Abstract
C23H27BrO2, P21/n (no. 14), a = 10.3200(18) Å, b = 15.905(3)(6) Å, c = 12.913(2) Å, β = 97.683(4), V = 2100.6(6) Å3, Z = 4, Rgt(F) = 0.0319, wRref(F2) = 0.0872, T = 296(2) K.
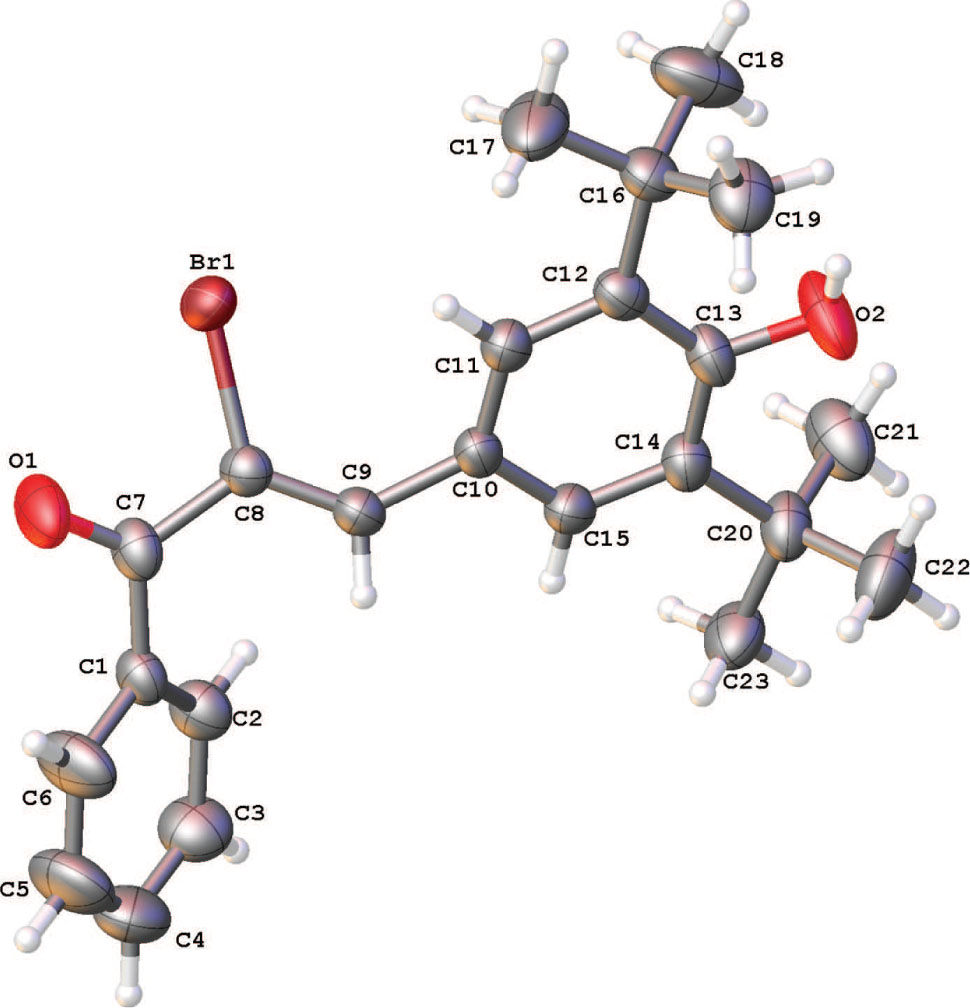
Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless prism |
| Size: | 0.17 × 0.15 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.97 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 21854, 4591, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3732 |
| N(param)refined: | 241 |
| Programs: | Bruker [1], SHELX [2], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 0.72766(2) | 0.11800(2) | 0.95063(2) | 0.04809(9) |
| O1 | 0.68221(17) | 0.02921(12) | 0.74815(15) | 0.0646(5) |
| O2 | 0.2748(2) | 0.31050(11) | 1.21175(16) | 0.0750(6) |
| H2O | 0.3136 | 0.3584 | 1.2133 | 0.113* |
| C1 | 0.4684(2) | −0.02245(13) | 0.73662(15) | 0.0396(4) |
| C2 | 0.3873(2) | −0.06645(14) | 0.79337(17) | 0.0468(5) |
| H2A | 0.3974 | −0.0614 | 0.8658 | 0.056* |
| C3 | 0.2910(3) | −0.11803(16) | 0.7434(2) | 0.0629(7) |
| H3A | 0.2366 | −0.1474 | 0.7824 | 0.075* |
| C4 | 0.2749(3) | −0.12634(19) | 0.6372(3) | 0.0729(8) |
| H4A | 0.2095 | −0.1609 | 0.6039 | 0.087* |
| C5 | 0.3556(4) | −0.0836(2) | 0.5804(2) | 0.0854(10) |
| H5A | 0.3455 | −0.0893 | 0.5081 | 0.102* |
| C6 | 0.4512(3) | −0.0323(2) | 0.6291(2) | 0.0727(8) |
| H6A | 0.5057 | −0.0036 | 0.5894 | 0.087* |
| C7 | 0.5797(2) | 0.03023(13) | 0.78504(16) | 0.0415(5) |
| C8 | 0.56493(19) | 0.08382(13) | 0.87725(15) | 0.0364(4) |
| C9 | 0.4505(2) | 0.10827(13) | 0.90392(16) | 0.0388(4) |
| H9A | 0.3790 | 0.0857 | 0.8615 | 0.047* |
| C10 | 0.41368(19) | 0.16345(13) | 0.98636(15) | 0.0375(4) |
| C11 | 0.4870(2) | 0.23193(13) | 1.02806(16) | 0.0401(4) |
| H11A | 0.5658 | 0.2439 | 1.0036 | 0.048* |
| C12 | 0.4467(2) | 0.28288(13) | 1.10493(16) | 0.0394(4) |
| C13 | 0.3266(2) | 0.26307(13) | 1.13903(16) | 0.0422(5) |
| C14 | 0.2498(2) | 0.19411(13) | 1.10084(16) | 0.0408(5) |
| C15 | 0.2956(2) | 0.14626(14) | 1.02373(17) | 0.0400(4) |
| H15A | 0.2458 | 0.1009 | 0.9958 | 0.048* |
| C16 | 0.5316(2) | 0.35910(15) | 1.14972(19) | 0.0489(5) |
| C17 | 0.6586(3) | 0.3630(2) | 1.1025(3) | 0.0834(10) |
| H17A | 0.7092 | 0.4103 | 1.1310 | 0.125* |
| H17B | 0.6395 | 0.3691 | 1.0281 | 0.125* |
| H17C | 0.7074 | 0.3122 | 1.1187 | 0.125* |
| C18 | 0.5654(4) | 0.3497(2) | 1.2696(3) | 0.0868(10) |
