Abstract
C18H21N5O2, monoclinic, P21/c (no. 4), a = 10.0914(6) Å, b = 11.8919(7) Å, c = 15.545(1) Å, β = 102.321(6)°, V = 1822.5(2) Å3, Z = 4, R gt (F) = 0.0503, wR ref (F 2) = 0.1419, T = 293(2) K.
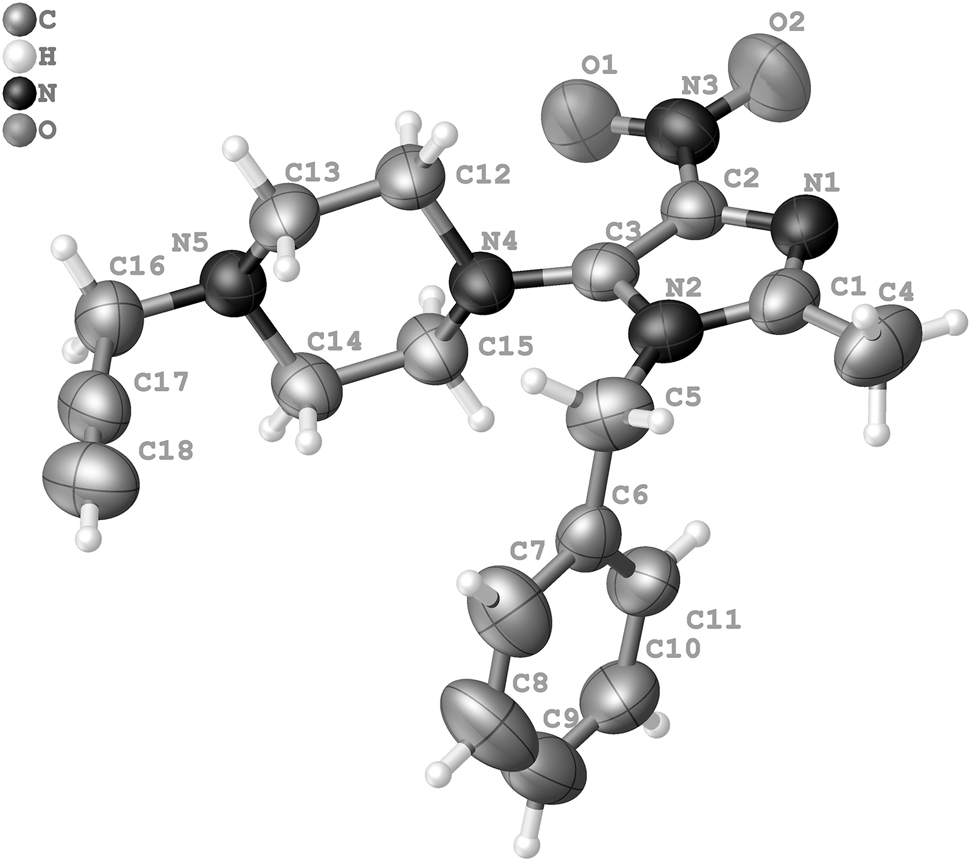
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow needle |
| Size: | 0.12 × 0.08 × 0.02 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θ max, completeness: | 29.5°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 27,507, 4593, 0.029 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 2681 |
| N(param)refined: | 238 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.39788 (18) | 0.85662 (14) | 0.39620 (13) | 0.0678 (5) |
| C2 | 0.50765 (16) | 0.85408 (13) | 0.52762 (11) | 0.0571 (4) |
| C3 | 0.59947 (16) | 0.81652 (12) | 0.48078 (10) | 0.0546 (4) |
| C4 | 0.2893 (3) | 0.8728 (2) | 0.31574 (19) | 0.0961 (8) |
| H4A | 0.275 (2) | 0.801 (2) | 0.2832 (15) | 0.104 (7)* |
| H4B | 0.206 (4) | 0.889 (3) | 0.334 (2) | 0.177 (15)* |
| H4C | 0.313 (2) | 0.931 (2) | 0.2772 (17) | 0.124 (9)* |
| C5 | 0.5772 (2) | 0.78057 (17) | 0.31923 (12) | 0.0787 (5) |
| H5A | 0.6695 | 0.8077 | 0.3255 | 0.094* |
| H5B | 0.5231 | 0.8146 | 0.2666 | 0.094* |
| C6 | 0.57666 (16) | 0.65507 (15) | 0.30680 (10) | 0.0610 (4) |
| C7 | 0.6547 (2) | 0.6106 (2) | 0.25229 (14) | 0.0929 (7) |
| H7 | 0.7078 | 0.6580 | 0.2260 | 0.111* |
| C8 | 0.6548 (3) | 0.4979 (3) | 0.23653 (18) | 0.1177 (9) |
| H8 | 0.7054 | 0.4698 | 0.1978 | 0.141* |
| C9 | 0.5823 (3) | 0.4256 (2) | 0.27636 (17) | 0.1012 (7) |
| H9 | 0.5849 | 0.3487 | 0.2663 | 0.121* |
| C10 | 0.5061 (2) | 0.46734 (19) | 0.33090 (16) | 0.0926 (7) |
| H10 | 0.4561 | 0.4187 | 0.3585 | 0.111* |
| C11 | 0.50232 (19) | 0.58224 (17) | 0.34585 (14) | 0.0791 (6) |
| H11 | 0.4487 | 0.6101 | 0.3828 | 0.095* |
| C12 | 0.83549 (17) | 0.87279 (14) | 0.52832 (13) | 0.0686 (5) |
| H12A | 0.8435 | 0.8849 | 0.5909 | 0.082* |
| H12B | 0.8079 | 0.9429 | 0.4979 | 0.082* |
| C13 | 0.97004 (17) | 0.83487 (14) | 0.51051 (13) | 0.0692 (5) |
| H13A | 0.9623 | 0.8260 | 0.4476 | 0.083* |
| H13B | 1.0385 | 0.8915 | 0.5314 | 0.083* |
| C14 | 0.90817 (17) | 0.64230 (14) | 0.52541 (13) | 0.0652 (4) |
| H14A | 0.9360 | 0.5726 | 0.5565 | 0.078* |
