Abstract
C27H24N4O2 monoclinic, P21/c (no. 14), a = 5.8073(2) Å, b = 31.2564(9) Å, c = 24.8105(8) Å, β = 90.13°, V = 4503.5(2) Å3, Z = 8, Rgt(F) = 0.0644, wRref(F2) = 0.1854, T = 150(2) K.
Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Block |
| Size: | 0.19 × 0.18 × 0.17 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.67 mm−1 |
| Diffractometer, scan mode: | Bruker, φ and ω-scans |
| θmax, completeness: | 66°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13522, 6998, 0.058 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5443 |
| N(param)refined: | 604 |
| Programs: | Bruker programs [1], SHELX [2], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 1.0425(5) | 0.11384(9) | 0.24328(13) | 0.0351(7) |
| N2 | 1.1338(5) | 0.04868(8) | 0.28875(12) | 0.0315(6) |
| N3 | 0.9303(5) | 0.04699(9) | 0.20495(12) | 0.0324(6) |
| N4 | 1.0215(5) | −0.01517(9) | 0.25004(13) | 0.0389(8) |
| N5 | 0.5394(5) | 0.36321(9) | 0.19920(13) | 0.0341(7) |
| N6 | 0.4232(5) | 0.29812(8) | 0.24128(12) | 0.0317(6) |
| N7 | 0.6241(5) | 0.29671(8) | 0.15714(12) | 0.0319(6) |
| N8 | 0.5073(6) | 0.23435(9) | 0.19873(13) | 0.0390(8) |
| O1 | 1.2335(5) | 0.11476(8) | 0.32150(11) | 0.0403(6) |
| O2 | 0.8532(5) | 0.11169(7) | 0.16529(11) | 0.0406(6) |
| O3 | 0.3489(5) | 0.36429(7) | 0.27750(11) | 0.0394(6) |
| O4 | 0.7292(5) | 0.36153(8) | 0.12125(11) | 0.0399(6) |
| C1 | 1.5751(6) | 0.07623(12) | 0.34842(16) | 0.0366(8) |
| H1 | 1.6264 | 0.0761 | 0.3121 | 0.044* |
| C2 | 1.7076(6) | 0.05881(11) | 0.38842(16) | 0.0364(8) |
| H2 | 1.8522 | 0.0464 | 0.3798 | 0.044* |
| C3 | 1.6317(6) | 0.05909(11) | 0.44264(16) | 0.0334(8) |
| C4 | 1.7662(7) | 0.04089(12) | 0.48427(17) | 0.0406(9) |
| H4 | 1.9108 | 0.0283 | 0.4761 | 0.049* |
| C5 | 1.6897(8) | 0.04135(14) | 0.5358(2) | 0.0532(12) |
| H5 | 1.7809 | 0.0287 | 0.5634 | 0.064* |
| C6 | 1.4750(8) | 0.06052(13) | 0.54948(18) | 0.0469(10) |
| H6 | 1.4252 | 0.0610 | 0.5859 | 0.056* |
| C7 | 1.3397(7) | 0.07839(12) | 0.50970(17) | 0.0417(9) |
| H7 | 1.1956 | 0.0909 | 0.5186 | 0.050* |
| C8 | 1.4162(6) | 0.07808(10) | 0.45559(16) | 0.0322(8) |
| C9 | 1.2810(6) | 0.09590(10) | 0.41331(15) | 0.0324(8) |
| H9 | 1.1364 | 0.1088 | 0.4208 | 0.039* |
| C10 | 1.3619(6) | 0.09421(11) | 0.36213(15) | 0.0315(8) |
| C11 | 1.1321(6) | 0.09058(10) | 0.28290(15) | 0.0320(8) |
| C12 | 0.9434(6) | 0.08907(10) | 0.20643(15) | 0.0315(7) |
