Deposits of iron oxides in the human globus pallidus
-
Helena Svobodová
Abstract
Samples taken from the human brain (Globus Pallidus) have been investigated by physical techniques such as light microscopy, scanning electron microscopy, transmission electron microscopy, Mössbauer spectroscopy and SQUID magnetometry. SEM-EDX/TEM investigation reveals multielemental composition of hematite and magnetite nanocrystals with sizes ranging from 40 nm to 100 nm and hematite microcrystals from 3 μm to 7 μm. Room temperature Mössbauer spectra show quadrupole doublets assigning to hematite and ferrihydrite. SQUID measurements of temperature dependence of the mass magnetic susceptibility between T = 2 – 300 K at DC field B0 = 0.1 T, the field dependence of the mass magnetization taken at the fixed temperature T0 = 2.0 and 4.6 K and the zero-field cooled and field cooled magnetization experiments (ZFCM/FCM) confirm a presence of ferrimagnetic phases such as maghemite and/or magnetite with hysteresis loops surviving until the room temperature. Differences between these measurements from the point of view of iron oxides detected can indicate important processes in human brain and interactions between ferritin as a physiological source of iron and surrounding environment.
1 Introduction
Iron is the most important metal with relatively high concentration in some regions of the brain. It catalyzes reactions forming reactive oxygen species and is one of the major factors associated with neurodegenerative diseases [1]. In humans, a large amount of the total iron content (66%) is present in the molecules of haemoglobin, which contains four Fe2+ ions. It accumulates in brain cells in the form of the much less reactive Fe(III) iron – ferritin - primarily an iron storage protein situated in the cytoplasm of the cells and in lesser amounts in the blood circulation. This protein shows spherical morphology with a diameter of 12 nm. The core of ferritin consists of 6 nm Fe(III)-oxide particle stored in the form of ferrihydrite (5 Fe2O3·9 H2O). Several studies show that the core physiological ferritin is composed of nanoparticles of ferrihydrite, magnetite (Fe3O4) or maghemite (γ-Fe2O3) and hematite (α-Fe2O3). Under some conditions iron can accumulate and create particles. This process - biomineralization influences properties of cells and tissues and supports some of their biological functions. Physiochemical properties of particles reflect conditions under biomineralization taking place. Under conditions prevailing in human body, the formation of an amorphous state or minute crystalline phase is expected. The deposits of iron oxides in the human brain are well documented over last decades. Ferrimagnetic components like magnetite Fe3O4 and/or
maghemite
Iron oxides are present in human brain and in animals. Magnetostatic bacteria, tuna, salmon and bird’s head contain magnetite for magnetotaxis and magnetic navigation. Lepidocrocite in chitons because of the mechanical strength, goethite in limpets and ferrihydrite in sea cucumbers can also be found.
The aim of this study is to examine to properties of the tissue from the perspective of distribution, structure, magnetic properties, oxidation and spin states of iron oxides in human globus pallidus with light and electron microscopy, Mössbauer spectroscopy and SQUID magnetometry.
2 Experimental
Postmortem brain tissue samples from male humans globus pallidus of different age (sample 1 – 61 y, sample 2 – 65 y, sample 3 – 69 y, sample 4 - 85 y) were routinely obtained at autopsy to prepare tissue sections for the pathology diagnosis. Tissues were taken from four individuals with no clinical findings of any motor abnormalities, iron metabolism pathologies, movements involving limbs, face, tongues. All procedures were conducted in accordance with the Declaration of Helsinki. Special attention was paid to avoid manipulations with iron instruments.
2.1 Light microscopy
The samples for light microscopy investigation were fixed in 10% formaldehyde for 24 hours and embedded in paraffin blocks, cut by a microtome into 5 μm thin sections, and mounted on gelatin-coated slides. Afterwards sections were stained for general morphological purposes by haematoxylin and eosin and for iron Fe (III) detection by Perls’ method. Tissue sections were then covered by cover glass and the samples were examined under the light microscope Eclipse E50i (Nikon).
2.2 Scanning and transmission electron microscopy (SEM/TEM)
The samples for electron microscopy investigation were fixed in 3% fixation solution of glutardialdehyde buffered by phosphate for scanning electron microscopy. Samples from the autopsy were dehydrated in graded acetone and subjected to critical point drying of CO2. Specimens were mounted on glass stubs and coated with 30 nm layer of gold in ion sputtering apparatus (SCD 050, BALZERS). Samples were analyzed by scanning electron microscope EVO LS 15 (ZEISS) with the accelerating voltage of 15 kV. Simultaneous EDX line analysis was performed with AME-TEK (EDAX) EDS Element Silicon Drift Detector. The time period of spectrum collection was 200 s with the energy range 0.160 to 9 keV.
The samples from the autopsy intended for TEM investigation were fixed in 3% solution of glutardialdehyde (SERVA, Heidelberg, Germany) for two hours and buffered by phosphate (pH 7.2 – 7.4). After dehydration of the tissue by alcohol, the samples were embedded into Durcupan ACM (Fluka AG, Busch, Switzerland) as recommended by the manufacturer and cut by ultramicrotome with a glass knife (C. Reichert, Wien, Austria). The thickness of the samples was 200 nm. Uncontrasted ultrathin sections were mounted on nickel grids and investigated by transmission electron microscope JEOL 840B (Jeol, Japan) with acceleration voltage of 150 kV. In order to determine the iron oxide phase, chemical analysis EDX KEVEX 3205-1200 (Kevex, Valencia, USA) was applied. For phase identification the International Centre for Diffraction Data (ICDD) for hematite ICDD card no. 33-0664, ferrihydrite ICDD card no. 29-0712 and magnetite ICDD card no. 190629 were used.
2.3 Mössbauer spectroscopy
Mössbauer spectra were collected at room temperature in the constant acceleration mode using a spectrometer equipped with 57Co/Rh gamma source (Ritverc). Isomer shift values are quoted with respect to a room temperature Mössbauer spectrum of a calibration α-Fe foil with the thickness of 12.5 μm (Goodfellow). Spectra of all unfixed samples were recorded at high velocity (± 11 mm/s) to look for possible presence of iron oxides. Because no traces of sextets were revealed, spectra were subsequently taken at low velocity range (± 4 mm/s) to enhance the resolution.
2.4 SQUID magnetometry
The superconducting quantum interference device (SQUID) magnetometer (MPMS-XL7, Quantum Design) has been used in recording magnetic functions: temperature dependence of the mass magnetic susceptibility χρ(T; B0) at small DC field B0 = 0.1 T transformed to the product function χT, and the field dependence of the mass magnetization Mρ(B; T0) taken at the fixed temperature T0 = 2.0 and 4.6 K. Also, the zero-field cooled and field cooled magnetization experiments (ZFCM/FCM) were conducted along with the search for the hysteresis at low as well as elevated temperatures. The unfixed samples were raw lyophilized powders encapsulated into a gelatin made container as a sample holder.
