Abstract
C26H18F2N2O3S, monoclinic, P21/c (no. 14), a = 8.412(3) Å, b = 22.056(8) Å, c = 12.125(4) Å, β = 102.316(5)°, V = 2198.0(14) Å3, Z = 4, Rgt(F) = 0.0475, wRref(F2) = 0.1156, T = 173(2) K.
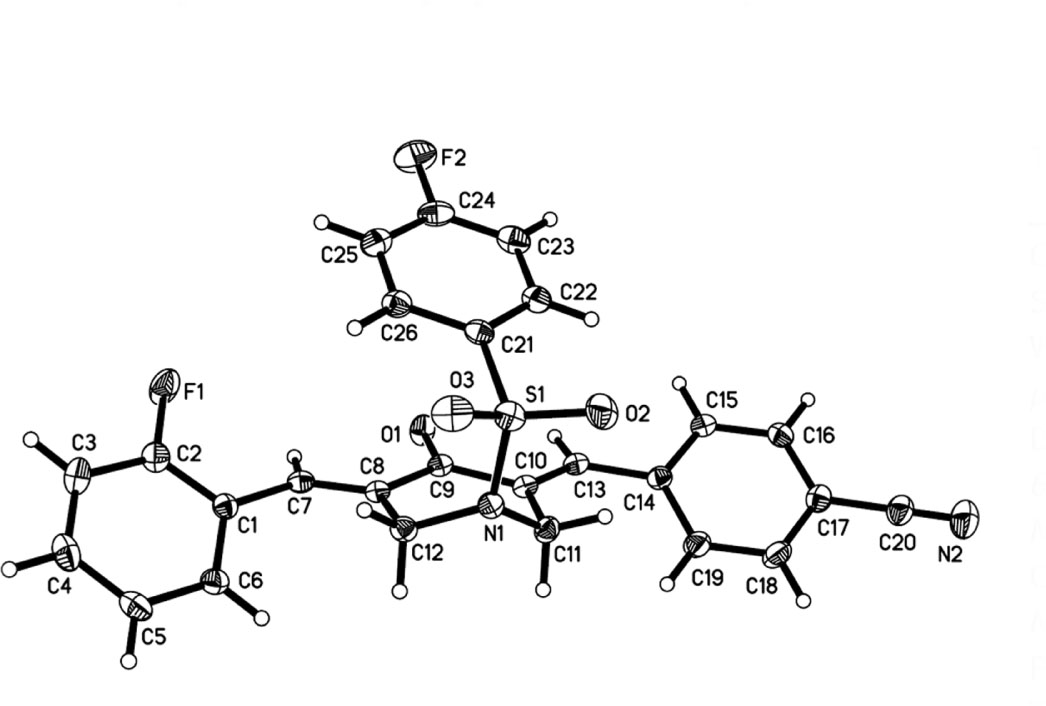
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.26 × 0.25 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.20 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11378, 4095, 0.034 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3110 |
| N(param)refined: | 307 |
| Programs: | Bruker programs [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| F1 | 0.39138(19) | 0.69104(6) | 0.33479(12) | 0.0567(4) |
| F2 | 0.6284(2) | 0.67176(7) | −0.07671(13) | 0.0642(5) |
| N1 | 0.9098(2) | 0.49932(8) | 0.36203(14) | 0.0287(4) |
| N2 | 1.0896(3) | 0.18516(11) | −0.0654(2) | 0.0623(7) |
| O1 | 0.47692(18) | 0.51400(7) | 0.14691(13) | 0.0376(4) |
| O2 | 1.13701(19) | 0.50701(8) | 0.26644(15) | 0.0496(5) |
| O3 | 1.0843(2) | 0.58965(8) | 0.39257(14) | 0.0477(5) |
| S1 | 1.02532(7) | 0.54487(3) | 0.30837(5) | 0.03483(17) |
| C1 | 0.5109(2) | 0.61043(9) | 0.45075(18) | 0.0282(5) |
| C2 | 0.4474(3) | 0.66868(10) | 0.4410(2) | 0.0370(6) |
| C3 | 0.4388(3) | 0.70506(12) | 0.5313(2) | 0.0455(6) |
| H3 | 0.3953 | 0.7449 | 0.5203 | 0.055* |
| C4 | 0.4952(3) | 0.68229(12) | 0.6391(2) | 0.0464(7) |
| H4 | 0.4896 | 0.7065 | 0.7029 | 0.056* |
| C5 | 0.5594(3) | 0.62475(11) | 0.6538(2) | 0.0417(6) |
| H5 | 0.5979 | 0.6093 | 0.7278 | 0.050* |
| C6 | 0.5677(3) | 0.58948(10) | 0.56101(18) | 0.0341(5) |
| H6 | 0.6133 | 0.5500 | 0.5724 | 0.041* |
| C7 | 0.5110(3) | 0.57439(9) | 0.34961(17) | 0.0279(5) |
| H7 | 0.4190 | 0.5790 | 0.2893 | 0.033* |
| C8 | 0.6254(2) | 0.53571(9) | 0.33204(17) | 0.0250(5) |
| C9 | 0.5941(2) | 0.50182(9) | 0.22258(18) | 0.0270(5) |
| C10 | 0.7093(2) | 0.45183(9) | 0.20863(17) | 0.0273(5) |
| C11 | 0.8562(3) | 0.44224(9) | 0.30376(18) | 0.0320(5) |
| H11A | 0.8286 | 0.4128 | 0.3585 | 0.038* |
| H11B | 0.9463 | 0.4250 | 0.2729 | 0.038* |
| C12 | 0.7842(2) | 0.52497(10) | 0.41614(17) | 0.0301(5) |
| H12A | 0.8236 | 0.5639 | 0.4528 | 0.036* |
| H12B | 0.7648 | 0.4969 | 0.4756 | 0.036* |
| C13 | 0.6782(3) | 0.42130(9) | 0.11060(18) | 0.0290(5) |
