Abstract
Graphene is considered a promising catalyst candidate due to its 2D nature, single-atom thickness, zero bandgap and very high surface to volume ratio. Further, graphene oxide (GO) has been used as a catalytic support material for metal/metal oxide nanoparticles due to its tunable electrical properties. In addition, its high chemical stability and ultrahigh thermal conductivity may possibly promote high loading of catalytically active sites. This review article focuses on the recent progress in the catalytic applications of GO especially (i) as catalytic-support material (GO/reduced graphene oxide supported metal/metal oxide nanohybrids) for the green synthesis of biologically relevant molecules, (ii) for metal-free catalysis and (iii) for electrocatalysis, with special focus on graphene contribution to catalytic efficiency. The critical overview and future perspectives are also discussed.
1 Introduction
Researchers have been paying more attention to environmental issues in recent years because pollution is growing day by day, which poses a serious threat to the environment in particular, humans, plants and animals [1]. The design and development of cutting-edge environmentally friendly synthetic approaches using well-organized catalytic systems has gained significant attention in recent years to mitigate the toxic effects of pollutants [2]. Many interesting and attractive catalysts have recently been implemented in this context and are rapidly growing at a faster pace to meet the ecological demands [3,4]. Although homogeneous catalysts bestow advantages of excellent selectivity and high catalytic activity and have been employed in petrochemical industry for the manufacture of several significant value-added consumer products, they still possess several disadvantages of low thermal stability, non-reusability and, hence, non-recyclability [5]. Application of selective, highly active and energy-efficient heterogeneous catalyst in organic synthesis is a thought-provoking area and is a key to sustainable development [6]. Among these are metal oxide nanoparticles (NPs) which are well-known to catalyze various organic transformations [7,8,9]. Further, variety of heterogeneous catalytic systems viz. metal-free carbon nanomaterials, including fullerene, carbon nanotube (CNT), graphite, graphene and carbon nanodots, have been explored either as an efficient carbocatalyst or as a metal oxide support for various catalytic applications [10,11,12,13,14,15,16,17,18]. However, there is still a huge scope for the expansion of suitable catalysts that not only provide high activity and selectivity but also provide a greener route to economic development. Graphene, a single layer of bonded sp2 hybridized carbon atoms with a 2D flattened honeycomb-like structure discovered in 2004 [19], recently attracted considerable interest from researchers worldwide [20] (Figure 1). While its presence was predicted decades ago [21] and experimentally recognized in 1962 by Boehm et al., it was first isolated by Andre Geim and Konstantin Novoselov (2004) by the mechanical cleavage of graphite crystal at the University of Manchester. It is the basic building block of other carbon allotropes, and graphene can be wrapped to create 0D fullerenes, rolled up to form 1D CNTs and stacked to make 3D graphite [22]. It has excellent thermal, optical and mechanical properties that indicate its potential candidacy for many applications [23,24]. Other expectional properties comprise superior thermal conductivity (∼5,000 W/m/K) [25], high planar surface (2,630 m2/g) [26], ultrahigh electron mobility (2,00,000 cm2 V−1 s−1) [27], superlative mechanical strength (Young’s modulus, ∼1,100 GPa) [28] and outstanding electronic properties [29,30] which bestow graphene with great application prospects in a number of fields [31,32,33]. The number of investigations on graphene applications and its derivatives in heterogeneous catalysis has increased exponentially [34]. Since the last decade, graphene emerged as incomparable 2D supports and catalysts for various catalytic reactions due to its single-atom thickness and an incredibly high surface to volume ratio [20]. In addition, its high chemical stability and ultrahigh thermal conductivity may possibly promote high loading of catalytically active sites [20,25], and its high electrical conductivity renders it suitable for electrochemical processes. Because of these properties, graphene remains intact and resistant to degradation at high temperature, even in the presence of extremely acidic or alkaline media as well.
![Figure 1 Schematic depictions about the origin (presenting the transformation) of graphene from graphite and peculiar structure of graphite and graphene. Reproduced from [58].](/document/doi/10.1515/gps-2020-0055/asset/graphic/j_gps-2020-0055_fig_013.jpg)
Schematic depictions about the origin (presenting the transformation) of graphene from graphite and peculiar structure of graphite and graphene. Reproduced from [58].
Graphene exhibits properties of zero bandgap and very low density of states around the Fermi level (Ef), which make graphene excellent material in catalytic applications among other 2D materials since the interactions between the graphene and the catalyst supported are highly tunable by physical or chemical method [20]. Chemical inertness of pristine graphene is well established from the small adsorption energies and limited charge transfer of the simulated adsorptions of various clusters and small gaseous molecules on it [20,35,36], and discovering effective ways of activating graphene is the major challenge in graphene-based catalysis that can be accomplished either by the introduction of defects [37,38] or by doping the substrate underneath epitaxial graphene [39] (Figure 2).
![Figure 2 Different forms of graphene: (a) graphene oxide, (b) pristine graphene, (c) functionalized graphene, (d) graphene quantum dot and (e) reduced graphene oxide. Reproduced from [58].](/document/doi/10.1515/gps-2020-0055/asset/graphic/j_gps-2020-0055_fig_014.jpg)
Different forms of graphene: (a) graphene oxide, (b) pristine graphene, (c) functionalized graphene, (d) graphene quantum dot and (e) reduced graphene oxide. Reproduced from [58].
Graphene oxide (GO) is a single graphitic layer with randomly distributed sp2 carbon atoms and sp3 carbon atoms containing hydroxyl, epoxy, carbonyl and carboxyl functional groups. The epoxy and hydroxyl groups lie above and below each layer of graphene and the carboxylic groups typically reside at the edges of the layers. The existence of various oxygen-containing groups on the GO surface offers outstanding hydrophilic character and analogous chemical reactivity [37]. In addition, the functional groups on the surface of GO serve as effective anchoring sites for immobilizing various catalytically active species. GO also possesses dynamic electronic properties and is usually insulated by the large portion of sp3 hybridized carbon atoms bonded to oxygen-containing groups, resulting in sheet resistance of ∼1,012 sq−1 or higher [40,41]. However, the sheet resistance of reduced GO (i.e., RGO) after GO reduction can be degraded by several orders of magnitude, thereby converting the material into a semiconductor or even a graphene-like assembly. It is well demonstrated that bandgap of GO can be tailored by controlling coverage, arrangement and relative ratio of the epoxy and hydroxyl groups [42–44]. The similar mechanical, optoelectronic or conductive graphene-like properties of reduced graphene make it highly desirable material to be used in a surfeit of application including catalysis due to its heterogeneous structure comprising graphene-like basal plane additionally decorated with structural defects and occupied with areas containing oxidized chemical groups [45].
Despite the aforementioned fascinating properties, GO and RGO do have some disadvantages for practical applications. The combination of structural defects, poor dispersion, restacking and multilayer thickness can affect the electrical properties and high surface area of GO materials [46]. The insulating nature of regular GO also limits its applications in electronic devices and energy storage. Furthermore, the residual defects and holes degrade the electronic quality of RGO [47]. The oxygenated groups will extend GO’s structural/chemical diversity to a large extent through more chemical modification or functionalization, providing an efficient way to tailor GO’s physical and chemical properties to planned rates. Consequently, GO and GO-based composites have demonstrated great potential in the energy storage/conversion and environmental protection applications [48].
GO, an oxidized form of pristine graphene, carries negative charge due to the presence of oxygen containing functional groups on the surfaces and hydrophilic GO can be further reduced to form hydrophobic rGO. Graphene has further attracted great interest as a promising nanomaterial for a variety of bioapplications because of its extraordinary properties. However, both GO and rGO are known to induce cytotoxicity, DNA damage and oxidative stress in mammalian cells. In vitro studies show that graphene induces cytotoxicity as a result of increased formation of reactive oxygen species and also as a result of disrupted mitochondrial membrane potential eventually leads cells to apoptosis while surface modifications can significantly reduce their toxic interactions with living systems [49].
The discovery of graphene has brought a massive development and new dimension to material science and nanotechnology. In fact, graphene has shown great talent in all fields of science and technology and has a wide variety of applications, from health to aerospace including catalysis. Hence, modern graphene work has concentrated on exploring new graphene derivatives for their use in product and device development and heterogeneous catalysis through functionalization or surface modification, due to their multidisciplinary capabilities.
Many interesting studies related to the multiple applications of graphene-based nanocomposites have been reported in the field of energy and environmental science [50], biomedical engineering [51], drug delivery and tissue engineering [52], agricultural production and crop protection [53], water treatment, supercapacitor electrodes [54], high-performance sensor applications [55], biosensors and bioelectronics [56] and many more [57]. Besides the above applications, recent research update on graphene/graphene-related materials and their engineering applications in different fields of science and technology is reported by Tiwari et al. [58]. Some fundamental aspects of graphene, GO, graphene quantum dots, graphene nanoribbon, etc., are also discussed followed by progress in materials engineering concerning graphene covering materials fabrication, detailed properties and applications. Navalón et al. [59] highlighted the achievements of graphene-based materials in green catalytic processes. The synthesis and features in carbocatalysis including hydrocarbon conversions and environmental purification, covering advanced oxidation processes, organic synthesis, selective oxidation and hydrogenation reactions have also been elucidated. In a review article published by Yu et al. [60], the basic structure, preparation methods and properties of graphene and GO, methods for the reduction of GO, functionalization of graphene and GO consisting of covalent binding modification, noncovalent binding modification and elemental doping are described.