3 Results and discussion
3.1 SEM-EDX-TEM experiments
Iron (ferritin and/or hemosiderin) accumulation is observed in places with high metabolic activity around glial cells and depends on age [3]. The ageing of cells is associated with the loss of ability of the cells to maintain a variety of factors that include iron homeostasis, transport, metabolic rate, distribution and utilization of iron-binding proteins. The inevitable consequence of ageing is an increase of iron in specific brain regions that are preferentially targeted in neurodegenerative diseases. Different structure of ferritin core was found in healthy physiological and in pathological conditions. Changes in the structure of ferritin may grow from ferritin iron cores to iron oxide (nano)particles in tissues and cells [4].
Our findings revealed Fe(III) positive globular structures in samples located probably around glial cells with sizes ranging from 3 μm to 10 μm (Figure 1). During the ex-vivo examination of tissues and cells it is crucial to stabilize the sample. For this purpose, the fixation in formalin is used. This process of formalin fixation following with embedding tissue in paraffin can alter the concentration of iron compared with that observed in fresh tissue. Multiple studies revealed comparable levels between fixed and fresh tissue [5, 6, 7, 8]. On the other hand, some authors argue that iron used in samples is not tightly bound within the fixed tissue and fixation leads to leach iron and its reduction [9, 10].
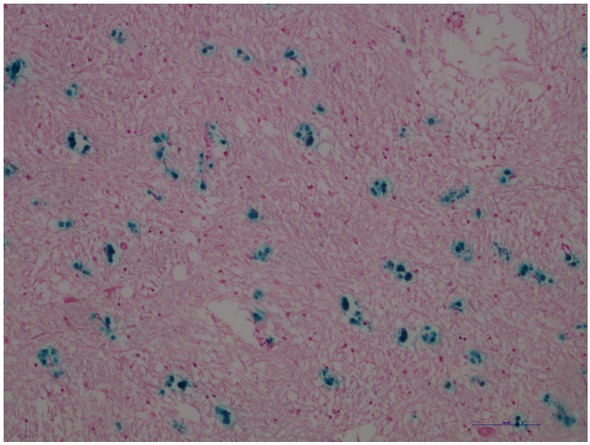
Human brain, globus pallidus. Blue dyed deposits correspond to the presence of Fe(III) probably around glial cells. Scale bar = 100 μm.
The results of iron depositions near glial cells agree with the results of other studies [11, 12]. It was observed that many spheroid structures in globus pallidus containing ferric iron regardless of the presence of diseases or different conditions are associated with iron mediated oxidative stress [13, 14]. This view is supported by multielemental composition of spheroid structures measured by EDX microanalysis [15, 16]. Our EDX-SEM investigation of complexes reveals multielemental composition with various amounts of O, Na, Si, P, S, Cl, K, Ca, Au and Fe. All iron-oxygen rich particles contained also phosphorus and sulfur (Figure 2). Elements like Ca, Fe, P, and S were detected in agreement with our results. The presence of gold in the samples is a side result of preparation. Our findings reveal the presence of Na and Si. These elements come probably from gelatin-coated slides and from their natural occurrence in human brain. They are vital elements present in human brain. Function of silicon in human brain is still unknown. Aluminum is a chemical element which can influence hematite formation and its structure. Ions can preferentially promote formation of some iron oxides and can influence their morphology, structure, size and magnetic properties.
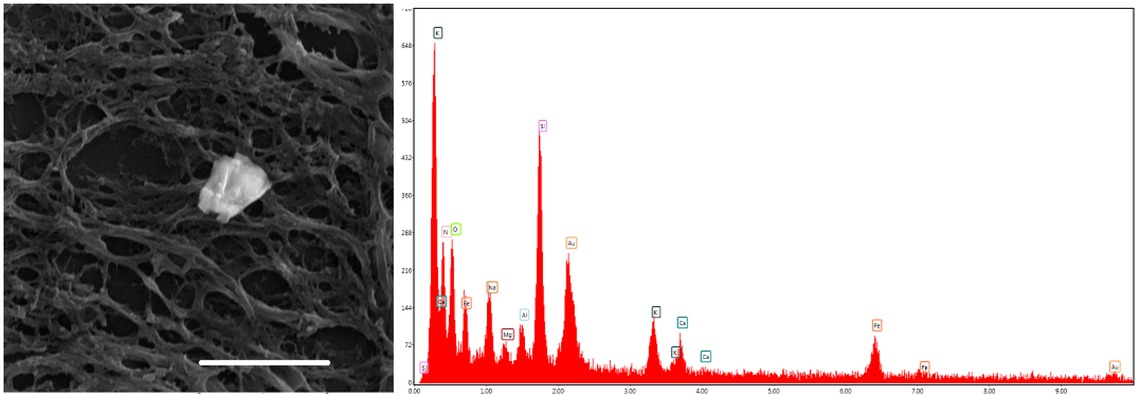
Human brain, globus pallidus. Left – Iron-rich particle. Right – EDX spectrum of this particle showed O, Na, Si, P, S, Cl, K, Ca, Au and Fe. Scale bar = 10 μm. SEM.
Investigation with scanning electron microscopy after removal of cover glass showed isolated, irregular iron-rich particles (Figure 2). Biomineralization process, as an origin of iron oxides, in the brain is the interaction between iron and the surrounding environment. It takes place in the human body - environment with low temperature and pressure. Under these conditions the formation of an amorphous state or minute crystalline phase is expected. Conditions prevailing in living organism (low temperature and pressure, almost neutral pH) may also cause the formation of metastable phases, and subsequent transformation on stable phases [17]. The final product of biomineralization
depends on many other factors such as chemical elements and compounds, temperature, pH [18], the presence of reactive oxygen species (ROS) and time. Selective electron diffraction in TEM shows the presence of crystalline material with irregular structures (Figure 3).
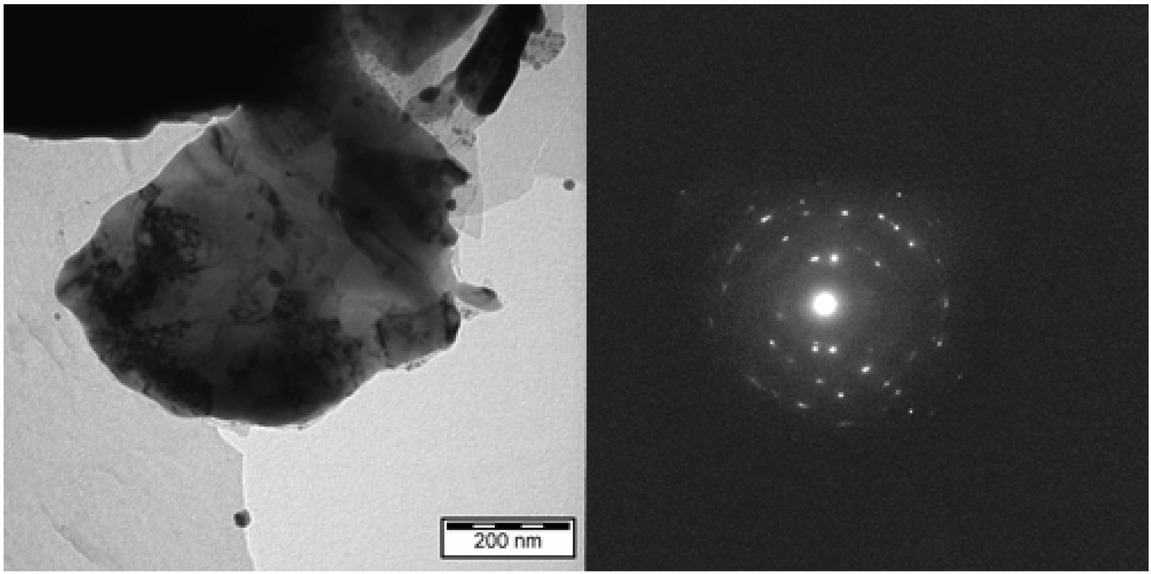
TEM records of the Human brain, globus pallidus. Irregular micrometer-sized hparticle (left) with diffraction pattern corresponding to hematite - α-Fe2O3 (right). Scale bar 3 = μm.
