Abstract
C24H41NO, orthorhombic, P212121 (no. 19), a = 6.3752(16) Å, b = 12.607(3) Å, c = 26.943(7) Å, V = 2165.4(10) Å3, Z = 4, Rgt(F) = 0.0573, wRref(F2) = 0.1232, T = 173 K.
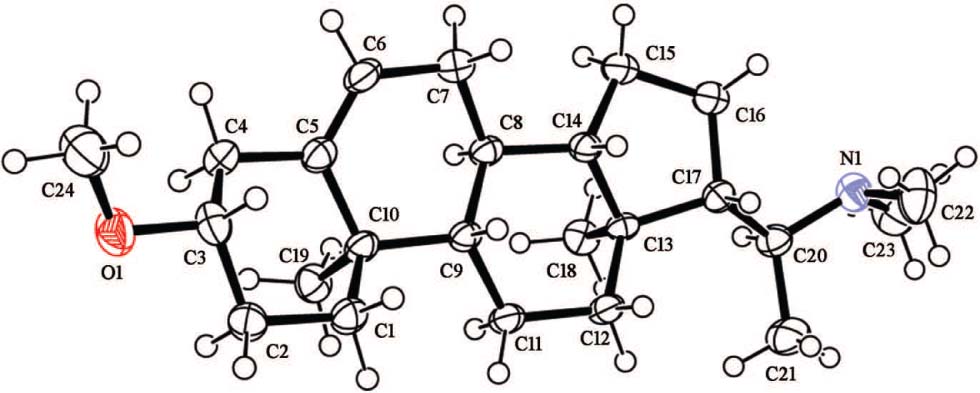
The crystal structure of the title compound (systematic name: 1-((3S,9S,10R,13S,14S)-3-methoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-N,N-dimethylethan-1-amine) is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Crystal collection and handling.
| Crystal: | Block, colorless |
| Size: | 0.28 × 0.15 × 0.12 mm |
| Wavelength: | Mo Kα radiation (λ = 0.71073 Å) |
| μ: | 0.065 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, Φ and ω-scans |
| θmax, completeness: | 27.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12457, 4735, 0.0721 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3072 |
| N(param)refined: | 242 |
| Programs: | Bruker programs [1], OLEX2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.0452(4) | 0.77064(18) | 0.28539(9) | 0.0418(7) |
| N1 | 0.7851(4) | −0.0559(2) | 0.47607(10) | 0.0337(7) |
| C1 | 0.1107(5) | 0.4805(2) | 0.31164(12) | 0.0298(8) |
| H1A | 0.0594 | 0.4181 | 0.2929 | 0.036 |
| H1B | 0.0447 | 0.4787 | 0.3449 | 0.036 |
| C2 | 0.0382(5) | 0.5811(2) | 0.28480(12) | 0.0319(8) |
| H2A | 0.0906 | 0.5805 | 0.2502 | 0.038 |
| H2B | −0.1170 | 0.5831 | 0.2838 | 0.038 |
| C3 | 0.1198(5) | 0.6787(3) | 0.31136(12) | 0.0323(8) |
| H3 | 0.0628 | 0.6796 | 0.3459 | 0.039 |
| C4 | 0.3575(5) | 0.6743(2) | 0.31374(12) | 0.0330(8) |
| H4A | 0.4152 | 0.6769 | 0.2796 | 0.040 |
| H4B | 0.4102 | 0.7371 | 0.3320 | 0.040 |
| C5 | 0.4329(5) | 0.5747(2) | 0.33921(11) | 0.0275(8) |
| C6 | 0.5569(6) | 0.5800(3) | 0.37860(12) | 0.0318(8) |
| H6 | 0.5959 | 0.6486 | 0.3899 | 0.038 |
| C7 | 0.6405(6) | 0.4869(2) | 0.40664(12) | 0.0324(8) |
| H7A | 0.5660 | 0.4813 | 0.4388 | 0.039 |
| H7B | 0.7911 | 0.4986 | 0.4138 | 0.039 |
| C8 | 0.6154(5) | 0.3824(2) | 0.37817(11) | 0.0246(7) |
| H8 | 0.7237 | 0.3789 | 0.3514 | 0.030 |
| C9 | 0.3953(5) | 0.3769(2) | 0.35448(11) | 0.0232(7) |
| H9 | 0.2928 | 0.3850 | 0.3823 | 0.028 |
| C10 | 0.3517(5) | 0.4702(2) | 0.31808(11) | 0.0257(7) |
| C11 | 0.3554(5) | 0.2666(2) | 0.33200(12) | 0.0280(8) |
| H11A | 0.2081 | 0.2631 | 0.3205 | 0.034 |
| H11B | 0.4467 | 0.2578 | 0.3026 | 0.034 |
| C12 | 0.3956(5) | 0.1740(2) | 0.36785(12) | 0.0277(8) |
| H12A | 0.2924 | 0.1768 | 0.3953 | 0.033 |
| H12B | 0.3757 | 0.1060 | 0.3500 | 0.033 |
| C13 | 0.6177(5) | 0.1780(2) | 0.38937(11) | 0.0217(7) |
