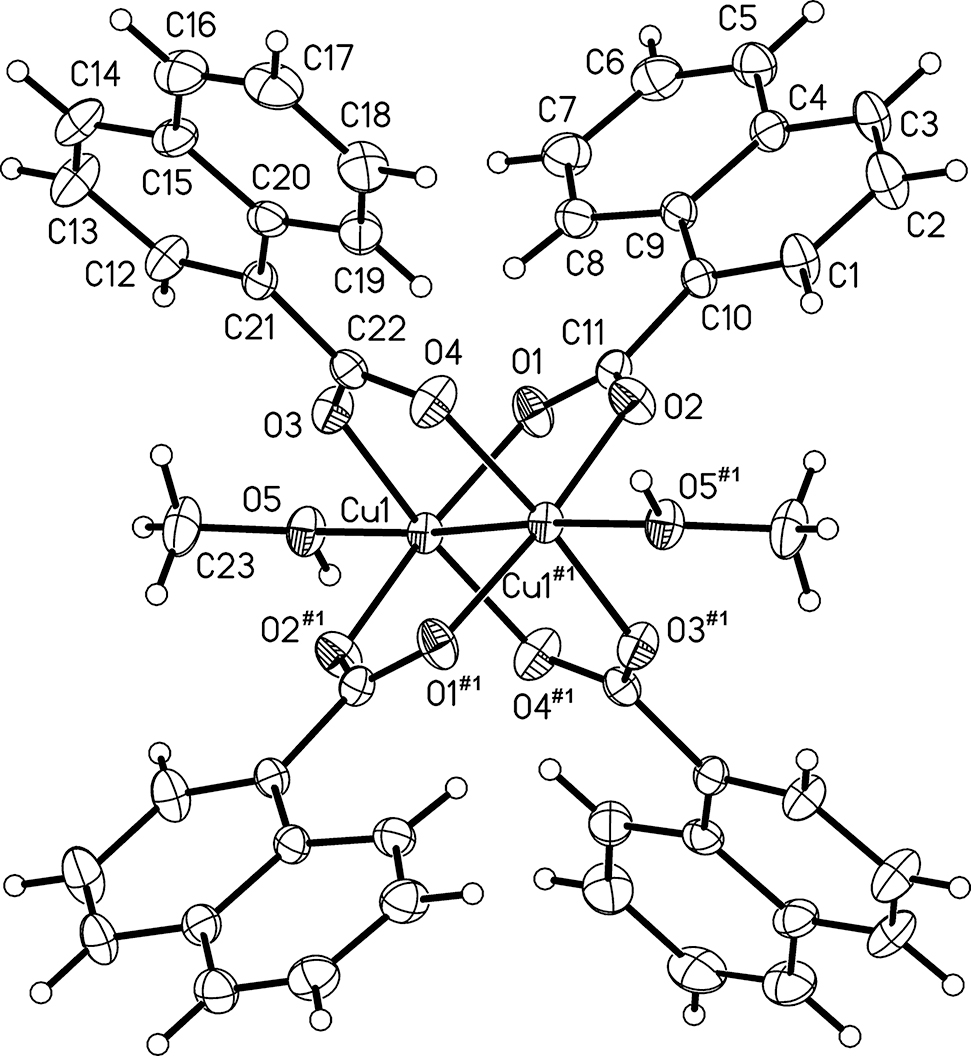
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Blue block |
| Size: | 0.47 × 0.24 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.14 mm−1 |
| Diffractometer, scan mode: | Rigaku R-AXIS RAPID, ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 14878, 3469, 0.054 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2299 |
| N(param)refined: | 262 |
| Programs: | Rigaku [1], SHELX [2], [, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cu1 | 0.00310 (3) | 0.83972 (8) | 0.48061 (4) | 0.0417 (2) |
| O1 | 0.03638 (19) | 0.7646 (5) | 0.5920 (3) | 0.0592 (11) |
| O2 | 0.03265 (18) | 1.0437 (5) | 0.6249 (2) | 0.0611 (12) |
| O3 | 0.07396 (16) | 0.9280 (5) | 0.5230 (3) | 0.0566 (11) |
| O4 | 0.06879 (17) | 1.2057 (5) | 0.5548 (3) | 0.0646 (13) |
| O5 | 0.01618 (17) | 0.5841 (5) | 0.4482 (3) | 0.0563 (11) |
| H5B | −0.008630 | 0.506780 | 0.431370 | 0.084* |
| C1 | 0.0639 (3) | 0.9459 (9) | 0.7684 (4) | 0.0638 (18) |
| H1A | 0.046669 | 1.055534 | 0.743762 | 0.077* |
| C2 | 0.0836 (3) | 0.9086 (10) | 0.8470 (5) | 0.079 (2) |
| H2A | 0.079015 | 0.992446 | 0.873748 | 0.095* |
| C3 | 0.1093 (3) | 0.7505 (10) | 0.8840 (4) | 0.0657 (18) |
| H3A | 0.122609 | 0.727272 | 0.936533 | 0.079* |
| C4 | 0.1162 (2) | 0.6210 (8) | 0.8446 (4) | 0.0509 (15) |
| C5 | 0.1428 (3) | 0.4556 (9) | 0.8837 (4) | 0.0626 (18) |
| H5A | 0.155936 | 0.433214 | 0.936163 | 0.075* |
| C6 | 0.1494 (3) | 0.3303 (10) | 0.8464 (4) | 0.0688 (19) |
| H6A | 0.165805 | 0.220615 | 0.872186 | 0.083* |
| C7 | 0.1317 (3) | 0.3647 (9) | 0.7688 (4) | 0.0680 (19) |
| H7A | 0.137125 | 0.278491 | 0.743771 | 0.082* |
| C8 | 0.1067 (2) | 0.5231 (8) | 0.7297 (4) | 0.0561 (16) |
| H8A | 0.095783 | 0.543825 | 0.678498 | 0.067* |
| C9 | 0.0969 (2) | 0.6574 (8) | 0.7646 (3) | 0.0460 (13) |