The lattice parameters a = 5.0016 Å, b = 5.0016 Å, c = 13.6202 Å of rhombohedral crystal system (space group
3.2 Mössbauer spectra
Two-line absorption spectra in Figure 4 are characteristic for a non-magnetic material. In fact, iron-containing nanoparticles exhibit superparamagnetic behaviour due to dynamic spin relaxation process of their magnetic moments at room temperature. These spectra were fitted with two doublets. One of them, with average isomer shift (< IS >) and quadrupole splitting (< QS >) values of (0.376±0.007) mm/s and (0.661±0.014) mm/s, respectively, represents Fe3+ nuclei in the high-spin state.
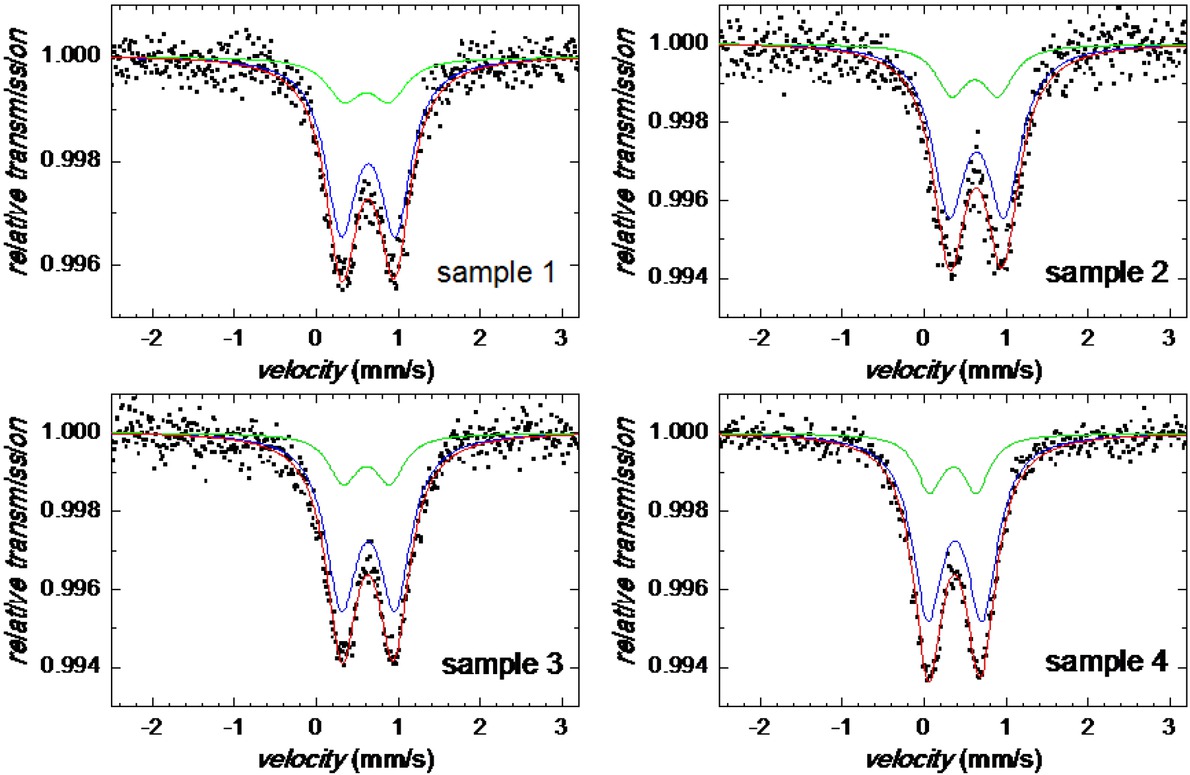
Mössbauer spectra for samples 1 through 4.
The second atomic site, also Fe3+, is characterized by < IS > = (0.354±0.007) mm/s and < QS > = (0.566±0.014) mm/s. All samples exhibit very similar hyperfine parameters that are almost equal for the individual doublets in the frame of experimental error as demonstrated in Figure 5. Both spectral components show rather large line widths which indicate that the structure of the particles is highly disordered and/or distribution in their size is observed. Typical IS and QS values for ferrihydrite core of hemosiderin and ferritin are of 0.35 mm/s and 0.71 mm/s, respectively [23]. Thus, the observed doublets can be assigned to hematite and ferrihydrite.
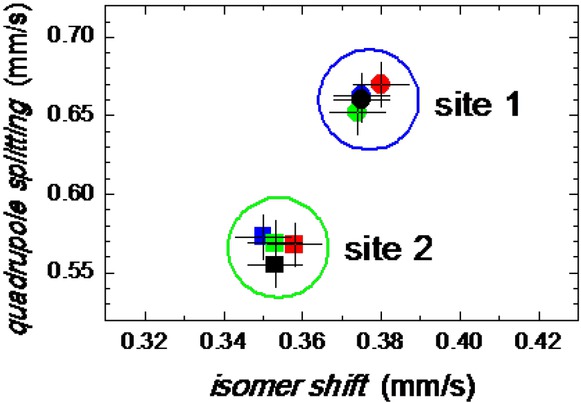
Hyperfine parameters of the Mössbauer spectra for samples 1 through 4.
3.3 SQUID magnetometry
Magnetometry reflects the presence of magnetic species such as paramagnetic Fe2+ centers of the hemoglobin and Fe3+ of the oxyhemoglobin, traces of other metal ions such as Cu2+, air dioxygen, and the ferrihydrite contained in ferritin. All these species produce a minor response with respect to the diamagnetism of the organic tissue that forms the brain mass. Some iron-oxide deposits are antiferromagnetic materials, e.g. FeO, α-FeOOH, and α-Fe2O3. However, their nanoparticles could bring magnetic response due to the presence of uncompensated magnetic moments at their surfaces. The ferrimagnetic materials, such as
It is crucial to know if ex-vivo post-mortem magnetic properties of iron correspond to in-vivo findings. Formaldehyde fixation causes chemical alterations of iron environment which can result in the alteration of magnetic properties of examined tissue ex-vivo and in-vivo. The process of tissue fixation is not fully understood. Fixation probably induces changes in tissues and cells such as dehydration, protein crosslinking and transmembrane water exchange [27]. It is known that fixation significantly reduces T1 and T2 relaxation times. Several studies revealed that the fixation in formaldehyde reduced T2 relaxation time up to 80% and to 20% in T1 [28, 29]. It seems that the reduction of relaxation times T1 and T2 is not only the result of formaldehyde fixation but also the result of postmortem interval, temperature, fixation solution, embedding media [29, 30, 31]. Still, some authors found no change in ex-vivo magnetic susceptibility over time [32, 33].