| H13 | 0.5859 | 0.4345 | 0.0564 | 0.035* |
| C14 | 0.7683(3) | 0.37020(9) | 0.07624(18) | 0.0295(5) |
| C15 | 0.7667(3) | 0.36228(9) | −0.03809(18) | 0.0301(5) |
| H15 | 0.7067 | 0.3897 | −0.0916 | 0.036* |
| C16 | 0.8503(3) | 0.31550(10) | −0.07497(19) | 0.0331(5) |
| H16 | 0.8481 | 0.3109 | −0.1532 | 0.040* |
| C17 | 0.9383(3) | 0.27491(10) | 0.00278(19) | 0.0348(5) |
| C18 | 0.9386(3) | 0.28133(10) | 0.1174(2) | 0.0425(6) |
| H18 | 0.9972 | 0.2534 | 0.1706 | 0.051* |
| C19 | 0.8538(3) | 0.32810(10) | 0.1533(2) | 0.0393(6) |
| H19 | 0.8533 | 0.3319 | 0.2313 | 0.047* |
| C20 | 1.0241(3) | 0.22505(11) | −0.0344(2) | 0.0428(6) |
| C21 | 0.9050(3) | 0.58326(9) | 0.19160(18) | 0.0301(5) |
| C22 | 0.8874(3) | 0.55904(10) | 0.08393(19) | 0.0367(5) |
| H22 | 0.9407 | 0.5222 | 0.0731 | 0.044* |
| C23 | 0.7924(3) | 0.58846(11) | −0.0074(2) | 0.0418(6) |
| H23 | 0.7786 | 0.5724 | −0.0816 | 0.050* |
| C24 | 0.7188(3) | 0.64138(11) | 0.0126(2) | 0.0427(6) |
| C25 | 0.7324(3) | 0.66633(11) | 0.1182(2) | 0.0422(6) |
| H25 | 0.6783 | 0.7031 | 0.1282 | 0.051* |
| C26 | 0.8265(3) | 0.63652(10) | 0.20921(19) | 0.0348(5) |
| H26 | 0.8374 | 0.6524 | 0.2833 | 0.042* |
Source of material
N-Methyl-4-piperidone (1.14 g, 0.01 mol), 2-fluorobenzaldehyde (1.24 mol, 0.01 mol) and 4-cyanobenzaldehyde (1.31 g, 0.01 mol) were dissolved in 15 mL of acetic acid. Dry HCl gas was passed through this mixture for 25 min. After stirring at room temperature for about 24 h, the mixture was added into 100 mL water, and then aqueous NaOH solution was added until the pH was adjusted to about 7. The mixture was filtered and subsequently washed by water to provide a yellow precipitate. The precipitates were purified on silica gel by column using methanol/petroleum ether/EtOAc (10:10:1, v/v/v) as the eluent to afford a yellow intermediate. Then, the yellow intermediate and 4-fluorophenylsulfonylfluoride (1.78 g, 0.01 mol) were dissolved in 50 mL of dichloromethane. Potassium carbonate (2.76 g, 0.02 mol) was added to the mixture and the mixture were stirred for about 12 h at room temperature. The precipitate was collected, washed with water and recrystallized from dichloromethane/methanol (1:1, v/v) to get light yellow crystals of title compound.