Despite excellent research on the use of graphene-based heterogeneous catalysts in organic transformations of simple and complex molecules [18,20,34], there are only a few literature reports [61–63] related to the investigations based on the use of GO/rGO as a catalyst/catalyst support for the green synthesis of biologically relevant molecules. The use of a number of oxygenated groups in particular, viz. hydroxyl (OH), alcoxy (C–O–C), carbonyl (C═O), carboxylic acid (COOH) and other oxygen-based functional groups necessary for surface functionality; a large number of defects; and a special 2D structure have made GO the perfect material in catalytic field [64–67]. In addition, the negatively charged surface of GO can be easily exploited to spread other catalytically active materials on its surface, including NPs of metal and metal oxide, to enhance their resultant properties [68,69]. For this purpose, many metal and/or metal oxide NPs such as Cu [70], Ni [71], Au [72,73], Ag/CeO2 [74], Pd [75] and Pt [76] have been incorporated into the GO/rGO. Furthermore, properties similar to that of pristine graphene can be achieved by rGO, which has been further examined for manifold catalytic applications [77–80]. Recently, many environmentally sound alternative green protocols for the synthesis of biologically active heterocyclic compounds using nanocatalysts [81,82] have been researched. Keeping in view the multiple role of graphene in catalysis and the importance of pharmacologically active compounds in medicinal field, it became a necessity to write a review article on expeditious protocols involving graphene-based nanocomposites as catalyst for the synthesis of biologically significant molecules. The present review article is aimed at supporting the scientific community on this subject. Today’s main challenge is to achieve sustainable chemical production with lower energy usage and less effects on the environment, and nanocatalysis contributes significantly to the implementation of green chemistry principles. In this context, academic and industrial scientists need a timely update on recent advances in the graphene-based nanocatalysis. Although several research articles have been published on the catalytic applications of GO and reduced nanosheets of GO as effective carbocatalyst, the current review article summarizes the detailed investigation on GO as (i) an excellent catalytic support material (GO-/rGO-supported metal/metal oxide nanohybrids) for the synthesis of diverse bioactive heterocyclic compounds, (ii) a green metal-free catalyst and (iii) an electrocatalyst for some reactions of industrial importance and also offers an insight on critical overview and future perspectives. An overview of catalysis followed by important properties of graphene and varied applications of graphene-based nanocomposites in different fields of science and technology is outlined in the beginning of this review. Applications of GO/rGO as a catalyst support (CuO-GO/rGO, Cu@Cu2ONPs-RGO, PtNPs@rGO, RGO/ZnO, sulfonated rGO, pyrene tagged metal complex/N-heterocyclic carbene metal complex/Ionic liquid supported onto GO) in organic synthesis, GO/rGO as a green metal-free catalyst and GO/rGO as an electrocatalyst are then described in detail. Lastly, challenges and opportunities are explored for the potential production of graphene-based nanomaterial in sustainable catalysis. This analysis offers crucial details for the catalysis community with great success in designing and manufacturing graphene-based novel nanomaterials as heterogeneous catalysts for organic synthesis.
2 GO/rGO as catalyst support
Carbon materials have attracted great attention as support materials used in heterogeneous catalysis because of their large surface area and chemical stability by virtue of which high loading of active sites is facilitated [34]. Among carbon materials, graphene and its derivatives (GO/rGO) have extraordinary properties, comparatively activated carbon, carbon black and CNTs that make it a promising candidate for catalysis [70–72,83–85]. Metal atoms are well recognized for catalyzing the growth of CNTs, and this was the basis for the study of metal–carbon interactions that encouraged applications focused on graphene-supported metals [86]. Although unsupported NPs have been reported as catalysts for organic transformations [7–9], agglomeration of NPs leads to reduced active surface area, resulting in poor catalytic performance. This can be circumvented by loading various metal/metal oxide NPs onto GO/rGO for many catalytic applications to improve the conversion efficiency, and several researchers have studied the subject extensively [78–80]. Recently, a review article on the utilization of four categories of carbon-based materials, viz. graphene (including, GO and RGO), graphitic carbon nitride, CNTs and activated carbon as supports in organic transformations including hydrogenation, oxidation, reduction, condensation, and multi-component reactions, is reported in detail by Bahuguna et al. [87].
The role of metal oxide NPs/pyrene tagged metal complexes/N-heterocyclic carbene metal complex/sulfonated groups/ionic liquid supported on GO/rGO as catalytic support for the synthesis of bioactive molecules is comprehensively reviewed in terms of catalytic performance, activity, selectivity and greenness of the process.
2.1 Metal oxide NPs supported on GO/rGO catalyzed organic transformations
In recent years, CuO-GO/rGO nanocomposite has been extensively used as a catalyst for various organic transformations, viz. synthesis of coumarin-based triazoles [88], CO oxidation [89], nitroaromatics hydrogenations [90], imidazo[1,2-a]pyridines syntheses [91], ynones, 1,3-diynes, 1,5-benzodiazepines [92] syntheses, etc. Further, it can also be utilized as a catalyst for N-arylation of N-heterocycles [77], decomposition reaction of dye molecules [93], synthesis of alkylaminophenols via Petasis-Borono–Mannich (PBM) reaction [94], synthesis of flavanones [95], etc. In one such study, Movahed et al. [77] carried out the synthesis of core–shell Cu@Cu2O NPs on RGO (Cu@Cu2O NPs-RGO) by in situ reduction of GO and copper sulfate using l-ascorbic acid as a reducing agent and applied it as a heterogeneous catalyst in the N-arylation of N-heterocycles. In another study, the CuOx nanocomposites based on rGO revealed a far higher catalytic activity against the decomposition reaction of dye molecules under visible light illumination compared to the unembellished CuOx NPs [93]. Choi and coworkers [93] performed the synthesis of CuOx/rGO nanocomposites by controlling the impregnation condition of a copper precursor (Cu(NO3)2·3H2O) on GO and explored for catalytic activity toward the dye molecules’ decomposition reaction under visible light illumination. Among CuOx/rGO nanocomposites, CuO/rGO showed excellent photocatalytic activity that can be credited to the synergistic combination of dye adsorptivity and electron acceptability of the rGO, the surface hydroxyl species in the CuO/rGO, the narrow bandgap and smaller size of the CuO NPs.
It is well-known that the PBM reaction, reported by Nicos Petasis in 1993, may be used in combinatorial chemistry and drug development [96]. It can also be used to access highly functionalized amines and amino esters with a high diastereoselectivity as well as enantioselectivity [97,98]. In yet another research, the composite CuO NPs/rGO acts as a catalyst as well as a susceptor and improves the overall potential of the reaction mixture to absorb microwave irradiation (MW) [94]. In this research, Dandia et al. [94] developed CuO NPs/rGO composite catalyzed green method for selective synthesis of alkylaminophenols in 73–93% yield through the PBM reaction of boronic acids, salicylaldehyde and amines under MW (Scheme 1).

A CuO NPs/rGO composite catalyzed Petasis-Borono–Mannich reaction. Reproduced by permission of The Royal Society of Chemistry. RSC Advances 2018;8(53):30280–8. doi: 10.1039/C8RA05203D.