Figure 6 demonstrates a typical record of the temperature dependence of the mass magnetic susceptibility between T = 2 – 300 K taken at low field B0 = 0.1 T. The magnetic susceptibility on heating from 2 K decreases until 30 K when it stays almost constant. The product function, T vs T, displays a considerably positive slope which indicates a presence of the ordered ferro-/ferrimagnetic phase instead of the paramagnetic one. (for a net paramagnetic phase, the Curie law, = C/T, will be obeyed and then the product function, T = C,would be a straight line with zero slope.)
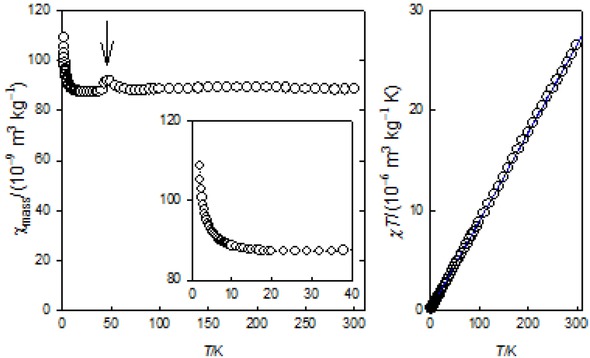
Magnetic susceptibility (left) and product function (right) for sample 1. (Small hook at ca 50 K refers to the solidus-solidus transition of the dioxygen impurity).
The zero-field cooled magnetization and field-cooled magnetization (ZFCM/FCM) records are shown in Figure 7. This data confirms a kind of the long-range ordering that survives until room temperature. As the data were taken in the field decreasing mode, some remnant magnetization survives at the zero field.
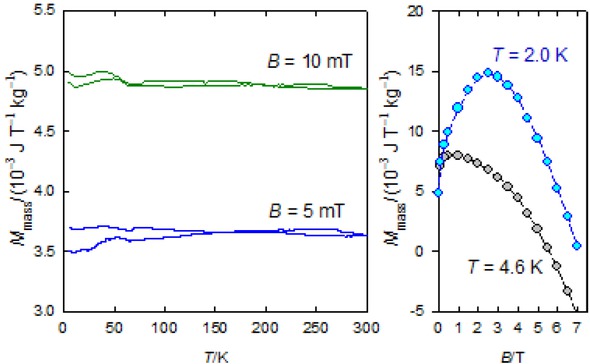
FCM/ZFCM curves (left) and a field development of the mass magnetization (right) for 1.
The magnetization curves do not follow a usual course approaching saturation; due to the dominant diamagnetic signal from the host tissue, the mass magnetization rises to the maximum when it turns down since the latter component prevails in high fields
A presence of the remnant magnetization approves a search for the magnetic hysteresis that was positive; the corresponding hysteresis curves are drawn in Figure 8 for T = 20 and 200 K. Notice that the magnetic hysteresis reflects the presence of only ferrimagnetic phases such as γ-Fe2O3 or Fe3O4. The superparamagnetic behavior of the ferritin escapes above 15 K and it does not manifest itself in the recorded hysteresis curves above 20 K.
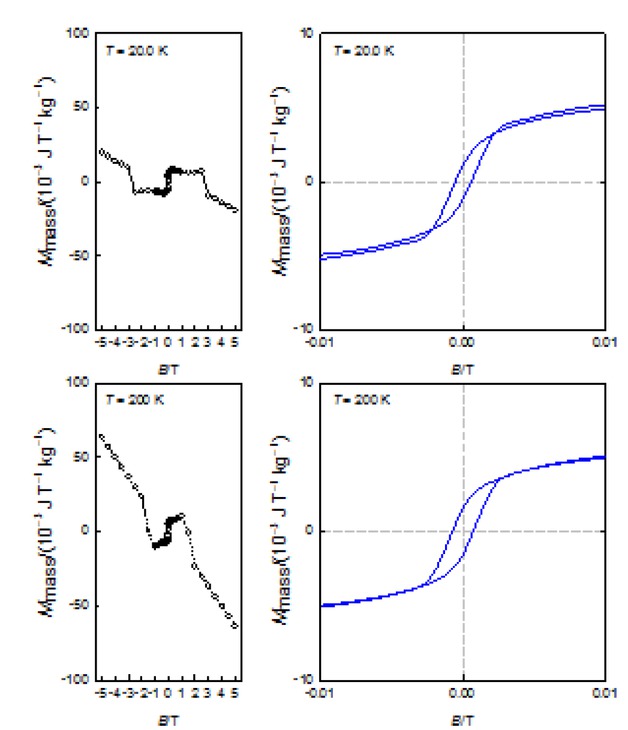
Hysteresis curves for the sample 1
3.4 General remarks
From the above results, we concluded that samples taken from the human brain contain ferrimagnetic
(maghemite
4 Conclusions
The samples taken from the human Globus Pallidus show a presence of various iron-oxide deposits of different size, shape, and count. Chemical composition of these particles is multielemental. Mössbauer spectroscopy shows that the observed doublets can be assigned mostly to hematite and ferrihydrite. Both spectral components show rather large line widths which indicate that the structure of the particles is highly disordered and/or distribution in their size is observed. SQUIDmagnetometry confirms a presence of ferromagnetic maghemite and/or magnetite. Still more measurements are needed to explain differences between the data. We propose that the interaction between ferritin as a physiological source of iron and surrounding environment is a crucial factor influencing the results of biomineralization.
Acknowledgement
Slovak Research and Development Agency projects No. APVV 16-0039 and VEGA 1/0919/17 are acknowledged for the financial support.