| H18A | 0.5946 | 0.4028 | 1.2994 | 0.130* |
| H18B | 0.6335 | 0.3087 | 1.2850 | 0.130* |
| H18C | 0.4891 | 0.3319 | 1.2987 | 0.130* |
| C19 | 0.4611(3) | 0.44145(15) | 1.1238(2) | 0.0626(7) |
| H19A | 0.5225 | 0.4830 | 1.1071 | 0.094* |
| H19B | 0.4212 | 0.4599 | 1.1828 | 0.094* |
| H19C | 0.3949 | 0.4336 | 1.0649 | 0.094* |
| C20 | 0.1187(2) | 0.17365(15) | 1.1410(2) | 0.0514(6) |
| C21 | 0.1387(3) | 0.16141(19) | 1.2597(2) | 0.0716(8) |
| H21A | 0.2054 | 0.1200 | 1.2783 | 0.107* |
| H21B | 0.0583 | 0.1428 | 1.2820 | 0.107* |
| H21C | 0.1648 | 0.2137 | 1.2932 | 0.107* |
| C22 | 0.0199(3) | 0.24498(19) | 1.1092(3) | 0.0796(9) |
| H22A | 0.0080 | 0.2511 | 1.0345 | 0.119* |
| H22B | 0.0526 | 0.2967 | 1.1410 | 0.119* |
| H22C | −0.0624 | 0.2315 | 1.1322 | 0.119* |
| C23 | 0.0592(2) | 0.09225(17) | 1.0920(2) | 0.0616(7) |
| H23A | 0.1191 | 0.0466 | 1.1091 | 0.092* |
| H23B | 0.0424 | 0.0986 | 1.0175 | 0.092* |
| H23C | −0.0214 | 0.0806 | 1.1188 | 0.092* |
Source of material
The title compound was prepared by dropwise addition of bromine (1.25 mmol) to 3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenyl-propenone (1.19 mmol) in methylene chloride (10 mL) and the reaction mixture was stirred for three hours at room temperature. The reaction mixture was evaporated. The raw product was recrystallized in ethanol to get crystals. The yield of the title compound is 70% m.p. 148 °C; 1H NMR (300 MHz, DMSO-d6) δ 1.41 (s, 18H, 6CH3), 7.48–7.84 (m, 9H, 5CHarom + 2CHarom + CH = +OHarom). 13C NMR (75 MHz, CDCl3) δ 30.92 (6CH3), 35.05 (2Cquat.), 119.07 (Carom ), 124.80 (Carom), 128.52 (CHarom), 129.36 (CHarom), 129.82 (CHarom), 132.86 (CHarom), 137.32 (Carom), 138.90 (Carom), 145.19 (CH=), 157.21 (=Cquat.), 191.38 (C = O).
Experimental details
H atoms were located in the difference Fourier map, but refined with fixed individual displacement parameters, using a riding model with C—H distances of 0.93 Å (for aromatic rings), 0.96 Å (CH3 group), with U(H) values of 1.2Ueq(C) (for CH in aromatic moiety), and 1.5Ueq(C) (for CH3) and O—H distance 0.8601 Å, with U(H) values of 1.2Ueq(O).
Comment
α-Halogen chalcones are valuable synthetic building blocks in organic synthesis [4, 5] . The existence of bulky and electron-withdrawing groups in α-position lead to an enhancement of the electrophilicity. Examples of the influence of α-modification on biological activity was presented in literature. α-Halo chalcones are shown to possess biological activity [6]. Development of chalcones and related analogues as antimitotic agents was reported [7].