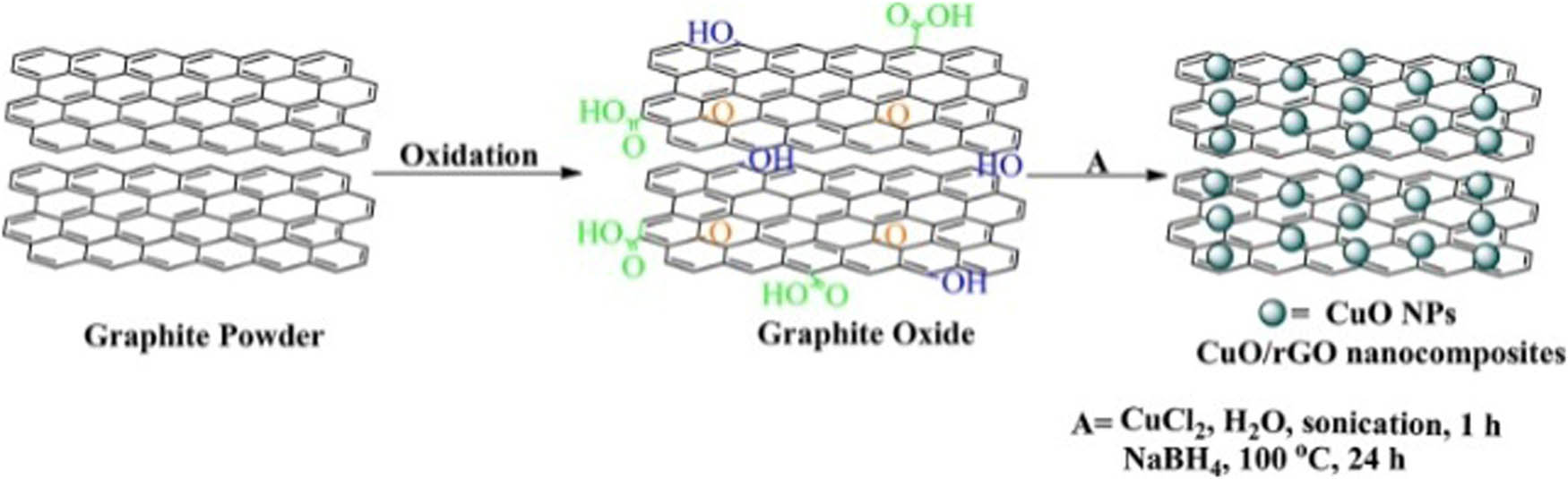
CuO NPs/rGO was recovered from the reaction mixture and recycled eight times without any noticeable loss of activity. The catalytic activity was found to be around sevenfold higher at 400 W power output under microwaves compared to traditional methods. Nanocomposite was synthesized using GO, Cu(OAc)2 monohydrate and hydrazine hydrate as a reducing agent; and GO reduction, protection and functionalization occur in a single step (Scheme 2). The CuO NPs/rGO composite X-ray diffraction (XRD) pattern revealed diffraction peaks for the CuO NP phase and rGO crystallographic planes which indicated the positive modification of CuO NPs on the rGO sheets. Transmission electron microscopy (TEM) imaging verified that the rGO sheet had been updated with a lot of the CuO NPs (about 23 nm). The overlap of CuO NPs, for transparent rGO sheet, demonstrated that the particles could adsorb on both sides of the rGO. The PBM reaction was even studied using various catalyst concentrations and in the presence of different solvents such as CH3CN, MeOH, 1,4-dioxane, THF, DMF and DCM. Excellent product yield (92% yield) was achieved with 10 wt% of the CuO NPs/rGO composite containing 2% CuO load in the presence of DCM. In this connection, a sustainable approach has further been developed by Gupta et al. [95] toward the synthesis of hybrid molecules containing versatile heterocyclic moiety, flavanone, possessing numerous biological activities such as antioxidant, antitumor, anti-inflammatory, anticancer, antidiabetic and antiulcer [99–102] with bioactive triazole [103] by combining Michael addition and click reaction using CuO/rGO nanocomposite as a catalyst (Scheme 3). The GO powder XRD (PXRD) pattern revealed characteristic diffraction peaks at 2θ corresponding to 9.33 and 42.20 due to [001] and [100] planes, suggesting graphite oxidation to GO. In the CuO/rGO nanocomposite PXRD pattern, the absence of the [100] plane at 2θ ∼ 42.20 and the appearance of a peak at 2θ ∼ 24.90 suggested that GO was reduced to rGO during CuO NPs doping on GO. Catalyst could be recycled and reused up to seven repeated runs without losing activity as compared with the reported methodologies [104–107]. Other prominent features of the process include water as a green solvent, good to excellent yields (87–96%), low catalyst loading, high atom efficiency, high substrate variation and good gram scale reaction. In addition, polar protic solvents, such as water and ethanol, showed improved conversion (92 and 94%, respectively) in the presence of 8 mg nanocomposite under refluxing conditions of 30 min (Scheme 4).

Synthesis of the CuO NPs/rGO composite. Reproduced by permission of The Royal Society of Chemistry. RSC Advances 2018;8(53):30280–8. doi: 10.1039/C8RA05203D.
Synthesis of CuO/rGo nanocomposites. Reproduced by permission of The American Chemical Society. ACS Omega 2018;3(7):7288–99, https://pubs.acs.org/doi/10.1021/acsomega.8b00334. Notice to readers: further permissions related to the material excerpted should be directed to the ACS.

General scheme for the cyclization of chalcones to flavanones. Reproduced by permission of The American Chemical Society. ACS Omega 2018;3(7):7288–99, https://pubs.acs.org/doi/10.1021/acsomega.8b00334. Notice to readers: further permissions related to the material excerpted should be directed to the ACS.
Another important aspect of the technique is the adaptation of various functional groups to the conditions of the reaction (Scheme 4). Reported plausible mechanism for CuO/rGO nanocomposite catalyzed reaction is outlined in Scheme 5. Similarly, flavanones containing a moiety of 1,2,3-triazole (4a–j) were also synthesized in a single step in magnificent yield (90–97%; Scheme 6). Further studies with different functional groups attached to phenyl azide were also carried out and reported to provide desired flavanone enclosed triazoles in excellent yield.

Plausible mechanism for the CuO/rGO nanocomposite catalyzed reaction. Reproduced by permission of The American Chemical Society. ACS Omega 2018;3(7):7288–99, https://pubs.acs.org/doi/10.1021/acsomega.8b00334. Notice to readers: further permissions related to the material excerpted should be directed to the ACS.

General scheme for 1,2,3-triazoles. Reproduced by permission of The American Chemical Society. ACS Omega 2018;3(7):7288–99, https://pubs.acs.org/doi/10.1021/acsomega.8b00334. Notice to readers: further permissions related to the material excerpted should be directed to the ACS.
Recently, nitrogen-doped graphene encapsulated Cu NPs are reported to act as an efficient and durable catalyst in selective oxidation of 5-hydroxymethylfurfual to 2,5-furandicarboxylic acid as a bioplastic monomer under mild conditions [108].
Moreover, catalytic applications of Pt NPs supported onto graphene are well established, which include its use as electrocatalyst for methanol oxidation reaction (Pt/graphene nanosheet surface [GNS]) [109] and for proton exchange membrane fuel cell (PEMFC) (Pt/rGO) [110], electrochemical sensors (Pt/G) [111], alcohol oxidation (Pt/rGO) [112], styrene hydrogenation (PtNPs/G) [113], etc. Aday et al. [114] reported the development of highly crystalline, colloidally stable and highly monodisperse Pt NPs@rGO and its use as a catalyst for the synthesis of acridinedione derivatives which are well recognized to possess numerous biological activities such as anticancer, antimicrobial, myorelaxant, free radical scavenging, etc. [115–117]. The synthesis of acridinedione derivatives was carried out in 94–96% via single-pot, multicomponent condensation of dimedone, various aromatic aldehydes and several aromatic amines in the presence of water/ethanol as a green solvent using Pt NPs@rGO as a recyclable heterogeneous catalyst [114] (Scheme 7).

Synthesis of acridinedione derivatives in the presence of Pt NPs@rGO.
Characterization of the synthesized catalyst was done on the basis of high-resolution electron microscopy, TEM, X-ray diffraction, Raman and X-ray photoelectron spectroscopy techniques. The results obtained using Pt NPs@rGO were compared to those obtained using other catalysts such as Pt NPs, glacial acetic acid, P-Dodecylbenzenesulfonic acid, H2SO4 and hydrochloric acid; and it was found that Pt NPs@rGO delivered the highest yield. It has been shown that no special care need to be taken in processing or handling the catalyst because it is not prone to air or moisture [114].
Metal oxide NPs supported on RGO has further been investigated for the green synthesis of the substituted indole derivatives [118] especially the 3-substituted indoles [119,120] that are established medicinally potent lead molecules and key intermediates for the synthesis of various therapeutic agents [121]. Green method for efficient synthesis of biologically active 3-substituted indoles using RGO/ZnO nanocomposite as recyclable heterogeneous catalyst in water (Scheme 8) has been developed by Rajesh et al. [122]. It is demonstrated that nanocomposite can act as amphiphilic heterogeneous catalyst in water, as the RGO surface is hydrophobic and the surface polarity of ZnO NPs is hydrophilic in nature [123] and can be recycled six times without substantial loss in catalytic activity. Other advantageous features of the methodology are higher environmental compatibility, sustainability factors such as smaller E-factor and higher atom economy.

RGO/ZnO-catalyzed synthesis of 3-amino alkylated indoles.
Khatun et al. [124] reported zinc metal containing aminically modified GO catalyzed CO2 fixation through N-formylation and carbamate formation reactions of amines. It was found that N-formylation of both aromatic and aliphatic amines gave high yield of the corresponding formylated products in the presence of polymethylhydrosiloxane as reducing agent under 1 bar CO2 pressure and mild temperature. Formation of carbamates from aniline or its derivatives and alkyl/aryl bromide was achieved with good product selectivity under the same CO2 pressure at room temperature under solvent-free condition. The catalyst can be reusable even after six consecutive runs, further making it a green catalytic process.
Variety of indole derivatives, aromatic aldehydes and secondary amines were employed to obtain good yields (83–92%) of a series of 3-amino alkylated indoles. RGO/ZnO was reported to be the best catalytic system for the synthesis of 3-amino alkylated indoles in terms of activity, selectivity and greenness of the protocol, with negligible waste generation from the reaction mixture [122]. Further investigations on indole with active methylene compounds and various aromatic aldehydes were also carried out to give the corresponding 3-substituted indoles in excellent yields via Knoevenagel condensation followed by Michael addition. It was established that the aromatic aldehydes bearing electron-donating substituents such as –OMe and –OH at meta and para positions showed less reactivity over electron withdrawing substituents such as –NO2 and chloro at ortho and para positions [122].
