Abstract
C26H21Fe2O6PS2, monoclinic, P21/c (no. 14), a = 9.0892(6) Å, b = 27.6631(18) Å, c = 11.3409(8) Å, β = 106.409(2)°, V = 2735.4(3) Å3, Z = 4, Rgt(F) = 0.0670, wRref(F2) = 0.1620, T = 296(2) K.
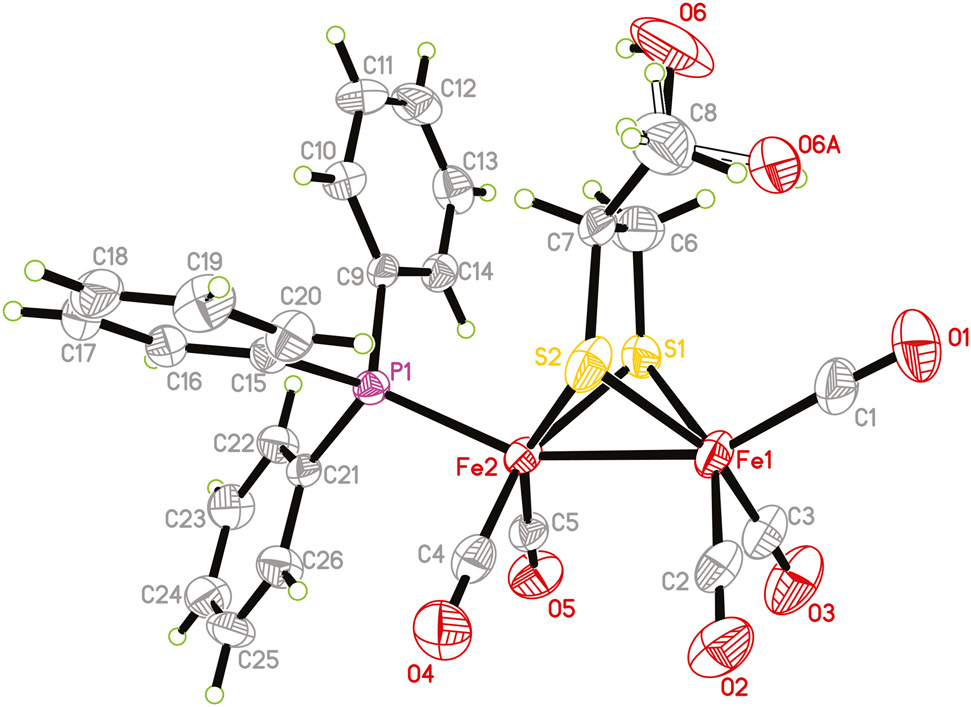
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.32 × 0.24 × 0.22 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.31 mm−1 |
| Diffractometer, scan mode: | Bruker D8 QUEST, φ and ω |
| θmax, completeness: | 25.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 63350, 4837, 0.090 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3418 |
| N(param)refined: | 344 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3], [, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Fe1 | 0.15445 (10) | 0.47736 (3) | 0.78454 (10) | 0.0539 (3) |
| Fe2 | 0.04267 (9) | 0.39648 (3) | 0.80761 (8) | 0.0408 (2) |
| S1 | 0.0102 (2) | 0.43417 (6) | 0.62538 (18) | 0.0630 (5) |
| S2 | −0.0601 (2) | 0.46758 (6) | 0.8423 (2) | 0.0666 (6) |
| P1 | −0.14211 (17) | 0.34028 (5) | 0.77659 (14) | 0.0377 (4) |
| O1 | 0.1284 (9) | 0.5793 (2) | 0.7129 (7) | 0.116 (2) |
| O2 | 0.3498 (7) | 0.4877 (3) | 1.0349 (6) | 0.095 (2) |
| O3 | 0.4231 (7) | 0.4501 (3) | 0.7076 (7) | 0.110 (2) |
| O4 | 0.1733 (7) | 0.3814 (2) | 1.0694 (5) | 0.0915 (19) |
| O5 | 0.2586 (6) | 0.3345 (2) | 0.7345 (5) | 0.0789 (16) |
| O6a | −0.3938 (18) | 0.5321 (5) | 0.5693 (15) | 0.177 (9) |
| H6a | −0.4199 | 0.5054 | 0.5395 | 0.265* |
| O6Ab | −0.190 (2) | 0.5637 (7) | 0.673 (3) | 0.181 (14) |
| H6Ab | −0.1504 | 0.5572 | 0.6184 | 0.271* |
| C1 | 0.1361 (9) | 0.5396 (3) | 0.7370 (8) | 0.076 (2) |
| C2 | 0.2699 (8) | 0.4846 (3) | 0.9364 (8) | 0.064 (2) |
| C3 | 0.3178 (9) | 0.4613 (3) | 0.7375 (8) | 0.071 (2) |
| C4 | 0.1203 (8) | 0.3869 (3) | 0.9665 (7) | 0.0583 (18) |
| C5 | 0.1747 (7) | 0.3581 (2) | 0.7667 (6) | 0.0493 (16) |
| C6 | −0.1707 (10) | 0.4643 (3) | 0.5850 (9) | 0.087 (3) |
| H6B | −0.1638 | 0.4937 | 0.5401 | 0.104* |
| H6C | −0.2476 | 0.4436 | 0.5319 | 0.104* |
| C7 | −0.2161 (8) | 0.4762 (3) | 0.6950 (10) | 0.083 (3) |
| H7 | −0.2984 | 0.4537 | 0.6975 | 0.099* |