Experimental details
All H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C—H) = 0.99 Å (methylene), Uiso(H) = 1.2Ueq(C) and d(C—H) = 0.95 Å (aromatic), Uiso(H) = 1.2Ueq(C).
Discussion
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)1,6-hepta-dien-3,5-dion) has proved to be a powerful chemopreventive and anticancer agent, including anti-inflammatory, antibacterial, and antioxidant properties. However, the clinical use of curcumin has been limited because of its low anticancer activity and poor bioavailability [4]. In order to improve these defects, a novel class of curcumin analogues, (3E,5E)-3,5-bis(arylidene)-4-piperidone derivatives, has been reported as better antitumor agents [5], [6], [7], [8]. Its pharmacophore is 1,5-diaryl-3-oxo-1,4-pentadienyl, which contains a α,β-unsaturated keto group and has a greater preference or sequential affinity for bio-thiols in contrast to amino and hydroxy groups resulting in a greater chemosensitivity to tumors rather than with normal cells. Our interests lie in incorporation of different substituent groups on both sides of (3E,5E)-3,5-bis(arylidene)-4-piperidone. In addition, N-benzenesulfonyl substituents should improve antitumor activities and anti-inflammatory activity [9], [10], [11], [12], [13]. In this study, we report herein the crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile.
There is one molecule in the asymmetric unit of the title crystal structure (cf. the figure). Bond lengths and angles are all in the expected ranges. Derived from the crystal structure deterimination we learned that the C1—C7—C8—C9 torsion angle value is 177.29(3)° and the C9—C10—C13—C14 torsion angle value is 178.64(2)°. The 2-fluorophenyl group and the 4-cyano phenyl group on both sides of 3,5-bis(arylidene)-4-piperidone adopt the E stereochemistry of olefinic double bonds and the E, E isomer [14]. The 4-fluorophenyl group is almost coplanar with two substituated aryl rings on both sides of 3,5-bis(arylidene)-4-piperidone, which can be proved by the dihedral angles (12.207(4)° and 13.925(5)°, respectively). On the whole, the title molecule group looks like an “organic clip” [15]. The heteroatoms (F, N, O, S) can act as hydrogen bonding acceptors for biological macromolecules with the aim of creating more potent cytostatica [16].
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21402010).
References
Bruker.: APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Nelson, K. M.; Dahlin, J. L.; Bisson, J.; Graham, J.; Pauli, G. F.; Walters. M. A.: The essential medicinal chemistry of curcumin. J. Med. Chem. 60 (2017) 1620–1637.10.1021/acs.jmedchem.6b00975Search in Google Scholar PubMed PubMed Central
Sun, J. F.; Zhang, S. P.; Yu, C.; Hou, G. G.; Zhang, X. F.; Li, K. K.; Zhao, F.: Design, Synthesis and bioevaluation of novel N-substituted-3,5-bis(arylidene)-4-piperidone derivatives as cytotoxic and antitumor agents with fluorescent properties. Chem. Biol. Drug. Des. 83 (2014) 392–400.10.1111/cbdd.12254Search in Google Scholar PubMed
Li, N.; Xin, W. Y.; Yao, B. R.; Wang, C. H.; Cong, W.; Zhao, F.; Li, H. J.; Hou, Y.; Meng, Q. G.; Hou, G. G.: Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur. J. Med. Chem. 147 (2018) 21–33.10.1016/j.ejmech.2018.01.088Search in Google Scholar PubMed