| C13 | 0.7102(6) | 0.09100(11) | 0.12761(16) | 0.0347(8) |
| C14 | 0.7709(6) | 0.09341(11) | 0.07486(16) | 0.0344(8) |
| H14 | 0.9132 | 0.1060 | 0.0647 | 0.041* |
| C15 | 0.6197(7) | 0.07690(11) | 0.03513(16) | 0.0357(9) |
| C16 | 0.6779(8) | 0.07841(13) | −0.02049(17) | 0.0462(10) |
| H16 | 0.8179 | 0.0912 | −0.0319 | 0.055* |
| C17 | 0.5286(10) | 0.06103(14) | −0.0574(2) | 0.0587(13) |
| H17 | 0.5672 | 0.0617 | −0.0945 | 0.070* |
| C18 | 0.3182(10) | 0.04211(14) | −0.0413(2) | 0.0601(14) |
| H18 | 0.2179 | 0.0303 | −0.0676 | 0.072* |
| C19 | 0.2588(8) | 0.04078(13) | 0.01185(19) | 0.0498(11) |
| H19 | 0.1169 | 0.0282 | 0.0224 | 0.060* |
| C20 | 0.3554(7) | 0.05669(12) | 0.10643(18) | 0.0431(9) |
| H20 | 0.2137 | 0.0443 | 0.1175 | 0.052* |
| C21 | 0.4092(7) | 0.05827(12) | 0.05161(17) | 0.0385(9) |
| C22 | 0.5038(7) | 0.07274(12) | 0.14474(17) | 0.0392(9) |
| H22 | 0.4665 | 0.0714 | 0.1820 | 0.047* |
| C23 | 1.0287(6) | 0.02736(11) | 0.24803(14) | 0.0304(8) |
| C24 | 1.1161(8) | −0.03971(12) | 0.29590(18) | 0.0467(10) |
| H24A | 1.1979 | −0.0652 | 0.2819 | 0.056* |
| H24B | 1.2300 | −0.0217 | 0.3152 | 0.056* |
| C25 | 0.9380(10) | −0.05380(15) | 0.3344(2) | 0.0658(14) |
| H25A | 0.8307 | −0.0733 | 0.3162 | 0.099* |
| H25B | 0.8536 | −0.0288 | 0.3478 | 0.099* |
| H25C | 1.0113 | −0.0686 | 0.3647 | 0.099* |
| C26a | 0.9123(8) | −0.04028(13) | 0.20664(19) | 0.0478(10) |
| H26Aa | 0.8312 | −0.0650 | 0.2227 | 0.057* |
| H26Ba | 0.7960 | −0.0223 | 0.1882 | 0.057* |
| H26Ca | 0.9740 | −0.0310 | 0.1714 | 0.057* |
| H26Da | 0.9500 | −0.0709 | 0.2114 | 0.057* |
| C27a | 1.076(2) | −0.0557(3) | 0.1673(4) | 0.0594(18) |
| H27Aa | 1.1447 | −0.0313 | 0.1484 | 0.089* |
| H27Ba | 0.9969 | −0.0741 | 0.1412 | 0.089* |
| H27Ca | 1.1972 | −0.0720 | 0.1856 | 0.089* |
| C27A | 0.6726(18) | −0.0350(3) | 0.2070(4) | 0.0594(18) |
| H27Da | 0.6130 | −0.0418 | 0.2429 | 0.089* |
| H27Ea | 0.6026 | −0.0542 | 0.1804 | 0.089* |
| H27Fa | 0.6346 | −0.0053 | 0.1979 | 0.089* |
| C28 | 0.2088(6) | 0.34473(11) | 0.31667(16) | 0.0351(8) |
| C29 | 0.2708(6) | 0.34842(11) | 0.36882(16) | 0.0361(8) |
| H29 | 0.4130 | 0.3616 | 0.3781 | 0.043* |
| C30 | 0.1234(7) | 0.33262(11) | 0.41012(16) | 0.0334(8) |
| C31 | 0.1821(7) | 0.33443(12) | 0.46515(16) | 0.0403(9) |
| H31 | 0.3229 | 0.3473 | 0.4759 | 0.048* |
| C32 | 0.0373(8) | 0.31770(13) | 0.50331(18) | 0.0502(12) |