| C8 | −0.2855 (14) | 0.5268 (4) | 0.6842 (11) | 0.114 (4) |
| H8AAa | −0.3343 | 0.5318 | 0.7490 | 0.137* |
| H8ABa | −0.2052 | 0.5508 | 0.6935 | 0.137* |
| H8BCb | −0.3752 | 0.5274 | 0.6134 | 0.137* |
| H8BDb | −0.3199 | 0.5328 | 0.7564 | 0.137* |
| C9 | −0.2793 (6) | 0.34205 (19) | 0.6247 (5) | 0.0384 (13) |
| C10 | −0.4319 (7) | 0.3516 (2) | 0.6067 (6) | 0.0518 (16) |
| H10 | −0.4697 | 0.3545 | 0.6745 | 0.062* |
| C11 | −0.5296 (8) | 0.3569 (3) | 0.4918 (7) | 0.069 (2) |
| H11 | −0.6325 | 0.3638 | 0.4822 | 0.082* |
| C12 | −0.4774 (10) | 0.3522 (3) | 0.3905 (7) | 0.072 (2) |
| H12 | −0.5449 | 0.3555 | 0.3123 | 0.087* |
| C13 | −0.3266 (9) | 0.3427 (2) | 0.4041 (6) | 0.0636 (19) |
| H13 | −0.2912 | 0.3394 | 0.3352 | 0.076* |
| C14 | −0.2258 (7) | 0.3379 (2) | 0.5203 (6) | 0.0486 (15) |
| H14 | −0.1225 | 0.3318 | 0.5294 | 0.058* |
| C15 | −0.2676 (7) | 0.3375 (2) | 0.8774 (5) | 0.0426 (14) |
| C16 | −0.3450 (7) | 0.2951 (3) | 0.8861 (6) | 0.0557 (17) |
| H16 | −0.3301 | 0.2679 | 0.8429 | 0.067* |
| C17 | −0.4441 (8) | 0.2930 (3) | 0.9585 (7) | 0.069 (2) |
| H17 | −0.4953 | 0.2643 | 0.9638 | 0.083* |
| C18 | −0.4675 (8) | 0.3328 (3) | 1.0225 (7) | 0.073 (2) |
| H18 | −0.5352 | 0.3313 | 1.0704 | 0.088* |
| C19 | −0.3904 (9) | 0.3747 (3) | 1.0156 (7) | 0.076 (2) |
| H19 | −0.4057 | 0.4018 | 1.0592 | 0.092* |
| C20 | −0.2896 (8) | 0.3769 (3) | 0.9438 (6) | 0.0584 (18) |
| H20 | −0.2365 | 0.4054 | 0.9408 | 0.070* |
| C21 | −0.0634 (6) | 0.2792 (2) | 0.7929 (5) | 0.0399 (14) |
| C22 | −0.1010 (8) | 0.2444 (2) | 0.7017 (6) | 0.0532 (17) |
| H22 | −0.1704 | 0.2518 | 0.6264 | 0.064* |
| C23 | −0.0365 (9) | 0.1987 (3) | 0.7211 (7) | 0.068 (2) |
| H23 | −0.0637 | 0.1757 | 0.6591 | 0.081* |
| C24 | 0.0663 (9) | 0.1872 (3) | 0.8303 (8) | 0.070 (2) |
| H24 | 0.1092 | 0.1565 | 0.8428 | 0.084* |
| C25 | 0.1060 (8) | 0.2210 (3) | 0.9210 (7) | 0.067 (2) |
| H25 | 0.1764 | 0.2133 | 0.9956 | 0.081* |
| C26 | 0.0422 (8) | 0.2668 (2) | 0.9028 (7) | 0.0588 (18) |
| H26 | 0.0708 | 0.2896 | 0.9655 | 0.071* |
aOccupancy: 0.589(16), bOccupancy: 0.411(16).
