Abstract
C66H44N6, triclinic, P1̄ (no. 2), a = 9.773(2) Å, b = 14.406(3) Å, c = 18.385(4) Å, α = 72.64(3)°, β = 82.10(3)°, γ = 76.90(3)°, V = 2399.5(8) Å3, Z = 2, Rgt(F) = 0.0546, wRref(F2) = 0.1559, T = 153(2) K.
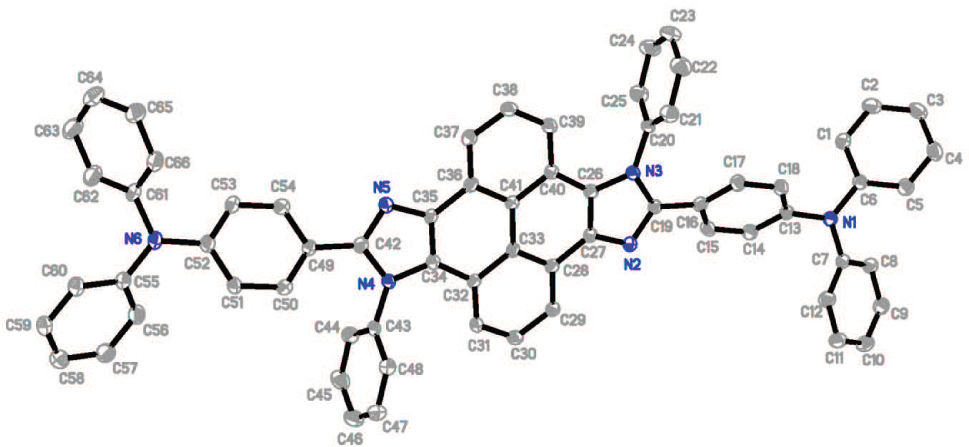
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.19 × 0.17 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Rigaku RAXIS-RAPID, ω-scans |
| θmax, completeness: | 25°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 18890, 8388, 0.073 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4143 |
| N(param)refined: | 649 |
| Programs: | CrystalClear [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | −0.3835(2) | 0.88995(19) | 0.58798(16) | 0.0384(7) |
| N2 | 0.0424(2) | 0.69369(18) | 0.35504(15) | 0.0315(6) |
| N3 | −0.0845(2) | 0.57386(17) | 0.38299(15) | 0.0304(6) |
| N4 | 0.5826(2) | 0.41811(17) | 0.12294(15) | 0.0299(6) |
| N5 | 0.4438(2) | 0.30767(18) | 0.14101(15) | 0.0334(7) |
| N6 | 0.8930(3) | 0.11087(19) | −0.08717(16) | 0.0409(7) |
| C1 | −0.6125(3) | 0.8471(2) | 0.5936(2) | 0.0408(9) |
| H1 | −0.5932 | 0.8429 | 0.5425 | 0.049* |
| C2 | −0.7394(4) | 0.8307(3) | 0.6327(2) | 0.0590(11) |
| H2 | −0.8062 | 0.8132 | 0.6087 | 0.071* |
| C3 | −0.7711(4) | 0.8392(3) | 0.7068(2) | 0.0607(12) |
| H3 | −0.8603 | 0.8306 | 0.7326 | 0.073* |
| C4 | −0.6717(4) | 0.8602(3) | 0.7418(2) | 0.0501(10) |
| H4 | −0.6914 | 0.8648 | 0.7928 | 0.060* |
| C5 | −0.5423(3) | 0.8749(2) | 0.7036(2) | 0.0417(8) |
| H5 | −0.4737 | 0.8885 | 0.7288 | 0.050* |
| C6 | −0.5128(3) | 0.8698(2) | 0.62919(19) | 0.0323(8) |
| C7 | −0.3199(3) | 0.9646(2) | 0.59748(19) | 0.0341(8) |
| C8 | −0.4014(3) | 1.0502(2) | 0.61258(19) | 0.0380(8) |
| H8 | −0.5012 | 1.0588 | 0.6178 | 0.046* |
| C9 | −0.3372(3) | 1.1229(2) | 0.6200(2) | 0.0436(9) |
| H9 | −0.3939 | 1.1809 | 0.6308 | 0.052* |
| C10 | −0.1921(4) | 1.1128(3) | 0.6120(2) | 0.0483(10) |
| H10 | −0.1490 | 1.1635 | 0.6165 | 0.058* |
| C11 | −0.1114(3) | 1.0276(3) | 0.5975(2) | 0.0478(10) |
| H11 | −0.0116 | 1.0195 | 0.5917 | 0.057* |
| C12 | −0.1744(3) | 0.9537(2) | 0.5913(2) | 0.0419(9) |
| H12 | −0.1172 | 0.8947 | 0.5827 | 0.050* |
| C13 | −0.3149(3) | 0.8327(2) | 0.53696(19) | 0.0335(8) |
| C14 | −0.2555(3) | 0.8786(2) | 0.46545(19) | 0.0359(8) |
| H14 | −0.2655 | 0.9487 | 0.4492 | 0.043* |
| C15 | −0.1827(3) | 0.8226(2) | 0.41845(19) | 0.0355(8) |
| H15 | −0.1413 | 0.8547 | 0.3703 | 0.043* |
| C16 | −0.1683(3) | 0.7192(2) | 0.44014(19) | 0.0313(7) |
| C17 | −0.2317(3) | 0.6751(2) | 0.51075(19) | 0.0354(8) |