| H32 | 0.0783 | 0.3192 | 0.5403 | 0.060* |
| C33 | −0.1677(8) | 0.29871(13) | 0.48833(19) | 0.0485(11) |
| H33 | −0.2659 | 0.2869 | 0.5151 | 0.058* |
| C34 | −0.2295(7) | 0.29683(12) | 0.43564(19) | 0.0450(10) |
| H34 | −0.3716 | 0.2838 | 0.4260 | 0.054* |
| C35 | −0.0875(6) | 0.31378(11) | 0.39474(16) | 0.0355(9) |
| C36 | −0.1483(7) | 0.31129(12) | 0.33946(16) | 0.0379(8) |
| H36 | −0.2907 | 0.2987 | 0.3292 | 0.046* |
| C37 | −0.0035(6) | 0.32677(12) | 0.30109(16) | 0.0371(8) |
| H37 | −0.0453 | 0.3255 | 0.2641 | 0.045* |
| C38 | 0.4394(6) | 0.33986(10) | 0.23771(15) | 0.0309(7) |
| C39 | 0.6280(6) | 0.33865(11) | 0.16087(15) | 0.0312(7) |
| C40 | 0.8579(6) | 0.33952(10) | 0.08214(15) | 0.0315(8) |
| C41 | 1.0685(6) | 0.32137(11) | 0.09718(16) | 0.0367(9) |
| H41 | 1.1169 | 0.3216 | 0.1338 | 0.044* |
| C42 | 1.2034(6) | 0.30331(11) | 0.05835(16) | 0.0359(8) |
| H42 | 1.3464 | 0.2908 | 0.0684 | 0.043* |
| C43 | 1.1358(6) | 0.30275(11) | 0.00397(16) | 0.0325(8) |
| C44 | 1.2720(7) | 0.28453(11) | −0.03711(17) | 0.0411(9) |
| H44 | 1.4157 | 0.2718 | −0.0281 | 0.049* |
| C45 | 1.2006(7) | 0.28486(13) | −0.08966(18) | 0.0481(10) |
| H45 | 1.2947 | 0.2723 | −0.1166 | 0.058* |
| C46 | 0.9911(8) | 0.30346(13) | −0.10412(18) | 0.0484(11) |
| H46 | 0.9425 | 0.3034 | −0.1407 | 0.058* |
| C47 | 0.8562(6) | 0.32180(12) | −0.06549(16) | 0.0376(8) |
| H47 | 0.7148 | 0.3349 | −0.0756 | 0.045* |
| C48 | 0.9234(6) | 0.32168(10) | −0.01065(15) | 0.0315(8) |
| C49 | 0.7856(6) | 0.34034(11) | 0.03025(15) | 0.0321(8) |
| H49 | 0.6428 | 0.3534 | 0.0212 | 0.039* |
| C50 | 0.5183(6) | 0.27707(10) | 0.19906(15) | 0.0307(8) |
| C51 | 0.4014(7) | 0.21090(12) | 0.24350(18) | 0.0448(10) |
| H51A | 0.3243 | 0.1849 | 0.2296 | 0.054* |
| H51B | 0.2838 | 0.2291 | 0.2610 | 0.054* |
| C52 | 0.5881(9) | 0.19825(14) | 0.2850(2) | 0.0581(12) |
| H52A | 0.7056 | 0.1807 | 0.2674 | 0.087* |
| H52B | 0.5172 | 0.1819 | 0.3143 | 0.087* |
| H52C | 0.6597 | 0.2241 | 0.2997 | 0.087* |
| C53 | 0.6119(8) | 0.20937(12) | 0.15564(18) | 0.0483(10) |
| H53A | 0.7001 | 0.1852 | 0.1711 | 0.058* |
| H53B | 0.7194 | 0.2275 | 0.1348 | 0.058* |
| C54 | 0.4187(12) | 0.19216(17) | 0.1181(2) | 0.0810(18) |
| H54A | 0.4861 | 0.1733 | 0.0908 | 0.122* |
| H54B | 0.3417 | 0.2162 | 0.1002 | 0.122* |
| H54C | 0.3063 | 0.1761 | 0.1395 | 0.122* |
aOccupancy: 0.5.