References
[1] Mikhaylova A., Davidson M., Toastmann H., Channell J.E.T., Guyodo Y.. Detection, identification and mapping of iron anomalies in brain tissue using X-ray absorption spectroscopy. J R Soc Interface 2005, 2, 33-37.10.1098/rsif.2004.0011Suche in Google Scholar
[2] Kirschvink J.L., Kobayashi-Kirschvink A., Woodford B.J., Magnetite biomineralization in the human brain. Proc. Natl. Acad. Sci. USA., 1992, 89, 7683–7687.10.1073/pnas.89.16.7683Suche in Google Scholar
[3] Connor J.R., Menzies S.L., Burdo J.R., Boyer P.J., Iron and iron management proteins in neurobiology. Pediatr. Neurol. 2001, 25, 118–129.10.1016/S0887-8994(01)00303-4Suche in Google Scholar
[4] Hautot D., Pankhurst Q.A., Morris C.M., Curtis A., Burn J., Dobson J., Preliminary observation of elevated levels of nanocrystalline iron oxide in the basal ganglia of neuroferritinopathy patients. Biochim. Biophys. Acta 2007, 1772, 21-25.10.1016/j.bbadis.2006.09.011Suche in Google Scholar
[5] Gellein K., Flaten T.P., Erikson K.M., Aschner M., Syversen T., Leaching of trace elements from biological tissue by formalin fixation. 2008, 121, 221-22510.1007/s12011-007-8051-1Suche in Google Scholar
[6] Sarafanov A.G., Todorov T., Kajdacsy-Balla A., Gray M.A., Macias V., Centeno J.A., Analysis of iron, zinc, selenium and cadmium in parafln-embedded prostate tissue specimens using inductively coupled plasma mass-spectrometry, J Trace Elements Med Biol. 2008, 22, 305-31410.1016/j.jtemb.2008.03.010Suche in Google Scholar
[7] Jakus J., Stransky A., Poliacek I., Barani H., Boselova L., Kainic acid lesions to the lateral tegmental field of medulla: effects on cough, expiration and aspiration reflexes inanesthetized cats. Physiol Res. 2000, 49, 387-398Suche in Google Scholar
[8] Poliacek I., Stransky A., Jakus J., Barani H., Tomori Z., Halasova E., Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiol Res 2003, 52, 749-76210.33549/physiolres.930389Suche in Google Scholar
[9] Schrag M., Dickson A., Jiffry A., Kirsch D., Vinters H.V., Kirsch W., The effect of formalin fixation on the levels of brain transition metals in archived samples. Biometals 2010, 23, 1123-112710.1007/s10534-010-9359-4Suche in Google Scholar
[10] Dobson J., Grassi P., Magnetic properties of human hippocampal tissue – evaluation of artefact and contamination sources. Brain Res Bulletin 1996, 39, 255-259.10.1016/0361-9230(95)02132-9Suche in Google Scholar
[11] Casanova M.F., Araque J.M.,Mineralization of the basal ganglia: implications for neuropsychiatry, pathology and neuroimaging. Psychiatry Res., 2003, 121, 59–8710.1016/S0165-1781(03)00202-6Suche in Google Scholar
[12] Morris C.M., Candy J.M., Oakley A.E., et al. Histochemical distribution of non-haem iron in the human brain. Acta Anat (Basel) 1992, 144, 235–257.10.1159/000147312Suche in Google Scholar PubMed
[13] Willwohl D., Kettner M., Braak H., Hubbard G.B., Dick E.J., Cox A.B., Schultz C., Pallido-nigral spheroids in nonhuman primates: accumulation of heat shock proteins in astroglial processes. Acta Neuropathol. 2002, 130, 276-28010.1007/s00401-001-0466-8Suche in Google Scholar
[14] Plevkova A., Antosiewicz J., Varechova S., Poliacek., Jakua J., Tatar M., Pokorski M., Convergence of nasal and trachealneural pathways in modulating the cough response in guinea pig. J Phys Pharm 2009, 60, 89-93.Suche in Google Scholar
[15] Singhrao S.K., Morgan B.P., Neal J.W., Newman G.R., Functional role for corpora amylacea based on evidence from complement studies, Neurodeg. 1995, 4, 335–345.10.1016/1055-8330(95)90024-1Suche in Google Scholar
[16] Tokutake S., Nagase H., Morisaki S., Oyanagi S., X-ray micro-probe analysis of corpora amylacea. Neuropathol Appl Neurobiol. 1995, 21, 269-273.10.1111/j.1365-2990.1995.tb01059.xSuche in Google Scholar
[17] Sunagawa I., Crystals, growth, morphology, and perfection. Cambridge University Press, Cambridge, 2005.10.1017/CBO9780511610349Suche in Google Scholar
[18] Schwertmann U., Friedl J., Stanjek H., Schulze D.G., The effect of Al on Fe oxides. XIX Formation of As-substituted hematite from ferrihdyrite at 25C and pH 4 to 7. Clays Clay Miner. 2000, 48, 159–172.Suche in Google Scholar
[19] Collingwood J.F., Telling N.D., Iron oxides in the human brain, in: D. Faivre (Eds.), Iron oxides. From nature to application,Wiley-VCH, Verlag GmbH&Co.KGaA, Weinheim, Germany, 2016, pp.143-16610.1002/9783527691395.ch7Suche in Google Scholar
[20] Sant’Ovaia H., Marques G., Santos A., Gomes C., Rocha A., Magnetic susceptibility and isothermal remanent magnetization in human tissues: a study case. Biometals 2015) 951-95810.1007/s10534-015-9879-zSuche in Google Scholar
[21] Baltpurvins K. A., Burns R.C., Lawrance G.A., Stuart A.D., Effect of pH and anion type on the aging of freshly precipitated iron(III) hydroxide sludges. Environ. Sci. Technol. 1996, 30, 939-944.10.1021/es950401sSuche in Google Scholar
[22] Galvez N., Barron V., Torrent J., Preparation and properties of hematite with structural phosphous. Clays Clay Miner. 1999, 47, 375–385.10.1346/CCMN.1999.0470314Suche in Google Scholar
[23] Meyrick D., Webb J., Cole C., Iron and iron proteins found in the genetic disease, hereditary spherocytosis. Inorg. Chimica Acta 2002, 339, 481-487.10.1016/S0020-1693(02)01049-6Suche in Google Scholar
[24] Schultheiss-Grassi P.P., Dobson J., Magnetic analysis of human brain tissue. Biometals 1999, 12, 67-72.10.1023/A:1009271111083Suche in Google Scholar
[25] Kopáni M., Hlinková J., Ehrlich H., Valigura D., Boča R. Magnetic Properties of Iron Oxides in the Human Globus pallidus. J. Bioanal. Biomed. 2017, 9, 80-90.10.4172/1948-593X.1000158Suche in Google Scholar
[26] R. Boča, M. Kopáni, M. Miglierini, M. Čaplovičová, V. Mrázová, Ľ. Dlháň, Magnetic and Non-Magnetic Iron-Oxide Deposits in Basal Ganglia. In Horizons in Neuroscience Research. Volume 12, Nova, New York, 2013, 135-214.Suche in Google Scholar
[27] Birkl C., Langkammer C., Golob-Schwarzl N., Leoni M., Haybaeck J., Goessler W., Fazekas F., Ropele S., Effects of formalin fixation and temperature on MR relaxation times in the human brain. NMR Biomed. 2016, 29, 458-46510.1002/nbm.3477Suche in Google Scholar
[28] Shepherd T.M., Thelwall P.E., Stanisz G.J., Blackband S.J., Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn Reson Med. 2009a, 62, 26–34.10.1002/mrm.21977Suche in Google Scholar
[29] Shepherd T.M., Flint J.J., Thelwall P.E., Stanisz G.J., Mareci T.H., Yachnis A.T., Blackband S.J., Postmortem interval alters the water relaxation and diffusion properties of rat nervous tissue - Implications for MRI studies of human autopsy samples. Neuroimage 2009b, 44, 820-82610.1016/j.neuroimage.2008.09.054Suche in Google Scholar
[30] Bottomley P.A., Foster T.H., Argersinger R.E., Pfeifer L.M., A review of normal tissue hydrogen nmr relaxation-times and relaxation mechanisms from 1-100 mhz - dependence on tissue-type, nmr frequency, temperature, species, excision, and age. Medical Physics 1984, 11, 425-44810.1118/1.595535Suche in Google Scholar
[31] Pfefferbaum A., Sullivan E.V., Adalsteinsson E., Garrick T., Harper C., Postmortem MR imaging of formalin-fixed human brain. Neuroimage 2004, 21, 1585-159510.1016/j.neuroimage.2003.11.024Suche in Google Scholar