| H17 | −0.2259 | 0.6053 | 0.5261 | 0.042* |
| C18 | −0.3031(3) | 0.7305(2) | 0.55899(19) | 0.0360(8) |
| H18 | −0.3441 | 0.6986 | 0.6073 | 0.043* |
| C19 | −0.0729(3) | 0.6641(2) | 0.39213(18) | 0.0298(7) |
| C20 | −0.1996(3) | 0.5217(2) | 0.41042(19) | 0.0300(8) |
| C21 | −0.1862(3) | 0.4434(2) | 0.4754(2) | 0.0406(8) |
| H21 | −0.1063 | 0.4278 | 0.5041 | 0.049* |
| C22 | −0.2905(4) | 0.3871(3) | 0.4985(2) | 0.0519(10) |
| H22 | −0.2825 | 0.3333 | 0.5436 | 0.062* |
| C23 | −0.4051(4) | 0.4089(3) | 0.4564(3) | 0.0569(11) |
| H23 | −0.4751 | 0.3692 | 0.4718 | 0.068* |
| C24 | −0.4187(3) | 0.4885(3) | 0.3917(2) | 0.0546(11) |
| H24 | −0.4989 | 0.5041 | 0.3631 | 0.066* |
| C25 | −0.3153(3) | 0.5457(3) | 0.3681(2) | 0.0437(9) |
| H25 | −0.3241 | 0.6005 | 0.3236 | 0.052* |
| C26 | 0.0331(3) | 0.5460(2) | 0.33697(18) | 0.0289(7) |
| C27 | 0.1086(3) | 0.6205(2) | 0.32081(18) | 0.0297(7) |
| C28 | 0.2402(3) | 0.6182(2) | 0.27494(18) | 0.0297(7) |
| C29 | 0.3151(3) | 0.6942(2) | 0.25954(19) | 0.0335(8) |
| H29 | 0.2803 | 0.7493 | 0.2798 | 0.040* |
| C30 | 0.4408(3) | 0.6888(2) | 0.21441(19) | 0.0368(8) |
| H30 | 0.4911 | 0.7412 | 0.2033 | 0.044* |
| C31 | 0.4945(3) | 0.6093(2) | 0.18538(18) | 0.0331(8) |
| H31 | 0.5816 | 0.6071 | 0.1552 | 0.040* |
| C32 | 0.4218(3) | 0.5312(2) | 0.19995(18) | 0.0298(7) |
| C33 | 0.2900(3) | 0.5356(2) | 0.24494(18) | 0.0276(7) |
| C34 | 0.4658(3) | 0.4458(2) | 0.16994(18) | 0.0303(7) |
| C35 | 0.3846(3) | 0.3765(2) | 0.18045(18) | 0.0295(7) |
| C36 | 0.2546(3) | 0.3778(2) | 0.22731(18) | 0.0296(7) |
| C37 | 0.1776(3) | 0.3022(2) | 0.24174(19) | 0.0367(8) |
| H37 | 0.2103 | 0.2485 | 0.2198 | 0.044* |
| C38 | 0.0539(3) | 0.3065(2) | 0.2882(2) | 0.0407(9) |
| H38 | 0.0023 | 0.2549 | 0.2984 | 0.049* |
| C39 | 0.0041(3) | 0.3841(2) | 0.31987(19) | 0.0372(8) |
| H39 | −0.0815 | 0.3854 | 0.3514 | 0.045* |
| C40 | 0.0775(3) | 0.4613(2) | 0.30641(18) | 0.0297(7) |
| C41 | 0.2072(3) | 0.4587(2) | 0.25987(18) | 0.0282(7) |
| C42 | 0.5632(3) | 0.3342(2) | 0.10661(18) | 0.0311(7) |
| C43 | 0.6985(3) | 0.4692(2) | 0.09590(19) | 0.0305(8) |
| C44 | 0.7011(3) | 0.5326(2) | 0.0234(2) | 0.0390(8) |
| H44 | 0.6321 | 0.5380 | −0.0101 | 0.047* |
| C45 | 0.8057(3) | 0.5889(3) | −0.0005(2) | 0.0505(10) |
| H45 | 0.8083 | 0.6331 | −0.0505 | 0.061* |
| C46 | 0.9058(4) | 0.5803(3) | 0.0485(3) | 0.0542(11) |
| H46 | 0.9756 | 0.6202 | 0.0328 | 0.065* |
| C47 | 0.9048(3) | 0.5140(3) | 0.1205(2) | 0.0471(10) |
| H47 | 0.9758 | 0.5067 | 0.1533 | 0.057* |
| C48 | 0.8002(3) | 0.4581(2) | 0.1448(2) | 0.0398(8) |