| H14B | 0.8999 | 0.6287 | 0.4630 | 0.078* |
| C15 | 0.77311 (17) | 0.67826 (14) | 0.54216 (13) | 0.0662 (5) |
| H15A | 0.7051 | 0.6215 | 0.5204 | 0.079* |
| H15B | 0.7791 | 0.6872 | 0.6049 | 0.079* |
| C16 | 1.14421 (18) | 0.69196 (17) | 0.54303 (13) | 0.0743 (5) |
| H16A | 1.1727 | 0.6279 | 0.5811 | 0.089* |
| H16B | 1.2087 | 0.7521 | 0.5621 | 0.089* |
| C17 | 1.14980 (19) | 0.66082 (16) | 0.45276 (15) | 0.0742 (5) |
| C18 | 1.1442 (3) | 0.63501 (19) | 0.37957 (18) | 0.0970 (7) |
| H18 | 1.1398 | 0.6145 | 0.3213 | 0.116* |
| N1 | 0.38429 (14) | 0.87939 (12) | 0.47618 (11) | 0.0666 (4) |
| N2 | 0.52531 (14) | 0.81809 (11) | 0.39517 (9) | 0.0626 (4) |
| N3 | 0.53046 (17) | 0.87016 (12) | 0.62039 (10) | 0.0692 (4) |
| N4 | 0.73480 (13) | 0.78517 (10) | 0.49693 (9) | 0.0572 (3) |
| N5 | 1.01089 (13) | 0.72848 (11) | 0.55473 (9) | 0.0609 (4) |
| O1 | 0.64251 (15) | 0.84369 (13) | 0.66527 (8) | 0.0857 (4) |
| O2 | 0.44060 (16) | 0.91050 (15) | 0.65223 (10) | 0.1051 (5) |
Source of materials
The commercially available 2-methyl-4-nitro-1H-imidazole was reacted with benzyl chloride to produced 1-benzyl-2-methyl-4-nitro-1H-imidazole 1 [5]. Bromination of 1 with liquid bromine in DMF, in the presence potassium carbonate as a base afforded 1-benzyl-5-bromo-2-methyl-4-nitro-1H-imidazole 2 [5]. Reaction of 2 with piperazine using isopropanol as solvent gave 1-(1-benzyl-2-methyl-4-nitro-1H-imidazol-5-yl)piperazine 3 [5]. The title compound 4 has been prepared via reaction of the piperazine derivative 3 with propargyl bromide using NaH as base to produce the new compound 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine 4 as yellow flakes in 82% yield. M.p. = 122–124°. HRMS-ESI m/z: 362.15719 (M + Na)+. 1H NMR (500 MHz, DMSO-d 6, 298 K): δ 2.25 (s, 3H, H–C4); 2.46 (br.s, 4H, H–C13, H–C14); 2.99 (br.s, 4H, H–C12, H–C15); 3.17 (t, 1H, J = 2.2, H–C18); 3.28 (d, 2H, J = 2.2, H–C16); 5.17 (s, 2H, H–C5); 7.12 (d, 2H, J = 7.4 Hz, H–C7, H–C11); 7.32 (t, 1H, J = 7.3 Hz, H–C9); 7.39 (pseudo t, 2H, H–C8, H–C10). 13C NMR (125 MHz, DMSO-d 6, 298 K): δ 14.1 (C4); 46.2 (C5); 46.4 (C16); 48.5 (C12, C15); 51.4 (C13, C14); 76.3 (C17); 79.6 (C18); 126.9 (C7, C11); 128.1 (C9); 129.4 (C8, C10); 136.6 (C6); 138.8 (C2); 140.2 (C1); 140.9 (C3).