Source of material
A mixture of complex [Fe2(CO)6{μ-SCH2CH(CH2OH)}] (1 mmol) and triphenylphosphine (1 mmol) was treated with Me3NO⋅2H2O (1 mmol). The resulting solution was stirred at room temperature for 0.5 h. Afterwards, the solvent was reduced in vacuo and the residue was subjected to TLC separation using CH2Cl2: petroleum ether = 1:3 (v/v) as eluent. The title complex was obtained from the main red band. Slow evaporation of CH2Cl2/hexane solution at 4 °C afforded crystals suitable for X-ray diffraction analysis.
Experimental details
The structure was solved by direct method with the SHELXS program. Hydrogen atoms were positioned geometrically (C–H = 0.93–0.98 Å and O–H = 0.82 Å). Their Uiso values were set to 1.2Ueq or 1.5Ueq of the parent atoms. The hydroxy group features a disorder (see the figure).
Comment
Over the past decades, dithiolate-bridging diiron complexes have attracted great interest due to their structural resemblance with the active site of [FeFe]-hydrogenases [5], [6], [7]. [FeFe]-hydrogenases are natural metalloenzymes that can catalyze the reduction of protons to H2 [8], [, 9]. The X-ray crystallographic structures of the active site of [FeFe]-hydrogenases have promoted chemists to produce a great number of diiron analogues as mimics for the active site of [FeFe]-hydrogenases [10], [11], [12], [13]. In this study, we have successfully prepared a diiron analogue with a monophosphine ligand.
The asymmetric unit of the title complex consists of a butterfly diiron ethane-1,2-dithiolate dinuclear complex with five terminal carbonyls and a triphenylphosphine ligand, respectively. The phosphine ligand occupies an apical position of the distorted octahedral geometry of Fe2, in good agreement with monosubstituted analogues [14], [15], [16]. The Fe1–Fe2 bond length [2.5013(12) Å] is identical to that of the parent complex [Fe2(CO)6{μ-SCH2CH(CH2OH)}] [2.4998(6) Å] [17], but much shorter than those in diphosphine-substituted analogues [18], [19], [20] as well as in natural [FeFe]-hydrogenases [8], [, 9]. Note that the O6 atom is disordered over two sites with an occupancy of 0.589(16). The disorder suggests that it is unlikely that this OH⃛O hydrogen bond is present. Moreover, weak hydrogen bonds are also observed.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
5. Tard, C., Pickett, C. J. Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem. Rev. 2009, 109, 2245–2274; https://doi.org/10.1021/cr800542q.Search in Google Scholar
6. Li, Y., Rauchfuss, T. B. Synthesis of diiron(I) dithiolato carbonyl complexes. Chem. Rev. 2016, 116, 7043–7077; https://doi.org/10.1021/acs.chemrev.5b00669.Search in Google Scholar
7. Rauchfuss, T. B. Diiron azadithiolates as models for the [FeFe]- hydrogenase active site and paradigm for the role of the second coordination sphere. Acc. Chem. Res. 2015, 48, 2107–2116; https://doi.org/10.1021/acs.accounts.5b00177.Search in Google Scholar
8. Peters, J. W., Lanzilotta, W. N., Lemon, B. J., Seefeldt, L. C. X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 1998, 282, 1853–1857; https://doi.org/10.1126/science.282.5395.1853.Search in Google Scholar
9. Nicolet, Y., Piras, C., Legrand, P., Hatchikian, C. E., Fontecilla- Camps, J. C. Desulfovibrio Desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 1999, 7, 13–23; https://doi.org/10.1016/s0969-2126(99)80005-7.Search in Google Scholar
10. Ghosh, S., Hogarth, G., Hollingsworth, N., Holt, K. B., Richard, I., Richmond, M. G., Sanchez, B. E., Unwin, D. Models of the iron-only hydrogenase: a comparison of chelate and bridge isomers of Fe2(CO)4{Ph2PN(R)PPh2}(μ-pdt) as proton-reduction catalysts. Dalton. Trans. 2013, 42, 6775–6792; https://doi.org/10.1039/c3dt50147g.Search in Google Scholar