Zhu, H. P.; Xu, T. T.; Qiu, C. Y.; Wu, B. B.; Zhang, Y. L.; Chen, L. F.; Xia, Q. Q.; Li, C. L.; Zhou, B.; Liu, Z. G.; Liang, G.: Synthesis and optimization of novel allylated mono-carbonyl analogs of curcumin (MACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) in rats. Eur. J. Med. Chem. 121 (2016) 181–193.10.1016/j.ejmech.2016.05.041Search in Google Scholar PubMed
Yin, D. L.; Liang, Y. J.; Zheng, T. S.; Song, R. P.; Wang, J. B.; Sun, B. S.; Pan, S. H.; Qu, L. D.; Liu, J. R.; Jiang, H. C.; Liu, L. X.: EF24 inhibits tumor growth and metastasis via suppressing NF-kB dependent pathways in human cholangiocarcinoma. Sci. Rep. 6 (2016) 32167.10.1038/srep32167Search in Google Scholar PubMed PubMed Central
Bano, S.; Javed, K.; Ahmad, S.; Rathish, I. G.; Singh, S.; Alam, M. S.: Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur J. Med. Chem. 46 (2011) 5763–5768.10.1016/j.ejmech.2011.08.015Search in Google Scholar PubMed
Bashir, R.; Ovais, S.; Yaseen, S.; Hamid, H.; Alam, M. S.; Samim, M.; Singh, S.; Javed, K.: Synthesis of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and antiinflammatory agents. Bioorg. Med. Chem. Lett. 21 (2011) 4301–4305.10.1016/j.bmcl.2011.05.061Search in Google Scholar PubMed
Antoniou, V.; Tsoukali-Papadopoulou, H.; Epivatianos, P.; Nathanael, B.: Synthesis and pharmacological evaluation of novel 5-substituted-1-(phenylsulfonyl)-2-methylbenzimidazole derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 45 (2010) 2245–2249.10.1016/j.ejmech.2010.01.067Search in Google Scholar PubMed
Park, E. B.; Kim, K. J.; Hui, R. J.; Lee, J. K.; Kim, H. J.; Lee, H. H.; Ji, W. L.; Shin, J. S.; Koeberle, A.; Werz, O.: Synthesis, structure determination, and biological evaluation of phenylsulfonyl hydrazide derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. Lett. 26 (2016) 5193–5197.10.1016/j.bmcl.2016.09.070Search in Google Scholar PubMed
Pinz, M. P.; Reis, A. S.; Oliveira, R. L. D.; Voss, G. T.; Vogt, A. G.; Sacramento, M. D.; Roehrs, J. A.; Alves, D.; Luchese, C.; Wilhelm, E. A.: 7-Chloro-4-phenylsulfonyl quinoline, a new antinociceptive and anti-inflammatory molecule: Structural improvement of a quinoline derivate with pharmacological activity. Regul. Toxicol. Pharm. 90 (2017) 72–77.10.1016/j.yrtph.2017.08.014Search in Google Scholar PubMed
Sun, J. F.; Wang, S. W.; Li, H. J.; Jiang, W. G.; Hou, G. G.; Zhao, F.; Cong, W.: Synthesis, antitumor activity evaluation of some new N-aroyl-α,β-unsaturated piperidones with fluorescence. J. Enzyme Inhib. Med. Chem. 31 (2016) 495–502.10.3109/14756366.2015.1043296Search in Google Scholar PubMed
Hou, G. G.; Zhao, H. J.; Sun, J. F.; Lin, D.; Dai, X. P.; Han, J. T.; Zhao, H.: Synthesis, structure and luminescence of Co-crystals with hexagonal channels: arranging disposition and π-π interactions. CrystEngComm 15 (2012) 577–585.10.1039/C2CE25759ASearch in Google Scholar
Das, U.; Sakagami, H.; Chu, Q.; Wang, Q.; Kawase, M.; Selvakumar, P.; Sharma, R. K.; Dimmock, J. R.: 3,5-Bis(benzylidene)-1-(4-2-(morpholin-4-yl) ethoxyphenylcarbonyl)-4-piperidone hydrochloride: a lead tumor-specific cytotoxin which induces apoptosis and autophagy. Bioorg. Med. Chem. Lett. 20 (2010) 912–917.10.1016/j.bmcl.2009.12.076Search in Google Scholar PubMed PubMed Central
©2018 Lian-Dong Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S