| H48 | 0.7985 | 0.4129 | 0.1943 | 0.048* |
| C49 | 0.6560(3) | 0.2814(2) | 0.05629(19) | 0.0314(8) |
| C50 | 0.8026(3) | 0.2608(2) | 0.05222(19) | 0.0352(8) |
| H50 | 0.8497 | 0.2858 | 0.0820 | 0.042* |
| C51 | 0.8814(3) | 0.2050(2) | 0.00577(19) | 0.0378(8) |
| H51 | 0.9814 | 0.1912 | 0.0046 | 0.045* |
| C52 | 0.8150(3) | 0.1689(2) | −0.03919(19) | 0.0356(8) |
| C53 | 0.6687(3) | 0.1887(2) | −0.03536(19) | 0.0386(8) |
| H53 | 0.6218 | 0.1643 | −0.0656 | 0.046* |
| C54 | 0.5912(3) | 0.2429(2) | 0.0114(2) | 0.0387(8) |
| H54 | 0.4913 | 0.2547 | 0.0134 | 0.046* |
| C55 | 1.0173(3) | 0.1357(2) | −0.13101(19) | 0.0374(8) |
| C56 | 1.0379(3) | 0.2326(2) | −0.1547(2) | 0.0434(9) |
| H56 | 0.9678 | 0.2836 | −0.1413 | 0.052* |
| C57 | 1.1596(4) | 0.2560(3) | −0.1978(2) | 0.0534(10) |
| H57 | 1.1730 | 0.3226 | −0.2129 | 0.064* |
| C58 | 1.2619(4) | 0.1830(3) | −0.2188(2) | 0.0645(12) |
| H58 | 1.3447 | 0.1990 | −0.2490 | 0.077* |
| C59 | 1.2414(4) | 0.0865(3) | −0.1951(2) | 0.0624(12) |
| H59 | 1.3112 | 0.0359 | −0.2092 | 0.075* |
| C60 | 1.1216(3) | 0.0620(3) | −0.1513(2) | 0.0491(10) |
| H60 | 1.1101 | −0.0050 | −0.1350 | 0.059* |
| C61 | 0.8316(3) | 0.0361(2) | −0.1003(2) | 0.0382(8) |
| C62 | 0.8267(4) | 0.0293(3) | −0.1732(2) | 0.0503(10) |
| H62 | 0.8658 | 0.0740 | −0.2159 | 0.060* |
| C63 | 0.7649(4) | −0.0426(3) | −0.1843(2) | 0.0591(11) |
| H63 | 0.7634 | −0.0479 | −0.2345 | 0.071* |
| C64 | 0.7056(4) | −0.1063(3) | −0.1231(2) | 0.0603(12) |
| H64 | 0.6619 | −0.1549 | −0.1309 | 0.072* |
| C65 | 0.7102(4) | −0.0991(3) | −0.0502(2) | 0.0552(11) |
| H65 | 0.6692 | −0.1428 | −0.0077 | 0.066* |
| C66 | 0.7737(3) | −0.0291(2) | −0.0386(2) | 0.0448(9) |
| H66 | 0.7777 | −0.0255 | 0.0118 | 0.054* |
Source of material
A mixture of aniline (4.6 mL, 50.0 mmol), pyrene-4,5,9,10-tetraone (1.3 g, 5.0 mmol), corresponding aromatic aldehyde 4-(diphenylamino)benzaldehyde (3.3 g, 12.0 mmol), ammonium acetate (3.1 g, 40.0 mmol), and acetic acid (20 mL) was refluxed under nitrogen in an oil bath. After 2 h, the mixture was cooled and filtered. The solid product was washed with an acetic acid/water mixture (1:1, 150 mL). And then, the crude product was separated by chromatography using CH2Cl2 as eluent and further purified by sublimation under vacuum. The product was collected as a yellow solid in yield 48.2%.
Experimental details
The C—H atoms were then constrained to an ideal geometry, with C—H distances of 0.95 Å. The Uiso values of the hydrogen atoms were set to 1.2Ueq(C).