Source of material
To the solution of cyanuric chloride (3.69 g, 0.02 mol) and 2-naphthol (5.77 g, 0.04 mol) in tetrahydrofuran (50 mL) was added K2CO3 (5.52 g, 0.04 mol). The mixture was stirred at room temperature for 6 h and filtered. The filtrate was evaporated under reduced pressure to get a yellow solid, which was purified by silica gel to afford the intermediate product 2-chloro-4,6-bi(2-naphthoxyl)-1,3,5-triazine (4.90 g, yield 72%). To the solution of 2-chloro-4,6-bi(2-naphthoxyl)-1,3,5-triazine (2.00 g, 0.005 mol) in tetrahydrofuran (30 mL) was added HN(C2H5)2 (0.439 g, 0.006 mol) at room temperature. After the mixture was stirred for 2 h, the solvent was evaporated under reduced pressure to get a white solid, which was recrystallized with petroleum ether to afford the title compound (1.88 g, yield 86%) 1H NMR: 7.86–7.76 ppm (m, 6H), 7.65 ppm (s, 2H), 7.48 ppm (dd, J = 3.6 HZ, J = 4.8 HZ, 4H), 7.36 ppm (d, J = 8.8 HZ, 2H) 3.45 ppm (q, J = 6.8 HZ, 4H), 1.08 ppm (t, J = 6.8 HZ, 6H). Crystals were obtained by recrystallization with petroleum ether at room temperature.
Experimental details
The data were scaled and corrected for absorption using SADABS-2016/2 [1]. The hydrogen atoms were placed at calculated positions and refined as riding atoms with fixed isotropic displacement parameters. There is a 1/1 disorder at one of the ethyl groups (cf. table 2)
Discussion
Azo compounds containing a heterocyclic moiety were widely used in material field (such as molecular memory storages, nonlinear optical elements, printing system), pharmacy, agriculture, chemical industry and so on. As one of the most important class of azo compounds, s-triazines and its new derivative have drawn more and more attention of scientists from all over the world due to its diverse pharmacological activity [4] (such as antitumor [5], anti-inflammatory), biological activities (such as antibacteria [6], [7], antimycotic, herbicidal, insecticidal activities), high catalyst activity, abundant optoelectronic properties [8] and so on. In this paper, a new s-triazines derivative was synthesized by substitution reactions using 2,4,6-trichloro-1,3,5-triazine as starting material. Its structure was characterized by 1H NMR and X-ray single crystal diffraction.
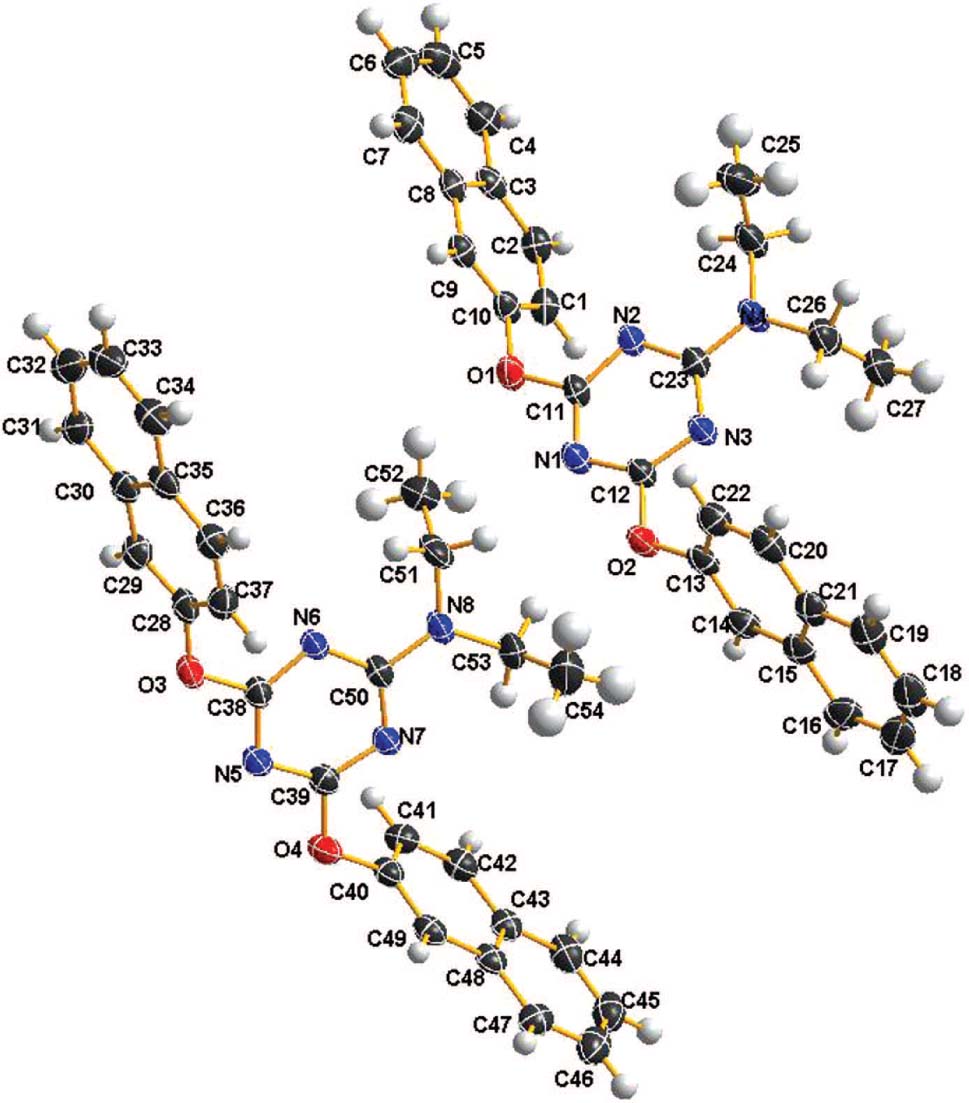