2.2 Pyrene-tagged metal complexes supported onto GO as catalyst
Although noncovalent methods for the immobilization of catalysts on solid surface are easy approaches, the drawback may be that the link between catalyst and solid is often not too strong [125]. Because of the intrinsic ability of pyrene to afford π stacking interactions with graphitic surfaces [126], pyrene-tagged metal complexes have been effectively supported on graphitized solids [127] and some have even been used in catalysis to reveal excellent recyclability properties [128]. It is demonstrated that noncovalently immobilized pyrene-tagged N-heterocyclic carbene (NHC)-based catalyst onto graphene surface modified important properties of the catalyst that can be related to π–π stacking interactions established between aromatic reactant and pyrene functionalities [129,130]. Ruiz-Botella and Peris [125] prepared two pyrene-tagged NHC complexes of rhodium(i) and immobilized these complexes onto rGO support and studied their catalytic activity in the 1,4-addition of phenylboronic acid to cyclohex-2-one and in the hydrosilylation of terminal alkynes. It was reported that bimetallic catalyst with the two pyrene tags supported on to reduced graphene exhibited improved catalytic activity against the monometallic one with only one pyrene tag for both the reactions that was further correlated to earlier studies demonstrating significant reduction in leaching when the metal complex is anchored to the surface of solid with more than one pyrene tag [129]. While the monometallic catalyst quickly became inactive, the solid containing bimetallic catalyst could be recycled up to five times with no noticeable loss of activity in the case of the addition of phenylboronic acid to cyclohexanone. Moreover, better selectivity toward β-(Z)-vinylsilane was observed in the hydrosilylation of terminal alkynes in case the immobilized bimetallic catalyst was used although the catalyst could only be reused upto two runs. Interesting results indicate that rGO-supported catalysts offer not only better catalytic performance but also improved selectivity. In yet another study, Sabater et al. [131] developed highly efficient catalyst by co-immobilizing Pd and Ru complexes with pyrene-tagged NHC ligands onto rGO surface and examined for its catalytic activity in the hydrodefluorination of a series of fluoroarenes with catalytic system recycled up to 12 times without the measurable loss of activity (Figure 3).
![Figure 3 Immobilization of pyrene-tagged palladium and ruthenium complexes onto reduced graphene oxide for hydrodefluorination. Reprinted (adapted) with permission from American Chemical Society” ref. [131] Organometallics 2015;34(7):1186–90. https://doi.org/10.1021/om501040x. Copyright @2014 American Chemical Society.](/document/doi/10.1515/gps-2020-0055/asset/graphic/j_gps-2020-0055_fig_015.jpg)
Immobilization of pyrene-tagged palladium and ruthenium complexes onto reduced graphene oxide for hydrodefluorination. Reprinted (adapted) with permission from American Chemical Society” ref. [131] Organometallics 2015;34(7):1186–90. https://doi.org/10.1021/om501040x. Copyright @2014 American Chemical Society.
Many organic transformations like the cyclization of acetylenic carboxylic acid and the coupling of diphenylcyclopropenone with substituted phenylacetylenes, hydrogenation of alkenes and alcohol oxidation have also been carried out using pyrene-tagged metal complexes supported on rGO with excellent recyclability [132,133].
2.3 NHC metal complex supported on GO as catalyst
N-heterocyclic carbenes [134] are among the most widely studied group of ligands, with applications in various fields including catalysis. The silylation modification technique reported on GO may provide catalytic activity for graphene nanocomposites [135]. In one such study, ionic liquid framework-modified GO (GO-IL)-supported NHC palladium complex (NHC-Pd/GO-IL) was synthesized by GO modification via a silylation reaction and used as an effective and recyclable catalyst for Suzuki reaction by Movahed et al. [136] (Scheme 9). The nanocomposite demonstrated strong catalytic activity in EtOH–H2O (1:1) for the Suzuki coupling of a variety of activated and deactivated aryl halides with aryl boronic acid. Further, the catalyst could be reused several times without significant decrease in its catalytic activity. Leaching experiments such as hot filtration and atomic absorption spectrometry analysis confirmed the heterogeneous nature of the catalytic reaction. In addition, the TEM image of the recovered catalyst showed the existence of well-distributed Pd NPs without any accumulation on the GO-IL sheets. It is shown that imidazolium IL plays a significant role in improving the dispersibility of Pd NPs found inside the GO-IL sheets, thereby avoiding the agglomeration of Pd NPs on the sheets of GO-IL. Evaluation by quantitative energy-dispersive X-ray spectroscopy (EDS) mapping indicated that the elements N, Pd and Si were found to be uniformly distributed across the entire surface of NHC-Pd/GO-IL nanocomposite, rather than only being localized at the edges of graphene sheets.

GO NHC-Pd/GO-IL catalyzed Suzuki reaction.
In yet another research, Shang and coworkers [137] immobilized NHC–palladium complex (NHC–Pd2+) onto the surface of GO via a chemical bonding method for the first time and employed as an effectual catalyst for Suzuki–Miyaura coupling reactions with yields of the products ranging between 83 and 96%. Recyclability of the catalyst was checked and it was found that it could be reused for at least six consecutive cycles without significantly losing its catalytic activity. GO-supported NHC–Pd2+ catalyst was characterized by TEM, X-ray diffraction spectroscopy, X-ray photoelectron spectroscopy and infrared (IR) spectroscopy. Several other investigations have also been carried out using the NHC-supported rGo nanocomposite catalyzed transformations. Recently, Page and coworkers [138] covalently modified partially rGO with 3-methyl-4-phenyl-1,2,3-triazolium salts, making use of epoxy functionalities on the carbon nanomaterial. Hydroxyl-triazolium-functionalized materials thus formed were used to prepare graphene-oxide-supported rhodium–triazolylidene hybrid catalysts which displayed outstanding activity for the hydrosilylation of terminal and internal alkynes. Furthermore, catalysts showed good selectivity toward β-(Z)-vinylsilane isomers (for the terminal substrates not hindered) or syn additions (Figure 4).
![Figure 4 Graphene modified with rhodium-based N-heterocyclic carbenes for alkyne hydrosilylation. Reprinted (adapted) with permission from American Chemical Society. Ref. [138] ACS Applied Nano Materials 2020;3(2):1640–55. https://doi.org/10.1021/acsanm.9b02398. Copyright @2020 American Chemical Society.](/document/doi/10.1515/gps-2020-0055/asset/graphic/j_gps-2020-0055_fig_016.jpg)
Graphene modified with rhodium-based N-heterocyclic carbenes for alkyne hydrosilylation. Reprinted (adapted) with permission from American Chemical Society. Ref. [138] ACS Applied Nano Materials 2020;3(2):1640–55. https://doi.org/10.1021/acsanm.9b02398. Copyright @2020 American Chemical Society.
2.4 Sulfonated rGO catalyzed organic transformations
Among solid acid catalysts, sulfonated graphene has emerged as an interesting environmentally benign water-tolerant solid-acid catalyst [139]. Since the last decade, sulfonated graphene has been discovered for various catalytic applications such as acid-catalyzed liquid reactions [140], hydrolysis of cellulose [141], conversion of 5-(hydroxymethyl)-2-furfural into biofuels [142], synthesis of 6,6′-(aryl(alkyl)methylene) bis(2,4-dialkylphenol) antioxidants [143], etherification of glycerol with isobutene [144], etc. 1,3,4-Oxadiazole moiety is a key intermediate for the synthesis of many antibacterial, antiviral, antiparasitic and other drugs [145,146]; and hence several methods were reported for the synthesis of 1,3,4-oxadiazoles [147,148]. Owing to the drawbacks such as requirement of large amount of solvent, strong basic medium and a long reaction time associated with 1,3,4-oxadiazole cyclization process with hydrazides [149], Brahmayya et al. [150] reported the direct synthesis of 1,3,4-oxadiazoles from hydrazides with carbon dioxide (1.0 MPa) using nano sulfonated rGO (rGOPhSO3H) as an efficient and mild carbon catalyst under ultrasonic irradiations (Scheme 10).

Direct synthesis of 1,3,4-oxadiazoles from hydrazides using rGOPhSO3H catalyst.
In other investigation, RGO-SO3H was used as an effective catalyst for one-pot synthesis of a series of 2-amino-3-cyano-7-hydroxy-4H-chromene derivatives using multicomponent reaction of phenols, aldehydes and malononitrile under mild and green conditions [151]. The reaction was performed in water as a green solvent providing good to excellent yield of the product, and the catalyst RGO-SO3H was reusable at least five times without measurable decrease in its catalytic activity. The synthesized compounds were characterized using IR, 1H NMR and mass spectral studies. The rGO-SO3H was even successfully applied as a reusable solid acid catalyst for direct amidation of carboxylic acids with amines using ultrasonic irradiation [152] affording the corresponding amides in good to high yields (56–95%) in short reaction time (Scheme 11). Sulfonic acid-containing aryl radicals were grafted onto chemically rGO under sonochemical conditions to prepare sulfonated rGO nanosheets (rGO-SO3H), which were characterized by fourier-transform infrared spectroscopy (FT-IR) spectroscopy, Raman spectroscopy, scanning electron microscope (SEM), XRD, thermogravimetric analysis (TGA), differential scanning calorimetry and X-ray photoelectron spectroscopy (XPS).