| C10 | 0.0693 (2) | 0.8251 (7) | 0.7270 (3) | 0.0426 (13) |
| C11 | 0.0446 (2) | 0.8798 (8) | 0.6416 (4) | 0.0443 (13) |
| C12 | 0.1682 (3) | 0.9853 (9) | 0.5590 (4) | 0.0593 (17) |
| H12A | 0.149284 | 0.876727 | 0.537478 | 0.071* |
| C13 | 0.2175 (3) | 1.0052 (11) | 0.5757 (5) | 0.080 (2) |
| H13A | 0.230352 | 0.911808 | 0.563903 | 0.096* |
| C14 | 0.2462 (3) | 1.1599 (11) | 0.6088 (5) | 0.074 (2) |
| H14A | 0.278707 | 1.172371 | 0.619237 | 0.089* |
| C15 | 0.2280 (2) | 1.3028 (9) | 0.6278 (4) | 0.0577 (17) |
| C16 | 0.2593 (3) | 1.4604 (11) | 0.6656 (5) | 0.076 (2) |
| H16A | 0.292466 | 1.470001 | 0.677709 | 0.092* |
| C17 | 0.2425 (3) | 1.5994 (11) | 0.6849 (5) | 0.082 (2) |
| H17A | 0.264090 | 1.701892 | 0.710673 | 0.098* |
| C18 | 0.1924 (3) | 1.5861 (10) | 0.6654 (5) | 0.075 (2) |
| H18A | 0.180502 | 1.681383 | 0.677885 | 0.090* |
| C19 | 0.1604 (3) | 1.4356 (9) | 0.6284 (4) | 0.0602 (17) |
| H19A | 0.127000 | 1.431288 | 0.615511 | 0.072* |
| C20 | 0.1771 (2) | 1.2866 (8) | 0.6092 (3) | 0.0489 (15) |
| C21 | 0.1473 (2) | 1.1197 (8) | 0.5732 (3) | 0.0441 (13) |
| C22 | 0.0932 (2) | 1.0845 (8) | 0.5493 (3) | 0.0446 (13) |
| C23 | 0.0381 (3) | 0.5763 (10) | 0.4102 (5) | 0.079 (2) |
| H23A | 0.041028 | 0.452762 | 0.400421 | 0.118* |
| H23B | 0.014436 | 0.639929 | 0.358870 | 0.118* |
| H23C | 0.073797 | 0.630818 | 0.445444 | 0.118* |
Source of material
The reaction of 0.172 g (1.0 mmol) CuCl2⋅2H2O with 0.344 g (2.0 mmol) 1-naphthoic acid (HNAP) in 20 mL methanol for 15 min afforded green solid, which was then filtered. The green filtrate was allowed to stand at room temperature by slow evaporation within two days; green block crystals suitable for X-ray diffraction were obtained (yield: 63.5% based on CuCl2⋅2H2O input).