There are two cystallographically independent molecules (cf. the figure) in the asymmetric unit, in which all bond lengths are in normal ranges. The geometric parameters of both crystallographically independent molecules are similar and are in accord with another symmetrically substituted triazine [9]. In molecular packing diagram, there are obvious π-π stacking interactions between the adjacent naphthalenyl moieties. The distance between adjacent naphthalenyl moieties is less than 3.585 Å, which is within normal range [10]. No classic hydrogen bonds were observed as following: C51—H51B⋯N6 hydrogen bond (d(H51B⋯N6) = 2.36 Å), C53—H53A⋯O2 hydrogen bond (d(H53A⋯O2) = 2.47 Å) and C53—H53B⋯N7 hydrogen bond (d(H53B⋯N7) = 2.30 Å).
Acknowledgements
We acknowledge the supports by Key project of Shaanxi Provincial Education Department (17JS029), Natural Science Basic Research Program of Shaanxi (2017JM8070). China Postdoctoral Science Foundation funded project (2016M602994).
References
Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA (2012).Suche in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with ShelXL. Acta Crystallogr. C27 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar PubMed PubMed Central
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: Olex2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
Huang, W.; Zheng, W.; Urbanb, D. J.; Inglesea, J.; Sidransky, E.; Austina, C. P.; Thomas, C. J.: Bioorg. Med. Chem. Lett. 17 (2007) 5783–5789.10.1016/j.bmcl.2007.08.050Suche in Google Scholar PubMed PubMed Central
Shiva, K.-M.; Claire, B.; Daniel, S.; Christian, G.: Synthesis and cytotoxicity evaluation of aryl triazolic derivatives and their hydroxymethine homologues against B16 melanoma cell line. Eur. J. Med. Chem. 122 (2016) 436–441.10.1016/j.ejmech.2016.06.057Suche in Google Scholar PubMed
Zhao, H.; Liu, Y.; Cui, Z.; Beattie, D.; Gu, Y.; Wang, Q.: Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agr. Food Chem. 59 (2011) 11711–11717.10.1021/jf203383sSuche in Google Scholar PubMed
Pate, R. V.; Park, S. W.: Access to a new class of biologically active quinoline based 1,2,4-triazoles. Eur. J. Med. Chem. 71 (2014) 24–30.10.1016/j.ejmech.2013.10.059Suche in Google Scholar PubMed
Kima, S. H.; Debnatha, D.; Geckeler, K. E.: Nanopumpkins and a sunscreen agent: the inclusion complex of cucurbituril and Tinosorb S. SupramolChem. 23 (2011) 337–341.10.1080/10610278.2010.514609Suche in Google Scholar
Zuo, Z.; Lei, F.; Dai, Y.; Wang, L.: The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4. Z. Kristallogr. NCS 233 (2018) 555–557.10.1515/ncrs-2017-0292Suche in Google Scholar
Deng, L.; Liu, H.; Li, S.: Crystal structure of 4-(chloromethyl)-3-nitrobenzoic acid, C8H6ClNO4. Z. Kristallogr. NCS 232 (2017) 647–648.10.1515/ncrs-2016-0389Suche in Google Scholar
©2018 Zhenyu Zuo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S