rGO-SO3H catalyzed direct amidation of carboxylic acids with amines.
2.5 Ionic liquid supported on GO as catalyst
Owing to their high thermal and chemical stability, low volatility, very high ability to dissolve a wide variety of compounds and more significantly, their environmentally friendly behavior [153], surface functionalization of G and GO using ionic liquids is gaining particular importance recently. The ionic liquid-functionalized graphene (G-IL/GO-IL) has been extensively used in pollutant decontamination, enhancement of styrene–butadiene rubber nanocomposites, high-temperature proton exchange membrane fuel cell, sensing and biosensing, lubrication, catalysis and carbon dioxide capturing and hydrogen production [154–159]. Moreover, ionic liquids interact strongly with the sp2-hydridized G and GO sheet carbon networks because of their high dipolar nature and make them more dispersible compared to their native networks [153]. It is established that acylation, isocyanate formation, esterification, amide formation, nucleophilic ring opening, diazotization and cycloaddition reactions generally facilitate the covalent functionalization of G and GO [33,160]. In one such investigation, Nakhate and Yadav [161] synthesized GO-supported functionalized ionic liquid (PTS–Im-3@GO) by anchoring 1-(4-sulfobutyl)-3-(3-propyltriethoxysilyl)imidazolium hydrogen sulfate onto GO via covalent bonds and used it for styrene oxide ring opening reaction with isopropyl alcohol, giving 95% conversion and 100% regioselectivity toward 2-isopropoxy-2-phenylethan-1-ol at 50°C. Among various heterogeneous catalysts used, viz. GO, PTS–Im-1@GO, PTS–Im-2@GO, PTS–Im-1, PTS–Im-2, PTS–Im-3, Hβ-zeolite and montmorillonite K--10, PTS–Im-3@GO showed good conversion toward the desired product. Characterization of PTS–Im-3@GO was done by FT-IR, SEM, EDS, TGA, XRD, silicon-29 magnetic resonance spectroscopy, XPS and carbon hydrogen nitrogen sulphur analysis. Likewise, Garkoti et al. [162] developed heterogenization of amine-functionalized ionic liquids via covalent immobilization of 2-chloroethylamine on GO sheets modified by imidazole. The prepared material was characterized by FTIR, XRD, TGA, TEM, SEM and EDX spectroscopy and used as a catalyst for the synthesis of 3-substituted indoles via Yonemitsu-type reaction. After recovery from the reaction mixture, the catalyst was reused seven times without any significant loss of activity. Hanoon et al. [163] reported an efficient method for the facile synthesis of biologically relevant benzimidazole derivatives [164] using an acidic ionic liquid covalently supported on graphene sheets (A-FGO). The catalyst was efficiently synthesized and characterized using FTIR, SEM, X-ray photoelectron spectroscopy, EDS and X-ray diffraction techniques. The catalyst was removed by filtration, washed with dichloromethane and reused in the next cycles (up to five runs). Shorter reaction times, high yields, safer reaction conditions, the eco-friendly nature and possible reuse of the catalyst are the salient features of the present methodology.
3 GO/rGO as a green metal-free catalyst
Carbocatalysis is a form of catalysis for the transformation or synthesis of organic or inorganic substrates using heterogeneous carbon materials. The introduction of GO as a new class of metal-free catalysts based on carbonaceous materials opens up a range of potential application possibilities in chemical synthesis. For the first time, Dreyer and his coworkers [165] reported GO as a carbocatalyst to catalyze the oxidation of various alcohols and alkenes, and the hydration of different alkynes into their respective aldehydes and ketones in good to excellent yields. Several experiments have since been conducted to investigate GO as a carbocatalyst. In one test, Basu and coworkers [166] produced for the first time from a mixture of secondary aryl alcohols and thiols an effective and mild one-pot GO catalyzed sequential dehydration–hydrothiolation reaction. The resultant unsymmetrical thioethers were synthesized under metal-free conditions and the catalyst could be reused for five cycles without losing any appreciable activity. Patel and coworkers [167] employed GO as metal-free carbocatalyst to promote amidation of esters with amines in good to superb yields without using any additives. It is demonstrated that the enhanced catalytic activity can be due to the oxygen functionalities on the GO surface that form H-bonds with the reactants, thereby speeding up the reaction. Improved yields and a wide range of functional group tolerance are some of the important features of the developed protocol. In yet another research, Karthik and Suresh [168] reported sustainable metal-free approach using GO as a benign solid-acid catalyst to synthesize phenols from aryl and heteroaryl boronic acids, ipso-hydroxylation occurs in a short reaction time by using aqueous H2O2 as an oxidant in the presence of water. The presence of carboxyl groups boosts ipso-hydroxylation as revealed by the control experiments, and GO can be reused several times without losing its activity.
Furthermore, GO has been successfully utilized as a green carbocatalyst for the synthesis of numerous biologically relevant scaffolds such as benzylbarbiturocoumarin derivatives [169], 3-dihydroquinazolinones and quinazolin-4-(3H)-ones [170] and benzylpyrazolyl coumarin derivatives [171], 2,5-dimethyl-N-phenyl pyrrole [172], poly heterocyclic spiro-indeno quinoxaline pyrrolizidines quinoxalin and spiro-oxindoles pyrrolizidines [173], 3-sulfenylimidazo[1,2-a]pyridines [174], dihydro-2-oxopyrroles [175], etc. Roy et al. [176] developed an eco-friendly method for the synthesis of library of bioactive nitrogen containing heterocycles, quinoxalines [177] from 2-nitroanilines under entirely metal-free conditions using GO or rGO as a green catalyst (Scheme 12).

GO/rGO catalyzed synthesis of quinoxalines from 2-nitroaniline.
A wide range of functional groups such as methyl, methoxy, furyl or bromide present with either nitroaniline or dicarbonyl compounds, along with ketone or aldehyde, provided excellent quinoxaline transformation (Scheme 12). Initially, GO or rGO catalyzed the reduction of nitroaniline with hydrazine hydrate takes place followed by the reaction with 1,2-dicarbonyl compounds or with α-hydroxy ketones in one-pot tandem way to afford quinoxalines in good yields (83–95%). After being recovered from the reaction mixture, the catalyst was recycled without any appreciable loss of activity for four uninterrupted runs.
Ebajo et al. [178] reported the synthesis of triazoloquinazolinone compounds by utilizing Brønsted acidic edges and Lewis-acid sites in GO as heterogeneous promoters. GOs with the maximum number of Lewis acid sites possess the highest degree of oxidation resulting in the best yields (up to 95%) under moderate reaction conditions (85°C in EtOH). According to them, the perceived Lewis acidity through the opening of basal plane epoxide ring in addition to the saturated Brønsted acidic carboxylic groups is responsible for the increased carbocatalytic activities as revealed by the results of FT-IR spectroscopy, temperature-programmed decomposition mass spectrometry and X-ray photoelectron spectroscopy. In one more investigation, the identification of GO as pseudocatalyst in organic transformations is further supported by the fact that recycled GO can be successfully regenerated to hold 97% activity of new GO [179]. It is demonstrated that graphene’s structural features can provide a broad range of conversion and selectivity by tailoring the surface morphology and functionalities [83]. In this sense, several review articles and accounts have been published on carbocatalytic activity of graphene [34,85,180]. Such eco-friendly approaches built by researchers opened the door to more studies focused on GO as a metal-free catalyst for sustainable organic synthesis. Further investigations toward the catalytic activity of GO can be done by manipulating surface modification and edge defects which may lead to the development of novel green synthetic protocols involving GO as a metal-free catalyst.
4 GO/rGO as electrocatalyst
Electrocatalytic activity of graphene-based nanomaterials was first reported in 2009 for methanol oxidation reaction with an unprecedented high activity in Pt subnanoclusters supported onto graphene nanosheets by Yoo et al. [181]. Since then several studies based on electrocatalytic property of graphene-based materials have been carried out by researchers all over the world. Owing to the fascinating properties of graphene such as chemical stability, electrical conductivity, tunable surface area and excellent mechanical behavior, graphene-based materials have outstanding applications as electrode materials in electrochemical devices such as supercapacitors, rechargeable lithium ion batteries, fuel cells and solar cells [182–185]. Among these devices, future-generation energy devices for clean power production include PEMFCs and rechargeable metal–air batteries [186,187]. Technically, precious Pt-based nanomaterials are the commercial electrocatalysts for the oxygen reduction reaction (ORR), which reports 36–56% of the cost of the PEMFCs [188]. Various heteroatom-doped carbon materials [189], sulfur-doped graphene (SG) [190], phosphorous-doped graphite layers [191], iodine-doped graphene [192] and edge-halogenated (Cl, Br, or I) graphene nanoplatelets [193] and mesoporous nitrogen-doped carbons prepared from ionic liquids and nucleobases for productive metal-free oxygen reductions [194] are investigated. An article published by Iqbal et al. [195] outlines graphene-based materials for fuel cell technology applications such as electrodes additives, bipolar plates and proton conducting electrolyte membrane. The graphene dispersed electrodes show improved electrocatalytic activity toward fuel oxidation and oxidant reduction reactions. In addition, graphene as an electrolyte has displayed an excellent performance toward high ionic conductivity and power density in proton exchange membrane devices. The integration of graphene into fuel cell systems has shown commendable efficiency and has promising future for commercial applications.