Experimental details
The structure was solved by direct methods with the SHELXS program. All H-atoms from C atoms were positioned with idealized geometry (Uiso(H) = 1.2 Ueq(C) and Uiso(H) = 1.5 Ueq(C) for aromatic and methyl H atoms, respectively) using a riding model with C–H = 0.93 or 0.96 Å. H atom attached to the O atom was refined using a riding model, with the O–H distance fixed with Uiso(H) values set at 1.5 Ueq(O).
Comment
Construction of supramolecular systems and crystal engineering have been one of the most active fields in chemistry and materials science, due to their fascinating structures and potential applications in ion exchange [4], catalysis [5], [, 6], gas storage and separation [7], [, 8], fluorescent sensing [9], [, 10], optical and magnetic properties [11], [, 12], and so on. In the past decades, considerable effort has been devoted to design the supramolecular assemblies by carefully selecting building blocks and organic ligands [13]. Aromatic carboxylate ligands, due to their versatile coordination modes and potential luminescence nature, have been extensively employed as linkers to construct functional materials. Up to now, many complexes which were assembled by 1-naphthoates (NAP) have been reported [14], [15], [16].
Crystallographic analysis of the title complex shows that it crystallizes in the monoclinic space group C2/c, containing a dinuclear paddle-wheel complex [Cu2(NAP)4(MeOH)2], which is composed of two CuII ions, four NAP ligands and two methanol molecules. Each Cu ion is coordinated by four O atoms from four different NAP ligands, while the O atom from methanol occupies the apical position, forming a slightly distorted square pyramid with d(Cu1–O1) =1.969(4) Å, d(Cu1–O2#1) = 1.956(4) Å, d(Cu1–O3) =1.949(4) Å, d(Cu1–O4#1) = 1.953(4) Å, d(Cu1–O5) = 2.163(4) Å (#1 = −x, −y + 2, −z + 1), which are in good agreement with those reported for similar complexes [17]. The Cu⃛Cu distance is 2.578(1) Å, which is observed within the normal range for dinuclear paddle-wheel units in the structures of CuII carboxylate complexes [18], [19]. The dinuclear CuII units are further connected to form one-dimensional chains by hydrogen bonds [d(O5⃛O1#2 = 2.900(4) Å, O5–H5B⃛O1#2 = 150(2)°, #2 = −x, −y + 2, −z + 1].
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The author declares no conflicts of interest regarding this article.
References
1. Rigaku. RAPID–AUTO; Rigaku Corporation: Tokyo, Japan, 1998.Suche in Google Scholar
2. Sheldrick, G. M. SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Das, S., Kim, H., Kim, K. Metathesis in single crystal: complete and reversible exchange of metal ions constituting the frameworks of metal-organic frameworks. J. Am. Chem. Soc. 2009, 131, 3814–3815; https://doi.org/10.1021/ja808995d.Suche in Google Scholar
5. Xu, W., Chen, H., Jie, K. C., Yang, Z. Z., Li, T. T., Dai, S. Entropy-driven mechanochemical synthesis of polymetallic zeolitic imidazolate frameworks for CO2 fixation. Angew. Chem. Int. Ed. 2019, 58, 5018–5022; https://doi.org/10.1002/anie.201900787.Suche in Google Scholar
6. Zhang, M. Y., Xu, W., Li, T. T., Zhu, H. L., Zheng, Y. Q. In situ growth of tetrametallic FeCoMnNi–MOF-74 on nickel foam as efficient bifunctional electrocatalysts for the evolution reaction of oxygen and hydrogen. Inorg. Chem. 2020, 59, 15467–15477; https://doi.org/10.1021/acs.inorgchem.0c02504.Suche in Google Scholar