[32] Evia A.M., Kotrotsou A., Tamhane A.A., Dawe R.J., Kapasi A., Leurgans S.E., Schneider J.A., Bennett D.A., Arfanakis K., Ex-vivo quantitative susceptibilitymapping of human brain hemispheres. Plos One 2017, 12, Article Number: e018839510.1371/journal.pone.0188395Suche in Google Scholar
[33] Chua-anusorn W., Webb J., Macey D.J., Pootrakul P., St. Pierre T.G., The effect of histological processing on the form of iron in iron-loaded human tissues. Biochim Biophys Acta 1997, 1360, 255-261.10.1016/S0925-4439(97)00009-4Suche in Google Scholar
[34] Yang C.Y., Bryan A.M., Theil E.C., Sayers D.E., Bowen L.H. Structural variations in soluble iron complexes of models for ferritin: an x-ray absorption and Mössbauer spectroscopy comparison of horse spleen ferritin to Blutal (ironchondroitin sulfate) and Imferon (iron-dextran). J Inorg Biochem 1986, 28, 393-405.10.1016/0162-0134(86)80025-3Suche in Google Scholar
[35] Kopáni M., Kopániová A., Caplovicová M., Maruscáková L., Sisovsky V., Jakubovsky J., Iron and its relation to glycoconjugates in human globus pallidus. Bratislava Med J. 2014, 115, 362-366.10.4149/BLL_2014_071Suche in Google Scholar
© 2019 H. Svobodová et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Non-equilibrium Phase Transitions in 2D Small-World Networks: Competing Dynamics
- Harmonic waves solution in dual-phase-lag magneto-thermoelasticity
- Multiplicative topological indices of honeycomb derived networks
- Zagreb Polynomials and redefined Zagreb indices of nanostar dendrimers
- Solar concentrators manufacture and automation
- Idea of multi cohesive areas - foundation, current status and perspective
- Derivation method of numerous dynamics in the Special Theory of Relativity
- An application of Nwogu’s Boussinesq model to analyze the head-on collision process between hydroelastic solitary waves
- Competing Risks Model with Partially Step-Stress Accelerate Life Tests in Analyses Lifetime Chen Data under Type-II Censoring Scheme
- Group velocity mismatch at ultrashort electromagnetic pulse propagation in nonlinear metamaterials
- Investigating the impact of dissolved natural gas on the flow characteristics of multicomponent fluid in pipelines
- Analysis of impact load on tubing and shock absorption during perforating
- Energy characteristics of a nonlinear layer at resonant frequencies of wave scattering and generation
- Ion charge separation with new generation of nuclear emulsion films
- On the influence of water on fragmentation of the amino acid L-threonine
- Formulation of heat conduction and thermal conductivity of metals
- Displacement Reliability Analysis of Submerged Multi-body Structure’s Floating Body for Connection Gaps
- Deposits of iron oxides in the human globus pallidus
- Integrability, exact solutions and nonlinear dynamics of a nonisospectral integral-differential system
- Bounds for partition dimension of M-wheels
- Visual Analysis of Cylindrically Polarized Light Beams’ Focal Characteristics by Path Integral
- Analysis of repulsive central universal force field on solar and galactic dynamics
- Solitary Wave Solution of Nonlinear PDEs Arising in Mathematical Physics
- Understanding quantum mechanics: a review and synthesis in precise language
- Plane Wave Reflection in a Compressible Half Space with Initial Stress
- Evaluation of the realism of a full-color reflection H2 analog hologram recorded on ultra-fine-grain silver-halide material
- Graph cutting and its application to biological data
- Time fractional modified KdV-type equations: Lie symmetries, exact solutions and conservation laws
- Exact solutions of equal-width equation and its conservation laws
- MHD and Slip Effect on Two-immiscible Third Grade Fluid on Thin Film Flow over a Vertical Moving Belt
- Vibration Analysis of a Three-Layered FGM Cylindrical Shell Including the Effect Of Ring Support
- Hybrid censoring samples in assessment the lifetime performance index of Chen distributed products
- Study on the law of coal resistivity variation in the process of gas adsorption/desorption
- Mapping of Lineament Structures from Aeromagnetic and Landsat Data Over Ankpa Area of Lower Benue Trough, Nigeria
- Beta Generalized Exponentiated Frechet Distribution with Applications
- INS/gravity gradient aided navigation based on gravitation field particle filter
- Electrodynamics in Euclidean Space Time Geometries
- Dynamics and Wear Analysis of Hydraulic Turbines in Solid-liquid Two-phase Flow
- On Numerical Solution Of The Time Fractional Advection-Diffusion Equation Involving Atangana-Baleanu-Caputo Derivative
- New Complex Solutions to the Nonlinear Electrical Transmission Line Model
- The effects of quantum spectrum of 4 + n-dimensional water around a DNA on pure water in four dimensional universe
- Quantum Phase Estimation Algorithm for Finding Polynomial Roots
- Vibration Equation of Fractional Order Describing Viscoelasticity and Viscous Inertia
- The Errors Recognition and Compensation for the Numerical Control Machine Tools Based on Laser Testing Technology
- Evaluation and Decision Making of Organization Quality Specific Immunity Based on MGDM-IPLAO Method
- Key Frame Extraction of Multi-Resolution Remote Sensing Images Under Quality Constraint
- Influences of Contact Force towards Dressing Contiguous Sense of Linen Clothing
- Modeling and optimization of urban rail transit scheduling with adaptive fruit fly optimization algorithm
- The pseudo-limit problem existing in electromagnetic radiation transmission and its mathematical physics principle analysis
- Chaos synchronization of fractional–order discrete–time systems with different dimensions using two scaling matrices
- Stress Characteristics and Overload Failure Analysis of Cemented Sand and Gravel Dam in Naheng Reservoir
- A Big Data Analysis Method Based on Modified Collaborative Filtering Recommendation Algorithms
- Semi-supervised Classification Based Mixed Sampling for Imbalanced Data
- The Influence of Trading Volume, Market Trend, and Monetary Policy on Characteristics of the Chinese Stock Exchange: An Econophysics Perspective
- Estimation of sand water content using GPR combined time-frequency analysis in the Ordos Basin, China
- Special Issue Applications of Nonlinear Dynamics
- Discrete approximate iterative method for fuzzy investment portfolio based on transaction cost threshold constraint
- Multi-objective performance optimization of ORC cycle based on improved ant colony algorithm
- Information retrieval algorithm of industrial cluster based on vector space
- Parametric model updating with frequency and MAC combined objective function of port crane structure based on operational modal analysis
- Evacuation simulation of different flow ratios in low-density state
- A pointer location algorithm for computer visionbased automatic reading recognition of pointer gauges
- A cloud computing separation model based on information flow
- Optimizing model and algorithm for railway freight loading problem
- Denoising data acquisition algorithm for array pixelated CdZnTe nuclear detector
- Radiation effects of nuclear physics rays on hepatoma cells
- Special issue: XXVth Symposium on Electromagnetic Phenomena in Nonlinear Circuits (EPNC2018)
- A study on numerical integration methods for rendering atmospheric scattering phenomenon
- Wave propagation time optimization for geodesic distances calculation using the Heat Method