Rising demand for renewable energy has driven extensive work into powerful, cost-effective and eco-friendly energy conversion and storage systems. In this context, nanomaterials based on graphene have been used in fuel cell applications as gifted electrocatalysts for ORR [196–198]. Kumar et al. [199] developed extendable and benign method by using N-doped reduced graphene oxide (NrGO) for the metal-free oxygen reduction reaction. Characterization of NrGOs was carried out by XPS, Raman, FT-IR, HR-SEM, atomic force microscopy, XRD and UV-Vis techniques. Based on XPS, the highest degree of nitrogen doping was obtained at 4.3 atom percent at 450°C with an atomic ratio of C/O as high as 16. This NrGO’s electrocatalytic behavior against the ORR in alkaline electrolytes has been verified by electrochemical characterizations with a high reaction onset potential of 0.78 V vs reversible hydrogen electrode. Recent review article by Ali and Shen [200] emphasized efforts to expand the nanoscale synthesis of graphene-supported electrocatalysts and their electrocatalytic characteristics for a remarkable hydrogen evolution reaction (HER). HER has tremendous potential for the future renewable technologies in terms of energy storage and energy conversion. The effect of graphene for HER in alkaline medium is researched by Huang et al. [201]. Currently, the efficiency of water electrolysis producing hydrogen is too poor to compete economically for real energy requirements [202]. The best catalysts are the elements from the Pt group metals which are perfect from thermodynamics and kinetics point of view, but their lack and high cost hinder their large-scale use, requiring cheaper and more viable catalysts [203,204]. In this context, Luis-Sunga et al. [205] recently reviewed nonprecious metal graphene-based catalysts for HER.
5 Critical overview and future perspectives
While graphene-based materials are in their evolving stages, they have excessive potential to facilitate a wide range of organic transformations and can offer amazing talent in the design of new catalytic systems. Brilliant physicochemical properties associated with graphene combined with excellent catalytic performance in terms of high activity, selectivity, stability, recyclability and greenness of the synthetic process (by lowering energy consumption) of graphene-based nanocatalysts makes them superior over other heterogeneous catalysts in solving today’s major problems of sustainable development. Further, the variety of functional oxygenated groups on graphene oxide makes it an desirable medium for anchoring other catalytically active groups. Furthermore, nanosized materials exhibit additional unique properties compared to the macroscale as demonstrated by unexpected catalytic activity of gold NPs not found in bulk gold. Taking into account the added value, as catalysts these materials can provide affordability and the durability of their use as opposed to metal catalysts, and it can be expected that this field will continue to expand in the years to come. Consequently, it empowers us with abundant future applications as demonstrated by the growing number of research publications and patents in recent years.
Although the past decade has brought much progress, there are still challenges, which include loss of catalytic activity due to metal leaching under severe conditions. Another major concern is the lack of control over distinct morphologies that must be addressed during scale-up synthesis of nanocomposites. A uniform distribution over the support surface is not always an easy task, and implementation of large-scale production in industry is the biggest challenge. Further, supported metal NPs face consequences of deactivation associated with their extended usage under conditions of reactions, such as coke forming, aggregation, coarsening and sintering. Despite the ideas of a promising future, threats to both the environment and human health are still not clearly understood based on the evidences presently existed. To overcome these challenges, it is suggested that toxicity and long-standing effects of nanomaterials and by-products required to be studied while designing the catalyst more widely as soon as possible keeping in view the human health and environmental impacts. Thoughtful strategy for the development of highly active and enantioselective recyclable nanocomposite catalyst for the green and sustainable organic synthesis is the need of hour.
6 Conclusions
Graphene-based nanocomposites act as talented catalysts for facilitating wide range of organic transformations and have amazing potential in designing novel catalytic systems. In this review article, first the use of GO/rGO supported metal/metal oxide nanocomposite as excellent catalytic support material for the synthesis of diverse bioactive molecules is broadly reviewed. The use of GO/rGO as metal-free catalyst for various organic transformations and as electrocatalyst for reactions of industrial relevance is then briefly summarized. Finally, an insight is also presented into the critical overview and future perspectives for the future development of graphene-based nanocomposite catalysts. The most relevant literature including green synthetic strategies for the synthesis of pharmacologically active compounds using graphene-based nanocomposite as catalyst have been summarized, which will surely help the scientists in preparing novel graphene-based nanocatalysts for satisfactory chemical manufacture. It will provide a greener and more sustainable alternative pathway as the catalyst can be easily separated from the reaction mixture on most routes and can be recycled and reused without apparent loss of activity making it economic.
Besides the abovementioned catalytic applications, graphene-based nano spinel ferrites (GNSFs) hold great potential in remediating contaminated aquatic environments. In this context, introduction of magnetic spinel ferrites into 2-D graphene family nanomaterials conveys various benefits of inhibited particle agglomeration, enhanced active surface area and easier magnetic separation for reuse, making the GNSFs highly efficient and eco-friendly materials, which in turn have the potential of removing several unmanageable contaminants including organic dyes, antibiotics and heavy metal ions [206]. Further, introducing graphene materials into hierarchical catalysts can enhance the removal of gaseous HCHO, which is one of the most abundant indoor pollutant, and potentially other indoor pollutants. The Pt/NiFe-LDH/rGO showed excellent catalytic performance for HCHO degradation at room temperature, which are because of better dispersion of both NiFe-LDH and rGO in the composite [207]. Graphene-based materials through hybridization or fabrication of various functionalities on their large surface open up new avenues for the adsorptive removal of volatile organic compounds (e.g., through the buildup of efficient air purification systems) [208]. A biosensor based on the CeO2−x/C/rGO nanocomposites exploited for the detection of uric acid, an important molecule in the biological and medical fields. The biosensor based on the CeO2−x/C/rGO nanocomposites shows a great potential for practical applications by presenting a high sensitivity of 284.5 µA cm−2 mM−1 at −0.4 V (vs saturated calomel electrode), a wide linear range between 49.8 and 1050.0 μM and a low detection limit of 2.0 μM [209].
Considering the practical utility of GBMs in various fields of science and technology and the sustainability of their use compared to metal-based catalysts in organic synthesis, it is easy to predict that this area will grow extensively in the years to come; and further research is needed to identify an optimized catalyst in various organic transformations and to develop an environmentally friendly, energy-efficient and cost-effective method for graphene-based nanocomposites synthesis.
Conflict of interest: Author declare no conflict of interest.