The chemicals were purchased from Aldrich (Germany), Fluka (Switzerland), and Scharlau; they were used without further purification. The NMR spectra were recorded on a Bruker Avance III-(500 MHz) spectrometer with tetramethylsilane (TMS) as an internal standard. 1H NMR (500 MHz), 13C-NMR (125 MHz), and 2D were recorded in Dimethylsulfoxide (DMSO-d 6). The following abbreviations are used to describe peak patterns: s = singlet, t = triplet, br.s = broad singlet. High-resolution mass spectra (HRMS) were measured (in positive/or negative ion mode) using the electrospray ion trap (ESI pos low mass) technique by collision-induced dissociation on a Bruker APEX-IV (7 Tesla) instrument. Melting points were determined on a Stuart (SMP-10) melting point apparatus.
Single crystal of C18H21N5O2 was obtained through crystallization of the pure compound from CHCl3/Et2O.
Experimental details
A suitable crystal was selected and mounted on a Xcalibur, Eos diffractometer. The crystal was kept at 293(2) K during data collection. Using Olex2 [2], the structure was solved with the ShelXT [3] structure solution program using Intrinsic Phasing and refined with the ShelXL [4] refinement package. All H atoms bonded to C atoms were refined as riding, with C–H distances of 0.93 Å (for aromatic ring).
Comment
Imidazole is an important nucleus with diverse biological properties. The high therapeutic properties of the imidazole-related drugs have encouraged medicinal chemists to design and synthesize a large number of novel chemotherapeutic agents like Pantoprazole [6] (a proton pump inhibitor used for treatment of gastroesophageal reflux disease), Trifenagrel [7] (a potent arachidonate cyclooxygenase inhibitor), Eprosartan [8] (an angiotensin II receptor antagonist), and Cimetidine [9] (a histamine H2 receptor antagonist that inhibits stomach acid production). In view of the interest in the activity spectrum and profile of the nitroimidazoles and in continuation of our research on the synthesis and biological evaluation of imidazole analogs [10], [11], [12], [13], [14], [15], [16], [17], we here present the imidazolyl containing title structure. Herein, the propargyl bromide was couples with imidazole ring to produce the target compound.
This title crystal structure consists of the C18H21N5O2 molecules, in which all bond lengths are in normal ranges. The crystal is stabilized via CH⃛O (H18⃛O1 = 2.482(1) Å) and CH⃛O (H4A⃛O1 = 2.66(3) Å). The average plane of imidazole ring is perpendicular (90.17(7)°) to the average plane of the methylene groups of piperazine (C12–C13–C14–C15). A further intramolecular CH⃛O interactions can be also seen (H12A⃛O1 = 2.586(1) Å) and (H15B⃛O1 = 2.607(1) Å).