| C14 | 0.6440(5) | 0.2883(2) | 0.41290(11) | 0.0237(7) |
| H14 | 0.5287 | 0.2943 | 0.4379 | 0.028 |
| C15 | 0.8459(6) | 0.2803(3) | 0.44299(12) | 0.0346(8) |
| H15A | 0.8460 | 0.3318 | 0.4707 | 0.042 |
| H15B | 0.9702 | 0.2933 | 0.4218 | 0.042 |
| C16 | 0.8438(6) | 0.1654(3) | 0.46253(13) | 0.0358(9) |
| H16A | 0.8206 | 0.1646 | 0.4989 | 0.043 |
| H16B | 0.9792 | 0.1301 | 0.4554 | 0.043 |
| C17 | 0.6628(5) | 0.1075(2) | 0.43561(11) | 0.0246(7) |
| H17 | 0.5368 | 0.1120 | 0.4576 | 0.029 |
| C18 | 0.7811(5) | 0.1557(3) | 0.34859(12) | 0.0309(8) |
| H18A | 0.7730 | 0.2112 | 0.3232 | 0.046 |
| H18B | 0.7525 | 0.0866 | 0.3334 | 0.046 |
| H18C | 0.9218 | 0.1553 | 0.3632 | 0.046 |
| C19 | 0.4556(5) | 0.4500(3) | 0.26713(11) | 0.0324(8) |
| H19A | 0.6026 | 0.4298 | 0.2720 | 0.049 |
| H19B | 0.4487 | 0.5147 | 0.2471 | 0.049 |
| H19C | 0.3813 | 0.3926 | 0.2500 | 0.049 |
| C20 | 0.7118(5) | −0.0103(3) | 0.42859(12) | 0.0298(8) |
| H20 | 0.8328 | −0.0144 | 0.4050 | 0.036 |
| C21 | 0.5321(6) | −0.0718(3) | 0.40469(15) | 0.0493(11) |
| H21A | 0.5636 | −0.1479 | 0.4055 | 0.074 |
| H21B | 0.5152 | −0.0489 | 0.3702 | 0.074 |
| H21C | 0.4021 | −0.0582 | 0.4230 | 0.074 |
| C22 | 0.6242(6) | −0.0649(3) | 0.51411(14) | 0.0523(11) |
| H22A | 0.5575 | 0.0043 | 0.5191 | 0.079 |
| H22B | 0.6883 | −0.0885 | 0.5453 | 0.079 |
| H22C | 0.5184 | −0.1166 | 0.5036 | 0.079 |
| C23 | 0.8974(6) | −0.1556(3) | 0.47009(16) | 0.0506(11) |
| H23A | 0.8001 | −0.2105 | 0.4586 | 0.076 |
| H23B | 0.9581 | −0.1768 | 0.5020 | 0.076 |
| H23C | 1.0098 | −0.1465 | 0.4456 | 0.076 |
| C24 | 0.0485(8) | 0.8629(3) | 0.31472(15) | 0.0541(12) |
| H24A | −0.0087 | 0.9225 | 0.2956 | 0.081 |
| H24B | 0.1932 | 0.8788 | 0.3244 | 0.081 |
| H24C | −0.0369 | 0.8518 | 0.3445 | 0.081 |
Source of material
The stems and roots of Sarcococca hookeriana (14.5 kg) were extracted three times using MeOH with ultrasonic treatment. The extract was concentrated and partitioned between EtOAc and 1% aq. H2SO4. The acid-soluble fraction was alkalinized with aq. Na2CO3 to pH 9 and followed by exhaustive extraction with CH2Cl2 to afford crude alkaloids. The crude alkaloids were roughly separated by CC (SiO2; CH2Cl2/MeOH/Et2NH, 100:0:0–10:1:0–5:1:1) to give five fractions: Frs. A—E. The title compound Pachyaximine A was obtained from Fr. A by silica gel column chromatography using petroleum ether/ethylacetate/diethylamine (50:1:1) as the eluent and recrystallization with dichloromethane.