11. Gloaguen, F., Lawrence, J. D., Rauchfuss, T. B. Biomimetic hydrogen evolution catalyzed by an iron carbonyl thiolate. J. Am. Chem. Soc. 2001, 123, 9476–9477; https://doi.org/10.1021/ja016516f.Search in Google Scholar
12. Lyon, E. J., Georgakaki, I. P., Reibenspies, J. H., Darensbourg, M. Y. Coordination sphere flexibility of active-site models for Fe-only hydrogenase: studies in intra- and intermolecular diatomic ligand exchange. J. Am. Chem. Soc. 2001, 123, 3268–3278; https://doi.org/10.1021/ja003147z.Search in Google Scholar
13. Zhao, P. H., Ma, Z. Y., Hu, M. Y., He, J., Wang, Y. Z., Jing, X. B., Chen, H. Y., Li, Y. L. PNP-chelated and -bridged diiron dithiolate complexes Fe2(μ-pdt)(CO)4{(Ph2P)2NR} together with related monophosphine complexes for the [2Fe]H subsite of [FeFe]-hydrogenases: preparation, structure, and electrocatalysis. Organometallics 2018, 37, 1280–1290; https://doi.org/10.1021/acs.organomet.8b00030.Search in Google Scholar
14. Hu, M. Y., Zhao, P. H., Li, J. R., Gu, X. L., Jing, X. B., Liu, X. F. Synthesis, structures, and electrocatalytic properties of phosphine- monodentate, -chelate, and -bridge diiron 2,2-dimethylpropanedithiolate complexes related to [FeFe]-hydrogenases. Appl. Organomet. Chem. 2020, 34, e5523; https://doi.org/10.1002/aoc.5523.Search in Google Scholar
15. Yan, L., Hu, K., Liu, X. F., Li, Y. L., Liu, X. H., Jiang, Z. Q. Diiron ethane-1,2-dithiolate complexes with 1,2,3-thiadiazole moiety: synthesis, X-ray crystal structures, electrochemistry, and fungicidal activity. Appl. Organomet. Chem. 2021, 35, e6084.10.1002/aoc.6084Search in Google Scholar
16. Lü, S., Huang, H. L., Zhang, R. F., Ma, C. L., Li, Q. L., He, J., Yang, J., Li, T., Li, Y. L. Phosphine-substituted Fe-Te clusters related to the active site of [FeFe]-H2ases. Inorg. Chem. Front. 2020, 7, 2352–2361; https://doi.org/10.1039/d0qi00276c.Search in Google Scholar
17. Niu, S. J., Yu, X. Y., Liu, X. F., Li, Y. L. Tris(2-methoxyphenyl)phosphine substituted diiron ethanedithiolate complexes containing hydroxymethyl, methyl or ethyl groups. Polyhedron 2017, 137, 127–133; https://doi.org/10.1016/j.poly.2017.08.041.Search in Google Scholar
18. Zhao, P. H., Hu, M. Y., Ma, Z. Y., Li, J. R., Wang, Y. Z., He, J., Li, Y. L., Liu, X. F. Influence of dithiolate bridges on the structures and electrocatalytic performance of small bite-angle PNP-chelated diiron complexes Fe2(μ-xdt)(CO)4{k2-(Ph2P)2NR} related to [FeFe]- hydrogenases. Organometallics 2019, 38, 385–394; https://doi.org/10.1021/acs.organomet.8b00759.Search in Google Scholar
19. Hu, M. Y., Yan, L., Li, J. R., Wang, Y. H., Zhao, P. H., Liu, X. F. Reactions of Fe2(μ-odt)(CO)6 (odt = 1, 3-oxadithiolate) with small bite-angle diphosphines to afford the monodentate, chelate, and bridge diiron complexes: selective substitution, structures, protonation, and electrocatalytic proton reduction. Appl. Organomet. Chem. 2019, 33, e4949; https://doi.org/10.1002/aoc.4949.Search in Google Scholar
20. Zhao, P. H., Hu, M. Y., Li, J. R., Wang, Y. Z., Lu, B. P., Han, H. F., Liu, X. F. Impacts of coordination modes (chelate versus bridge) of PNP- diphosphine ligands on the redox and electrocatalytic properties of diiron oxadithiolate complexes for proton reduction. Electrochim. Acta 2020, 353, 136615; https://doi.org/10.1016/j.electacta.2020.136615.Search in Google Scholar
© 2021 Wei Gao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10