Funding source: Ministry of Higher Education, Jordan
Award Identifier / Grant number: Bas 1/1/2017
Acknowledgments
Part of this work has been carried out during sabbatical leave granted to RAA from the University of Jordan during the academic year 2020–2021.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was financially supported by Scientific Research Support Fund/Ministry of Higher Education, Jordan (grant no. Bas 1/1/2017).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO; Yarnton: England, 2015.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
5. Al-Soud, Y. A., Al-Ahmad, A. H., Abu-Qatouseh, L., Shtaiwi, A., Alhelal, K. A. S., Al-Suod, H. H., Alsawakhneh, S. O., Al-Qawasmeh, R. A. Nitroimidazoles Part 9. Synthesis, molecular docking, and anticancer evaluations of piperazine-tagged imidazole derivatives. Z. Naturforsch., B: Chem. Sci. 2021, B72, 293–302; https://doi.org/10.1515/znb-2020-0200.Suche in Google Scholar
6. Senn-Bilfiger, J., Sturn, E. The development of a new proton-pump inhibitor: the case history of pantoprazole. In Analogue-Based Drug Discovery; Fischer, J., Ganellin, C. R., Eds. Wiley-VCH: Weinheim, 2006, pp. 115–136.10.1002/3527608001.ch6Suche in Google Scholar
7. Abrahams, S. L., Hazen, R. J., Batson, A. G., Phillips, A. P. Trifenagrel:a chemically novel platelet aggregation inhibitor. J. Pharmacol. Exp. Therapeut. 1989, 249, 359–365.Suche in Google Scholar
8. Grange, R. L., Ziogas, J., North, A. J., Angus, J. A., Schiesser, C. H. Selenosartans: novel selenophene analogues of milfasartan and eprosartan. Bioorg. Med. Chem. Lett 2008, 18, 1241–1244.https://doi.org/10.1016/j.bmcl.2007.11.136.Suche in Google Scholar
9. Beggs, W. H., Andrews, F. A., Sarosi, G. A. Action of imidazole-containing antifungal drugs. Life Sci. 1981, 28, 111–118; https://doi.org/10.1016/0024-3205(81)90542-7.Suche in Google Scholar
10. Al-Masoudi, N. A., Al-Soud, Y. A., Kalogerakisa, A., Pannecouquec, C., De Clercqc, E. Nitroimidazoles part 2. Synthesis, antiviral and antitumor activity of new 4-nitroimidazoles. Chem. Biodivers. 2006, 3, 515–526; https://doi.org/10.1002/cbdv.200690055.Suche in Google Scholar PubMed
11. Al-Soud, Y. A., Al-Sa’doni, H., Amajaour, H. A. S., Al-Masoudi, N. A. Nitroimidazole part 3. Synthesis and anti-HIV activity of new N-alkyl 4-nitroimidazoles, bearing benzothiazole and benzoxazole backbones. Z. Naturforsch., B: Chem. Sci. 2007, B62, 523–528; https://doi.org/10.1515/znb-2007-0406.Suche in Google Scholar
12. Al-Soud, Y. A., Al-Masoudi, N. A., De Clercqc, E., Pannecouquec, C. Nitroimidazoles part 4. Synthesis and anti-HIV activity of new 5-alkylsulfanyl and 5-(4′-arylsulphonyl) piperazine 4-nitroimidazole derivatives. Heteroat. Chem. 2007, 18, 333–340; https://doi.org/10.1002/hc.20301.Suche in Google Scholar
13. Al-Soud, Y. A., Al-Masoudi, N. A., Hassan, H., De Clercq, E., Pannecouque, C. Nitroimidazoles v. Synthesis and anti-HIV evaluation of new 5-substituted piperazinyl-4-nitroimidazole derivatives. Acta Pharm. 2007, 57, 379–393; https://doi.org/10.2478/v10007-007-0031-7.Suche in Google Scholar PubMed
14. Al-Masoudi, N. A., Al-Soud, Y. A., De Clercq, E., Pannecouque, C. Nitroimidazoles part 6. Synthesis, structure and in vitro anti-HIV activity of new 5-substituted piperazinyl-4-nitroimidazole derivatives. Antiviral Chem. Chemother. 2007, 18, 191–200; https://doi.org/10.1177/095632020701800403.Suche in Google Scholar PubMed
15. Al-Soud, Y. A., Al-Masoudi, N. A., Al-Suod, H. H., Pannecouque, C. Nitroimidazoles part 8. Synthesis and anti-HIV activity of new 4-nitroimidazole derivatives using the suzuki cross-coupling reaction. Z. Naturforsch., B: Chem. Sci. 2012, B67, 925–934; https://doi.org/10.5560/znb.2012-0185.Suche in Google Scholar
16. Al-Soud, Y. A., Alhelal, K. A. S., Bahjat, A. S., Abu-Qatouseh, L., Al-Suod, H. H., Al-Ahmad, A. H., Al-Masoudi, N. A., Al-Qawasmeh, R. A. Synthesis, anticancer activity and molecular docking studies of new 4-nitroimidazole derivatives. Arkivoc 2021, viii, 296–309; https://doi.org/10.24820/ark.5550190.p011.479.Suche in Google Scholar
17. Huesca, M., Al-Qawasmeh, R. A., Young, A. H., Lee, Y. Aryl Imidazoles and Their Use as Anti-cancer Agents. U.S. Patent, 2015; p. 8969372.Suche in Google Scholar
© 2021 Raed A. Al-Qawasmeh et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27