- Analysis of electricity generation efficiency in photovoltaic building systems made of HIT-IBC cells for multi-family residential buildings
- A structural quality evaluation model for three-dimensional simulations
- WiFi Electromagnetic Field Modelling for Indoor Localization
- Modeling Human Pupil Dilation to Decouple the Pupillary Light Reflex
- Principal Component Analysis based on data characteristics for dimensionality reduction of ECG recordings in arrhythmia classification
- Blinking Extraction in Eye gaze System for Stereoscopy Movies
- Optimization of screen-space directional occlusion algorithms
- Heuristic based real-time hybrid rendering with the use of rasterization and ray tracing method
- Review of muscle modelling methods from the point of view of motion biomechanics with particular emphasis on the shoulder
- The use of segmented-shifted grain-oriented sheets in magnetic circuits of small AC motors
- High Temperature Permanent Magnet Synchronous Machine Analysis of Thermal Field
- Inverse approach for concentrated winding surface permanent magnet synchronous machines noiseless design
- An enameled wire with a semi-conductive layer: A solution for a better distibution of the voltage stresses in motor windings
- High temperature machines: topologies and preliminary design
- Aging monitoring of electrical machines using winding high frequency equivalent circuits
- Design of inorganic coils for high temperature electrical machines
- A New Concept for Deeper Integration of Converters and Drives in Electrical Machines: Simulation and Experimental Investigations
- Special Issue on Energetic Materials and Processes
- Investigations into the mechanisms of electrohydrodynamic instability in free surface electrospinning
- Effect of Pressure Distribution on the Energy Dissipation of Lap Joints under Equal Pre-tension Force
- Research on microstructure and forming mechanism of TiC/1Cr12Ni3Mo2V composite based on laser solid forming
- Crystallization of Nano-TiO2 Films based on Glass Fiber Fabric Substrate and Its Impact on Catalytic Performance
- Effect of Adding Rare Earth Elements Er and Gd on the Corrosion Residual Strength of Magnesium Alloy
- Closed-die Forging Technology and Numerical Simulation of Aluminum Alloy Connecting Rod
- Numerical Simulation and Experimental Research on Material Parameters Solution and Shape Control of Sandwich Panels with Aluminum Honeycomb
- Research and Analysis of the Effect of Heat Treatment on Damping Properties of Ductile Iron
- Effect of austenitising heat treatment on microstructure and properties of a nitrogen bearing martensitic stainless steel
- Special Issue on Fundamental Physics of Thermal Transports and Energy Conversions
- Numerical simulation of welding distortions in large structures with a simplified engineering approach
- Investigation on the effect of electrode tip on formation of metal droplets and temperature profile in a vibrating electrode electroslag remelting process
- Effect of North Wall Materials on the Thermal Environment in Chinese Solar Greenhouse (Part A: Experimental Researches)
- Three-dimensional optimal design of a cooled turbine considering the coolant-requirement change
- Theoretical analysis of particle size re-distribution due to Ostwald ripening in the fuel cell catalyst layer
- Effect of phase change materials on heat dissipation of a multiple heat source system
- Wetting properties and performance of modified composite collectors in a membrane-based wet electrostatic precipitator
- Implementation of the Semi Empirical Kinetic Soot Model Within Chemistry Tabulation Framework for Efficient Emissions Predictions in Diesel Engines
- Comparison and analyses of two thermal performance evaluation models for a public building
- A Novel Evaluation Method For Particle Deposition Measurement
- Effect of the two-phase hybrid mode of effervescent atomizer on the atomization characteristics
- Erratum
- Integrability analysis of the partial differential equation describing the classical bond-pricing model of mathematical finance
- Erratum to: Energy converting layers for thin-film flexible photovoltaic structures
Artikel in diesem Heft
- Regular Articles
- Non-equilibrium Phase Transitions in 2D Small-World Networks: Competing Dynamics
- Harmonic waves solution in dual-phase-lag magneto-thermoelasticity
- Multiplicative topological indices of honeycomb derived networks
- Zagreb Polynomials and redefined Zagreb indices of nanostar dendrimers
- Solar concentrators manufacture and automation
- Idea of multi cohesive areas - foundation, current status and perspective
- Derivation method of numerous dynamics in the Special Theory of Relativity
- An application of Nwogu’s Boussinesq model to analyze the head-on collision process between hydroelastic solitary waves
- Competing Risks Model with Partially Step-Stress Accelerate Life Tests in Analyses Lifetime Chen Data under Type-II Censoring Scheme
- Group velocity mismatch at ultrashort electromagnetic pulse propagation in nonlinear metamaterials
- Investigating the impact of dissolved natural gas on the flow characteristics of multicomponent fluid in pipelines
- Analysis of impact load on tubing and shock absorption during perforating
- Energy characteristics of a nonlinear layer at resonant frequencies of wave scattering and generation
- Ion charge separation with new generation of nuclear emulsion films
- On the influence of water on fragmentation of the amino acid L-threonine
- Formulation of heat conduction and thermal conductivity of metals
- Displacement Reliability Analysis of Submerged Multi-body Structure’s Floating Body for Connection Gaps
- Deposits of iron oxides in the human globus pallidus
- Integrability, exact solutions and nonlinear dynamics of a nonisospectral integral-differential system
- Bounds for partition dimension of M-wheels
- Visual Analysis of Cylindrically Polarized Light Beams’ Focal Characteristics by Path Integral
- Analysis of repulsive central universal force field on solar and galactic dynamics
- Solitary Wave Solution of Nonlinear PDEs Arising in Mathematical Physics
- Understanding quantum mechanics: a review and synthesis in precise language
- Plane Wave Reflection in a Compressible Half Space with Initial Stress
- Evaluation of the realism of a full-color reflection H2 analog hologram recorded on ultra-fine-grain silver-halide material
- Graph cutting and its application to biological data
- Time fractional modified KdV-type equations: Lie symmetries, exact solutions and conservation laws
- Exact solutions of equal-width equation and its conservation laws
- MHD and Slip Effect on Two-immiscible Third Grade Fluid on Thin Film Flow over a Vertical Moving Belt
- Vibration Analysis of a Three-Layered FGM Cylindrical Shell Including the Effect Of Ring Support
- Hybrid censoring samples in assessment the lifetime performance index of Chen distributed products
- Study on the law of coal resistivity variation in the process of gas adsorption/desorption
- Mapping of Lineament Structures from Aeromagnetic and Landsat Data Over Ankpa Area of Lower Benue Trough, Nigeria
- Beta Generalized Exponentiated Frechet Distribution with Applications