Discussion
Pyrene belongs to one of the polycyclic aromatic hydrocarbons (PAHs) which possesses large planar π-system, strong π-stacking interactions, high photoluminescence (PL) efficiency and exceptionally long fluorescence lifetimes [4]. Its unique properties have inspired researchers from many scientific areas, making pyrene the chromophore of choice in fundamental and applied photochemical research [4], [5], [6], [7], [8]. Fused imidazoles, such as benzimidazole and phenanthro[9,10-d]imidazole, has been shown to be good electron-transporting materials and luminescent materials for OLEDs [9], [10], [11]. In continuation of our studies on the development of PAHs and N-containing heterocyclic compounds, herein we report a pyrene-imidazole compound.
In the crystal structure, the pyrene ring and the imidazole ring are almost coplanar, while the triphenylamine moieties keep propeller-shaped. The crystal showed a centrosymmetric, coplanar configuration and a slip-stacked packing mode along the long molecular axis. While the dihedral angle between the pyrene-imidazole and phenyl ring connected to imidazole was near to 90°, keeping a certain distance between the molecules. The molecules adopt a stacking mode similar to the J-type aggregation along the cystallographic c axis in each column with π⋯π interactions formed between adjacent molecules. And each molecule interacts with one neighboring molecule via another C—H⋯π interactions.
Acknowledgements
The authors are grateful for support from the Student Innovation and Practical Training program of Northeast Agricultural University 2018 and Young Talent Plan of Northeast Agricultural University (17QC24) and Postdoctoral Foundation of Heilongjiang Province (LBH-Z17014). We thank the editor for providing the figure.
References
Rigaku/MSC. CrystalClear. Rigaku/MSC Inc., The Woodlands, Texas, USA (2006).Search in Google Scholar
Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Figueira-Duarte, T. M.; Mullen, K.: Pyrene-based materials for organic electronics. Chem. Rev. 111 (2011) 7260–7314.10.1021/cr100428aSearch in Google Scholar PubMed
Liu, Y. L.; Shan, T.; Yao, L.; Bai, Q.; Guo, Y. C.; Li, J. Y.; Han, X.; Li, W. J.; Wang, Z. M.; Yang, B.; Lu, P.; Ma, Y. G.: Isomers of pyrene-imidazole compounds: synthesis and configuration effect on optical properties. Org. Lett. 17 (2015) 6138–6141.10.1021/acs.orglett.5b02879Search in Google Scholar PubMed
Gao, B. X.; Wang, M.; Cheng, Y. X.; Wang, L. X.; Jing, X. B.; Wang, F. S.: Pyrazine-containing acene-type molecular ribbons with up to 16 rectilinearly arranged fused aromatic rings. J. Am. Chem. Soc. 130 (2008) 8297–8306.10.1021/ja800311aSearch in Google Scholar PubMed
Kim, H. M.; Lee, Y. O.; Lim, C. S.; Kim, J. S.; Cho, B. R.: Two-photon absorption properties of alkynyl-conjugated pyrene derivatives. J. Org. Chem. 73 (2008) 5127–5130.10.1021/jo800363vSearch in Google Scholar PubMed
Zhang, S. Q.; Qiao, X. L.; Chen, Y.; Wang, Y. Y.; Edkins, R. M.; Liu, Z. Q.; Li, H. X.; Fang, Q.: Synthesis, structure, and opto-electronic properties of regioisomeric pyrene-thienoacenes. Org. Lett. 16 (2014) 342–345.10.1021/ol402971nSearch in Google Scholar PubMed
Gao, Z. Q.; Lee, C. S.; Bello, I.; Lee, S. T.; Chen, R. M.; Luh, T. Y.; Shi, J.; Tang, C. W.: Bright-blue electroluminescence from a silyl-substituted ter-(phenylene-vinylene) derivative. Appl. Phys. Lett. 74 (1999) 865–867.10.1063/1.123392Search in Google Scholar
Li, W. J.; Liu, D. D.; Shen, F. Z.; Ma, D. G.; Wang, Z. M.; Feng, T.; Xu, Y. X.; Yang, B.; Ma, Y. G.:A twisting donor-acceptor molecule with an intercrossed excited state for highly efficient, deep-blue electroluminescence. Adv. Funct. Mater. 22 (2012) 2797–2803.10.1002/adfm.201200116Search in Google Scholar
Gao, Z.; Liu, Y. L.; Wang, Z. M.; Shen, F. Z.; Liu, H.; Sun, G. N.; Yao, L.; Lv, Y.; Lu, P.; Ma, Y. G.: High-efficiency violet-light-emitting materials based on phenanthro[9,10-d]imidazole. Chem. Eur. J. 19 (2013) 2602–2605.10.1002/chem.201203335Search in Google Scholar PubMed
©2018 Yulong Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S