References
[1] Ghorani-Azam A, Riahi-Zanjani B, Balali-Mood M. Effects of air pollution on human health and practical measures for prevention in Iran. J Res Med Sci Off J Isfahan Univ Med Sci. 2016;21:65. 10.4103/1735-1995.189646.Suche in Google Scholar PubMed PubMed Central
[2] Yilmaz B, Müller U. Catalytic applications of zeolites in chemical industry. Top Catal. 2009;52(6–7):888–95. 10.1007/s11244-009-9226-0.Suche in Google Scholar
[3] Martin K. Green catalysts for industry. Handb green Chem Technol. 2002;14:321–77. 10.1002/9780470988305.ch13.Suche in Google Scholar
[4] Misono M, Ono I, Koyano G, Aoshima A. Heteropolyacids. Versatile green catalysts usable in a variety of reaction media. Pure Appl Chem. 2000;72(7):1305–11. 10.1351/pac200072071305.Suche in Google Scholar
[5] Grills DC, Matsubara Y, Kuwahara Y, Golisz SR, Kurtz DA, Mello BA. Electrocatalytic CO2 reduction with a homogeneous catalyst in ionic liquid: high catalytic activity at low overpotential. J Phys Chem Lett. 2014;5(11):2033–8. 10.1021/jz500759x.Suche in Google Scholar PubMed
[6] Cravotto G, Gaudino EC, Tagliapietra S, Carnaroglio D, Procopio A. A green approach to heterogeneous catalysis using ligand-free, metal-loaded cross-linked cyclodextrins. Green Proc Synth. 2012;1(3):269–73. 10.1515/gps-2012-0029.Suche in Google Scholar
[7] Mikami Y, Dhakshinamoorthy A, Alvaro M, Garcia H. Catalytic activity of unsupported gold nanoparticles. Catal Sci Technol. 2013;3(1):58–69. 10.1039/C2CY20068F.Suche in Google Scholar
[8] Yi J, Miller JT, Zemlyanov DY, Zhang R, Dietrich PJ, Ribeiro FH, et al. A reusable unsupported rhenium nanocrystalline catalyst for acceptorless dehydrogenation of alcohols through γ-C–H activation. Angew Chem Int Ed. 2014;53(3):833–6. 10.1002/anie.201307665.Suche in Google Scholar PubMed
[9] Schauermann S, Nilius N, Shaikhutdinov S, Freund HJ. Nanoparticles for heterogeneous catalysis: new mechanistic insights. Acc Chem Res. 2013;46(8):1673–81. 10.1021/ar300225s.Suche in Google Scholar PubMed
[10] Zhu QL, Xu Q. Immobilization of ultrafine metal nanoparticles to high-surface-area materials and their catalytic applications. Chem. 2016;1(2):220–45. 10.1016/j.chempr.2016.07.005.Suche in Google Scholar
[11] Machado BF, Serp P. Graphene-based materials for catalysis. Catal Sci Technol. 2012;2(1):54–75. 10.1039/C1CY00361E.Suche in Google Scholar
[12] Chen Q, Yin Q, Dong A, Gao Y, Qian Y, Wang D, et al. Metal complex hybrid composites based on fullerene-bearing porous polycarbazole for H2, CO2 and CH4 uptake and heterogeneous hydrogenation catalysis. Polymer. 2019;169:255–62. 10.1016/j.polymer.2019.02.056.Suche in Google Scholar
[13] Yang P, Yang L, Gao Q, Luo Q, Zhao X, Mai X, et al. Anchoring carbon nanotubes and post-hydroxylation treatment enhanced Ni nanofiber catalysts towards efficient hydrous hydrazine decomposition for effective hydrogen generation. Chem Commun. 2019;55(61):9011–4. 10.1039/C9CC04559G.Suche in Google Scholar
[14] Majumdar B, Mandani S, Bhattacharya T, Sarma D, Sarma TK. Probing carbocatalytic activity of carbon nanodots for the synthesis of biologically active dihydro/spiro/glyco quinazolinones and aza-Michael adducts. J Org Chem. 2017;82(4):2097–106. 10.1021/acs.joc.6b02914.Suche in Google Scholar PubMed
[15] Shah N, Basu P, Prakash P, Donck S, Gravel E, Namboothiri IN, et al. Supramolecular assembly of gold nanoparticles on carbon nanotubes: application to the catalytic oxidation of hydroxylamines. Nanomaterials. 2016;6(3):37. 10.3390/nano6030037.Suche in Google Scholar PubMed PubMed Central
[16] Brinkley KW, Burkholder M, Siamaki AR, Belecki K, Gupton BF. The continuous synthesis and application of graphene supported palladium nanoparticles: a highly effective catalyst for Suzuki-Miyaura cross-coupling reactions. Green Proc Synth. 2015;4(3):241–6. 10.1515/gps-2015-0021.Suche in Google Scholar
[17] Mohammadi O, Golestanzadeh M, Abdouss M. Recent advances in organic reactions catalyzed by graphene oxide and sulfonated graphene as heterogeneous nanocatalysts: a review. N J Chem. 2017;41(20):11471–97. 10.1039/C7NJ02515G.Suche in Google Scholar
[18] Garg B, Ling YC. Versatilities of graphene-based catalysts in organic transformations. Green Mater. 2013;1(1):47–61. 10.1680/gmat.12.00008.Suche in Google Scholar
[19] Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9. 10.1126/science.1102896.Suche in Google Scholar PubMed
[20] Yam KM, Guo N, Jiang Z, Li S, Zhang C. Graphene-based heterogeneous catalysis: role of graphene. Catalysts. 2020;10(1):53. 10.3390/catal10010053.Suche in Google Scholar
[21] Wallace PR. The band theory of graphite. Phys Rev. 1947;71(9):622. 10.1103/PhysRev.71.622.Suche in Google Scholar
[22] Georgakilas V, Perman JA, Tucek J, Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev. 2015;115(11):4744–822. 10.1021/cr500304f.Suche in Google Scholar PubMed
[23] Sur UK. Graphene: a rising star on the horizon of materials science. Int J Electrochem. 2012;2012:237689. 10.1155/2012/237689.Suche in Google Scholar
[24] Banerjee AN. Graphene and its derivatives as biomedical materials: future prospects and challenges. Interface Focus. 2018;8(3):20170056. 10.1098/rsfs.2017.0056.Suche in Google Scholar PubMed PubMed Central
[25] Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8(3):902–7. 10.1021/nl0731872.Suche in Google Scholar PubMed
[26] Stoller MD, Park S, Zhu Y, An J, Ruoff RS. Graphene-based ultracapacitors. Nano Lett. 2008;8(10):3498–502. 10.1021/nl802558y.Suche in Google Scholar PubMed
[27] Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146:351–5. 10.1016/j.ssc.2008.02.024.Suche in Google Scholar
[28] Lee C, Wei X, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321(5887):385–8. 10.1126/science.1157996.Suche in Google Scholar PubMed
[29] Bhuyan MS, Uddin MN, Islam MM, Bipasha FA, Hossain SS. Synthesis of graphene. Int Nano Lett. 2016;6(2):65–83. 10.1007/s40089-015-0176-1.Suche in Google Scholar
[30] Wang J, Gong C, Wen S, Liu H, Qin C, Xiong C, et al. Proton exchange membrane based on chitosan and solvent-free carbon nanotube fluids for fuel cells applications. Carbohydr Polym. 2018;186:200–7. 10.1016/j.carbpol.2018.01.032.Suche in Google Scholar PubMed
[31] Aïssa B, Memon NK, Ali A, Khraisheh MK. Recent progress in the growth and applications of graphene as a smart material: a review. Front Mater. 2015;2:58. 10.3389/fmats.2015.00058.Suche in Google Scholar
[32] Choi W, Lahiri I, Seelaboyina R, Kang YS. Synthesis of graphene and its applications: a review. Crit Rev Solid State Mater Sci. 2010;35(1):52–71. 10.1080/10408430903505036.Suche in Google Scholar
[33] Lokhande AC, Qattan IA, Lokhande CD, Patole SP. Holey graphene: an emerging versatile material. J Mater Chem A. 2020;8(3):918–77. 10.1039/C9TA10667G.Suche in Google Scholar
[34] Fan X, Zhang G, Zhang F. Multiple roles of graphene in heterogeneous catalysis. Chem Soc Rev. 2015;44(10):3023–35. 10.1039/C5CS00094G.Suche in Google Scholar PubMed
[35] Okamoto Y. Density-functional calculations of icosahedral M13 (M = Pt and Au) clusters on graphene sheets and flakes. Chem Phys Lett. 2006;420(4-6):382–6. 10.1016/j.cplett.2006.01.007.Suche in Google Scholar
[36] Leenaerts O, Partoens B, Peeters FM. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: a first-principles study. Phys Rev B. 2008;77(12):125416. 10.1103/PhysRevB.77.125416.Suche in Google Scholar
[37] Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, et al. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev. 2012;112(11):6156–214.10.1021/cr3000412Suche in Google Scholar PubMed
[38] Jia Y, Zhang L, Gao G, Chen H, Wang B, Zhou J, et al. A heterostructure coupling of exfoliated Ni–Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv Mater. 2017;29(17):1700017. 10.1002/adma.201700017.Suche in Google Scholar PubMed
[39] Kong XK, Chen CL, Chen QW. Doped graphene for metal-free catalysis. Chem Soc Rev. 2014;43(8):2841–57. 10.1039/C3CS60401B.Suche in Google Scholar
[40] Becerril HA, Mao J, Liu Z, Stoltenberg RM, Bao Z, Chen Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS nano. 2008;2(3):463–70. 10.1021/nn700375n.Suche in Google Scholar PubMed
[41] Chen D, Feng H, Li J. Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev. 2012;112(11):6027–53. 10.1021/cr300115g.Suche in Google Scholar PubMed
[42] Yan JA, Xian L, Chou MY. Structural and electronic properties of oxidized graphene. Phys Rev Lett. 2009;103(8):086802. 10.1103/PhysRevLett.103.086802.Suche in Google Scholar PubMed
[43] Yan JA, Chou MY. Oxidation functional groups on graphene: structural and electronic properties. Phys Rev B. 2010;82(12):125403. 10.1103/PhysRevB.82.125403.Suche in Google Scholar
[44] Boukhvalov DW, Katsnelson MI. Modeling of graphite oxide. J Am Chem Soc. 2008;130(32):10697–701. 10.1021/ja8021686.Suche in Google Scholar PubMed
[45] Tarcan R, Todor-Boer O, Petrovai I, Leordean C, Astilean S, Botiz I. Reduced graphene oxide today. J Mater Chem C. 2020;8(4):1198–224. 10.1039/C9TC04916A.Suche in Google Scholar