- INS/gravity gradient aided navigation based on gravitation field particle filter
- Electrodynamics in Euclidean Space Time Geometries
- Dynamics and Wear Analysis of Hydraulic Turbines in Solid-liquid Two-phase Flow
- On Numerical Solution Of The Time Fractional Advection-Diffusion Equation Involving Atangana-Baleanu-Caputo Derivative
- New Complex Solutions to the Nonlinear Electrical Transmission Line Model
- The effects of quantum spectrum of 4 + n-dimensional water around a DNA on pure water in four dimensional universe
- Quantum Phase Estimation Algorithm for Finding Polynomial Roots
- Vibration Equation of Fractional Order Describing Viscoelasticity and Viscous Inertia
- The Errors Recognition and Compensation for the Numerical Control Machine Tools Based on Laser Testing Technology
- Evaluation and Decision Making of Organization Quality Specific Immunity Based on MGDM-IPLAO Method
- Key Frame Extraction of Multi-Resolution Remote Sensing Images Under Quality Constraint
- Influences of Contact Force towards Dressing Contiguous Sense of Linen Clothing
- Modeling and optimization of urban rail transit scheduling with adaptive fruit fly optimization algorithm
- The pseudo-limit problem existing in electromagnetic radiation transmission and its mathematical physics principle analysis
- Chaos synchronization of fractional–order discrete–time systems with different dimensions using two scaling matrices
- Stress Characteristics and Overload Failure Analysis of Cemented Sand and Gravel Dam in Naheng Reservoir
- A Big Data Analysis Method Based on Modified Collaborative Filtering Recommendation Algorithms
- Semi-supervised Classification Based Mixed Sampling for Imbalanced Data
- The Influence of Trading Volume, Market Trend, and Monetary Policy on Characteristics of the Chinese Stock Exchange: An Econophysics Perspective
- Estimation of sand water content using GPR combined time-frequency analysis in the Ordos Basin, China
- Special Issue Applications of Nonlinear Dynamics
- Discrete approximate iterative method for fuzzy investment portfolio based on transaction cost threshold constraint
- Multi-objective performance optimization of ORC cycle based on improved ant colony algorithm
- Information retrieval algorithm of industrial cluster based on vector space
- Parametric model updating with frequency and MAC combined objective function of port crane structure based on operational modal analysis
- Evacuation simulation of different flow ratios in low-density state
- A pointer location algorithm for computer visionbased automatic reading recognition of pointer gauges
- A cloud computing separation model based on information flow
- Optimizing model and algorithm for railway freight loading problem
- Denoising data acquisition algorithm for array pixelated CdZnTe nuclear detector
- Radiation effects of nuclear physics rays on hepatoma cells
- Special issue: XXVth Symposium on Electromagnetic Phenomena in Nonlinear Circuits (EPNC2018)
- A study on numerical integration methods for rendering atmospheric scattering phenomenon
- Wave propagation time optimization for geodesic distances calculation using the Heat Method
- Analysis of electricity generation efficiency in photovoltaic building systems made of HIT-IBC cells for multi-family residential buildings
- A structural quality evaluation model for three-dimensional simulations
- WiFi Electromagnetic Field Modelling for Indoor Localization
- Modeling Human Pupil Dilation to Decouple the Pupillary Light Reflex
- Principal Component Analysis based on data characteristics for dimensionality reduction of ECG recordings in arrhythmia classification
- Blinking Extraction in Eye gaze System for Stereoscopy Movies
- Optimization of screen-space directional occlusion algorithms
- Heuristic based real-time hybrid rendering with the use of rasterization and ray tracing method
- Review of muscle modelling methods from the point of view of motion biomechanics with particular emphasis on the shoulder
- The use of segmented-shifted grain-oriented sheets in magnetic circuits of small AC motors
- High Temperature Permanent Magnet Synchronous Machine Analysis of Thermal Field
- Inverse approach for concentrated winding surface permanent magnet synchronous machines noiseless design
- An enameled wire with a semi-conductive layer: A solution for a better distibution of the voltage stresses in motor windings
- High temperature machines: topologies and preliminary design
- Aging monitoring of electrical machines using winding high frequency equivalent circuits
- Design of inorganic coils for high temperature electrical machines
- A New Concept for Deeper Integration of Converters and Drives in Electrical Machines: Simulation and Experimental Investigations
- Special Issue on Energetic Materials and Processes
- Investigations into the mechanisms of electrohydrodynamic instability in free surface electrospinning
- Effect of Pressure Distribution on the Energy Dissipation of Lap Joints under Equal Pre-tension Force
- Research on microstructure and forming mechanism of TiC/1Cr12Ni3Mo2V composite based on laser solid forming
- Crystallization of Nano-TiO2 Films based on Glass Fiber Fabric Substrate and Its Impact on Catalytic Performance
- Effect of Adding Rare Earth Elements Er and Gd on the Corrosion Residual Strength of Magnesium Alloy
- Closed-die Forging Technology and Numerical Simulation of Aluminum Alloy Connecting Rod
- Numerical Simulation and Experimental Research on Material Parameters Solution and Shape Control of Sandwich Panels with Aluminum Honeycomb
- Research and Analysis of the Effect of Heat Treatment on Damping Properties of Ductile Iron
- Effect of austenitising heat treatment on microstructure and properties of a nitrogen bearing martensitic stainless steel
- Special Issue on Fundamental Physics of Thermal Transports and Energy Conversions
- Numerical simulation of welding distortions in large structures with a simplified engineering approach
- Investigation on the effect of electrode tip on formation of metal droplets and temperature profile in a vibrating electrode electroslag remelting process
- Effect of North Wall Materials on the Thermal Environment in Chinese Solar Greenhouse (Part A: Experimental Researches)
- Three-dimensional optimal design of a cooled turbine considering the coolant-requirement change
- Theoretical analysis of particle size re-distribution due to Ostwald ripening in the fuel cell catalyst layer
- Effect of phase change materials on heat dissipation of a multiple heat source system
- Wetting properties and performance of modified composite collectors in a membrane-based wet electrostatic precipitator
- Implementation of the Semi Empirical Kinetic Soot Model Within Chemistry Tabulation Framework for Efficient Emissions Predictions in Diesel Engines
- Comparison and analyses of two thermal performance evaluation models for a public building
- A Novel Evaluation Method For Particle Deposition Measurement
- Effect of the two-phase hybrid mode of effervescent atomizer on the atomization characteristics
- Erratum
- Integrability analysis of the partial differential equation describing the classical bond-pricing model of mathematical finance
- Erratum to: Energy converting layers for thin-film flexible photovoltaic structures