In the title compound the dihedral angle between the mean planes of the aromatic rings is 40.36(12)°. A search in the latest version of the Cambridge structural database, for related compounds yielded only one structure namely 3-(3,5-di-t-butyl-4-hydroxyphenyl)-1-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one (REFCODE PASSOU) [8]. The geometrical and molecular parameters are very similar between both compounds and the main difference is the crystal packing. In the title compound the molecules are linked by van der Waals interactions only, whereas in the related compound, the molecules are linked by hydrogen bond interactions. The conformation about the C=C bond is Z, with a C7—C8—C9—C10 torsion angle of 176.26(3)°.
Acknowledgements
Ali Khalilov thankful to Baku State University for the “50+50” individual grant support in this work.
References
Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA (2005).Search in Google Scholar
Sheldrick, G. M.: SHELXT – Integrated space-group and crystal-structure determination and crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053273314026370Search in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Search in Google Scholar
Ramanarayanan, G. V.; Shukla, V. G.; Akamanchi, K. G.: A novel and one step procedure for preparation of α-bromo-α, β-unsaturated carbonyl compounds. Synlett 12 (2002) 2059–2061.10.1002/chin.200310051Search in Google Scholar
Atsuta, H.; Fujiwara, K.; Murai, A.: Diastereoselective synthesis of the GH ring part of ciguatoxin. Synlett 3 (1997) 307–309.10.1055/s-1997-777Search in Google Scholar
Opletalova, V.: Chalcones and their heterocyclic analogs as potential therapeutic agents for bacterial diseases. Ceska Slov. Farm. 49 (2000) 278–284.Search in Google Scholar
Ducki, S.: Antimitotic chalcones and related compounds as inhibitors of tubulin assembly. Anti-Cancer Agents Med. Chem. 9 (2009) 336–347.10.2174/1871520610909030336Search in Google Scholar PubMed
Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C.: The Cambridge structural database. Acta Crystallogr. B72 (2016) 171–179.10.1016/B978-0-12-409547-2.02529-4Search in Google Scholar
©2018 Ali N. Khalilov et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S