7. Forse, A. C., Gonzalez, M. I., Siegelman, R. L., Witherspoon, V. J., Jawahery, S., Mercado, R., Milner, P. J., Martell, J. D., Smit, B., Blümich, B., Long, J. R., Reimer, J. A. Unexpected diffusion anisotropy of carbon dioxide in the metal-organic framework Zn2(dobpdc). J. Am. Chem. Soc. 2018, 140, 1663–1673; https://doi.org/10.1021/jacs.7b09453.Suche in Google Scholar
8. Li, H., Wang, K. C., Sun, Y. J., Lollar, C. T., Li, J. L., Zhou, H. C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121; https://doi.org/10.1016/j.mattod.2017.07.006.Suche in Google Scholar
9. Lu, K. D., Aung, T., Guo, N. N., Weichselbaum, R., Lin, W. B. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634; https://doi.org/10.1002/adma.201707634.Suche in Google Scholar
10. Wang, L., Tu, B. T., Xu, W., Fu, Y., Zheng, Y. Q. Uranyl organic framework as a highly selective and sensitive turn-on and turn-off luminescent sensor for dual functional detection arginine and MnO4−. Inorg. Chem. 2020, 59, 5004–5017; https://doi.org/10.1021/acs.inorgchem.0c00236.Suche in Google Scholar
11. Espallargas, G. M., Coronado, E. Magnetic functionalities in MOFs: from the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557.10.1039/C7CS00653ESuche in Google Scholar PubMed
12. Xu, W., Si, Z. X., Xie, M., Zhou, L. X., Zheng, Y. Q. Experimental and theoretical approaches to three uranyl coordination polymers constructed by phthalic acid and N,N′-donor bridging ligands: crystal structures, luminescence, and photocatalytic dedgradation of tetracycline hydrochloride. Cryst. Growth Des. 2017, 17, 2147–2157; https://doi.org/10.1021/acs.cgd.7b00097.Suche in Google Scholar
13. Inge, A. K., Köppen, M., Su, J., Feyand, M., Xu, H. Y., Zou, X. D., O’Keeffe, M., Stock, N. Unprecedented topological complexity in a metal- organic framework constructed from simple buiding units. J. Am. Chem. Soc. 2016, 138, 1970–1976; https://doi.org/10.1021/jacs.5b12484.Suche in Google Scholar
14. Takasaki, S., Takamizawa, S. Reversible crystal deformation of a single-crystal host of copper(II) 1-naphthoate-pyrazine through crystal phase transition induced by methanol vapor sorption. Chem. Commun. 2015, 51, 5024–5027; https://doi.org/10.1039/c4cc09948f.Suche in Google Scholar
15. Liu, Z. Y., Xia, Y. F., Jiao, J., Yang, E. C., Zhao, X. J. Two water-bridged cobalt(II) chains with isomeric naphthoate spacers: from metamagnetic to single-chain magnetic behaviour. Dalton Trans. 2015, 44, 19927–19934; https://doi.org/10.1039/c5dt03297k.Suche in Google Scholar
16. Xu, W., Yu, X. K. Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)- bis(pyridine)copper(II), C54H38Cu2N2O8. Z. Kristallogr. NCS 2013, 228, 9–10; https://doi.org/10.1524/ncrs.2013.0006.Suche in Google Scholar
17. Karthik, K., Qadir, A. M. Synthesis and crystal structure of a new binuclear copper(II) carboxylate complex as precursor for copper(II) oxide nanoparticles. J. Struct. Chem. 2019, 60, 1126–1132; https://doi.org/10.1134/s002247661907014x.Suche in Google Scholar
18. Wang, G. M., Xue, Z. Z., Pan, J., Wei, L., Han, S. D., Qian, J. J., Wang, Z. H. Ligand-oriented assembly of a porous metal-organic framework by [Cu4II4] clusters and paddle-wheel [Cu2II(COO)4(H2O)2] subunits. CrystEngComm 2016, 18, 8362–8365; https://doi.org/10.1039/c6ce01954d.Suche in Google Scholar
19. Ohmura, T., Setoyama, N., Mukae, Y., Usuki, A., Senda, S., Matsumoto, T., Tatsumi, K. Supramolecular porphyrin-based metal-organic frameworks: Cu(II) naphthoate–Cu(II) tetrapyridyl porphine structures exhibiting selective CO2/N2 separation. CrystEngComm 2017, 19, 5173–5177; https://doi.org/10.1039/c7ce01138e.Suche in Google Scholar
© 2020 Li‐Qin Shi, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10