Experimental details
All hydrogen atoms were positioned geometrically, with the d(C—H) = 0.95–1.00 Å. The Uiso(H) were set to 1.2 times Ueq(C) for the methylene groups as well as methyl groups, at 1.5 times Ueq(C) for the methyl groups.
Discussion
Previous studies have shown that S. hookeriana contains pregnane-type steroidal alkaloids [5]. These compounds have cholinesterase inhibition [6], antileishmanial [7], antibacterial [8] and estrogen biosynthesis-promoting [9] activities.
The title compound crystallizes with one molecule in the asymmetric unit of the space group P212121 (cf. the figure). Geometric parameters of the title structure are in the usual ranges. The compound contains one methoxy, one double bond and one dimethylamino group. The double bond were confirmed by the distance of 1.325(4) Å (C5—C6). The dimethylamino group was confirmed by the distance of 1.455(4) Å, 1.454(4) Å (C22—N1 and C23—N1), respectively. The compound contains three six membered rings and one five membered ring. The intermolecular connection was achieved by van der Waals interactions only.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 30960529
Funding statement: The authors gratefully acknowledge support from The National Natural Science Foundation of China [No. 30960529] and the Science and Technology Project of Guizhou Province [No. 2016–1015].
References
Bruker. SAINT-Plus, SHELXTL and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA (2013).Suche in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
Sheldrick, G. M.: SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Suche in Google Scholar PubMed PubMed Central
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
Kumar, A.; Sati, S. C.; Sati, M. D.; Kumar, S.; Singh, D.; Bhatt, U.; Kaur, G.: Chemical and potential biological perspectives of genus Sarcococca (Buxaceae). Nat. Prod. J. 5 (2015) 28–49.10.2174/2210315505666150219233014Suche in Google Scholar
Khan, M. T. H.: Putative molecular interactions involving naturally occurring steroidal alkaloids from Sarcococca hookeriana against acetyl- and butyryl- cholinesterase. Curr. Bioinform. 8 (2013) 416–428.10.2174/1574893611308040004Suche in Google Scholar
Devkota, K. P.; Choudhary, M. I.; Ranjit, R.; Samreen; Sewald, N.: Structure activity relationship studies on antileishmanial steroidal alkaloids from Sarcococca hookeriana. Nat. Prod. Res. 21 (2007) 292–297.10.1080/14786410701192736Suche in Google Scholar PubMed
Devkota, K. P.; Wansi, J. D.; Lenta, B. N.; Khan, S.; Choudhary, M. I.; Sewald, N.: Bioactive steroidal alkaloids from Sarcococca hookeriana. Planta Med. 76 (2010) 1022–1025.10.1055/s-0029-1240896Suche in Google Scholar PubMed
Zhang, P. Z.; Wang, F.; Yang, L. J.; Zhang, G. L.: Pregnane alkaloids from Sarcococca hookeriana var. digyna. Fitoterapia 89 (2013) 143–148.10.1016/j.fitote.2013.04.010Suche in Google Scholar PubMed
©2018 Shao-Jie Huo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S