[46] Novoselov KS, Fal VI, Colombo L, Gellert PR, Schwab MG, Kim K. A roadmap for graphene. Nature. 2012;490(7419):192–200. 10.1038/nature11458.Suche in Google Scholar PubMed
[47] Loh KP, Bao Q, Eda G, Chhowalla M. Graphene oxide as a chemically tunable platform for optical applications. Nat Chem. 2010;2(12):1015. 10.1038/nchem.907.Suche in Google Scholar PubMed
[48] Li F, Jiang X, Zhao J, Zhang S. Graphene oxide: a promising nanomaterial for energy and environmental applications. Nano Energy. 2015;16:488–515. 10.1016/j.nanoen.2015.07.014.Suche in Google Scholar
[49] Guo X, Mei N. Assessment of the toxic potential of graphene family nanomaterials. J food drug Anal. 2014;22(1):105–15. 10.1016/j.jfda.2014.01.009.Suche in Google Scholar PubMed PubMed Central
[50] Chang H, Wu H. Graphene-based nanocomposites: preparation, functionalization, and energy and environmental applications. Energy Environ Sci. 2013;6(12):3483–507. 10.1039/C3EE42518E.Suche in Google Scholar
[51] Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A. Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano. 2013;7(4):2891–7. 10.1021/nn401196a.Suche in Google Scholar PubMed PubMed Central
[52] Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Controlled Release. 2014;173:75–88. 10.1016/j.jconrel.2013.10.017.Suche in Google Scholar PubMed
[53] Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70. 10.1016/j.cropro.2012.01.007.Suche in Google Scholar
[54] Hussain SZ, Ihrar M, Hussain SB, et al. A review on graphene based transition metal oxide composites and its application towards supercapacitor electrodes. SN Appl Sci. 2020;2:764. 10.1007/s42452-020-2515-8.Suche in Google Scholar
[55] Yoon H, Jang J. Conducting-polymer nanomaterials for high-performance sensor applications: issues and challenges. Adv Funct Mater. 2009;19(10):1567–76. 10.1002/adfm.200801141.Suche in Google Scholar
[56] Lawal AT. Graphene-based nano composites and their applications. A review. Biosens Bioelectron. 2019;141:111384. 10.1002/adfm.200801141.Suche in Google Scholar
[57] Guazzo R, Gardin C, Bellin G, Sbricoli L, Ferroni L, Ludovichetti FS, et al. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials. 2018;8(5):349. 10.3390/nano8050349.Suche in Google Scholar PubMed PubMed Central
[58] Tiwari SK, Sahoo S, Wang N, Huczko A. Graphene research and their outputs: status and prospect. J Sci Adv Mater Devices. 2020;5(1):10–29. 10.1016/j.jsamd.2020.01.006.Suche in Google Scholar
[59] Navalón S, Ong WJ, Duan X. Sustainable catalytic processes driven by graphene-based materials. Processes. 2020;8(6):672. 10.3390/pr8060672.Suche in Google Scholar
[60] Yu W, Sisi L, Haiyan Y, Jie L. Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv. 2020;10(26):15328–45. 10.1039/D0RA01068E.Suche in Google Scholar PubMed PubMed Central
[61] Hu M, Yao Z, Wang X. Graphene-based nanomaterials for catalysis. Ind Eng Chem Res. 2017;56(13):3477–502. 10.1021/acs.iecr.6b05048.Suche in Google Scholar
[62] Gupta S, Banu R, Ameta C, Ameta R, Punjabi PB. Emerging trends in the syntheses of heterocycles using graphene-based carbocatalysts: an update. Top Curr Chem. 2019;377(3):13. 10.1007/s41061-019-0238-3Suche in Google Scholar PubMed
[63] Choudhury P, Basu B. 3-Graphene oxide nanosheets as sustainable carbocatalysts: synthesis of medicinally important heterocycles, green approaches in medicinal chemistry for sustainable drug design. Adv Green Chem. 2020;47–74. 10.1016/B978-0-12-817592-7.00003-4.Suche in Google Scholar
[64] Su C, Loh KP. Carbocatalysts: graphene oxide and its derivatives. Acc Chem Res. 2013;46(10):2275–85. 10.1021/ar300118v.Suche in Google Scholar PubMed
[65] Aliyev E, Filiz V, Khan MM, Lee YJ, Abetz C, Abetz V. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials. 2019;9(8):1180. 10.3390/nano9081180.Suche in Google Scholar PubMed PubMed Central
[66] Adil SF, Assal ME, Khan M, Shaik MR, Kuniyil M, Sekou D, et al. Eco-friendly mechanochemical preparation of Ag2O–MnO2/graphene oxide nanocomposite: an efficient and reusable catalyst for the base-free, aerial oxidation of alcohols. Catalysts. 2020;10:281. 10.3390/catal10030281.Suche in Google Scholar
[67] Su C, Acik M, Takai K, Lu J, Hao S-J, Zheng Y, et al. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat Commun. 2012;3:1298. 10.1038/ncomms2315.Suche in Google Scholar PubMed
[68] Ali AA, Madkour M, Sagheer FA, Zaki MI, AbdelNazeer A. Low-temperature catalytic CO oxidation over non-noble, efficient chromia in reduced graphene oxide and graphene oxide nanocomposites. Catalysts. 2020;10:105. 10.3390/catal10010105.Suche in Google Scholar
[69] Gopiraman M, Saravanamoorthy S, Deng D, Ilangovan A, Kim IS, Chung IM. Facile mechanochemical synthesis of nickel/graphene oxide nanocomposites with unique and tunable morphology: applications in heterogeneous catalysis and supercapacitors. Catalysts. 2019;9:486. 10.3390/catal9050486.Suche in Google Scholar
[70] Abbas M, Chen Z, Chen J. Shape-and size-controlled synthesis of Cu nanoparticles wrapped on RGO nanosheet catalyst and their outstanding stability and catalytic performance in the hydrogenation reaction of dimethyl oxalate. J Mater Chem A. 2018;6(39):19133–42. 10.1039/C8TA07371F.Suche in Google Scholar
[71] Sengupta D, Bhowmik K, De G, Basu B. Ni nanoparticles on RGO as reusable heterogeneous catalyst: effect of Ni particle size and intermediate composite structures in C–S cross-coupling reaction. Beilstein J Org Chem. 2017;13(1):1796–806. 10.3762/bjoc.13.174.Suche in Google Scholar PubMed PubMed Central
[72] Hurtado RB, Cortez-Valadez M, Aragon-Guajardo JR, Cruz-Rivera JJ, Martínez-Suárez F, Flores-Acosta M. One-step synthesis of reduced graphene oxide/gold nanoparticles under ambient conditions. Arab J Chem. 2017;13:1633–40. 10.1016/j.arabjc.2017.12.021.Suche in Google Scholar
[73] Movahed SK, Fakharian M, Dabiri M, Bazgir A. Gold nanoparticle decorated reduced graphene oxide sheets with high catalytic activity for Ullmann homocoupling. RSC Adv. 2014;4(10):5243–7. 10.1002/chin.201438086.Suche in Google Scholar
[74] Ji Z, Shen X, Yang J, Zhu G, Chen K. A novel reduced graphene oxide/Ag/CeO2 ternary nanocomposite: green synthesis and catalytic properties. Appl Catal B. 2014;144:454–61. 10.1016/j.apcatb.2013.07.052.Suche in Google Scholar
[75] Vilian AE, Choe SR, Giribabu K, Jang SC, Roh C, Huh YS, et al. Pd nanospheres decorated reduced graphene oxide with multi-functions: highly efficient catalytic reduction and ultrasensitive sensing of hazardous 4-nitrophenol pollutant. J Hazard Mater. 2017;333:54–62. 10.1016/j.jhazmat.2017.03.015.Suche in Google Scholar PubMed
[76] Hao Y, Wang X, Zheng Y, Shen J, Yuan J, Wang AJ, et al. Uniform Pt nanoparticles incorporated into reduced graphene oxides with MoO3 as advanced anode catalysts for methanol electro-oxidation. Electrochim Acta. 2016;198:127–34. 10.1016/j.electacta.2016.03.054.Suche in Google Scholar
[77] Movahed SK, Dabiri M, Bazgir A. A one-step method for preparation of Cu@ Cu2O nanoparticles on reduced graphene oxide and their catalytic activities in N-arylation of N-heterocycles. Appl Catal A. 2014;481:79–88. 10.1016/j.apcata.2014.04.023.Suche in Google Scholar
[78] Pei S, Cheng HM. The reduction of graphene oxide. Carbon. 2012;50(9):3210–28. 10.1016/j.carbon.2011.11.010.Suche in Google Scholar
[79] Shen Y, Zhu C, Chen B. Immobilizing 1–3 nm Ag nanoparticles in reduced graphene oxide aerogel as a high-effective catalyst for reduction of nitroaromatic compounds. Environ Pollut. 2020;256:113405. 10.1016/j.envpol.2019.113405.Suche in Google Scholar PubMed
[80] Jeffery AA, Rao SR, Rajamathi M. Preparation of MoS2–reduced graphene oxide (rGO) hybrid paper for catalytic applications by simple exfoliation–costacking. Carbon. 2017;112:8–16. 10.1016/j.carbon.2016.11.001.Suche in Google Scholar
[81] Banerjee B. Recent developments on nano-ZnO catalyzed synthesis of bioactive heterocycles. J Nanostruct Chem. 2017;7(4):389–413. 10.1007/s40097-017-0247-0.Suche in Google Scholar
[82] Guo X, Wang L, Hu J, Zhang M. CuI nanoparticle-catalyzed synthesis of tetracyclic benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6-imine heterocycles by SN Ar-type C–S, C–N bond formation from isothiocyanatobenzenes and benzimidazoles. RSC Adv. 2018;8(39):22259–67. 10.1039/C8RA02552E.Suche in Google Scholar
[83] Lonkar S, Abdala A. Applications of graphene in catalysis. J Thermodyn Catal. 2014;5:2. 10.4172/2157-7544.1000132.Suche in Google Scholar
[84] Chandel M, Makkar P, Ghosh BK, Moitra D, Ghosh NN. A facile synthesis methodology for preparation of Ag–Ni-reduced graphene oxide: a magnetically separable versatile nanocatalyst for multiple organic reactions and density functional study of its electronic structures. RSC Adv. 2018;8(66):37774–88. 10.1039/C8RA08235A.Suche in Google Scholar PubMed PubMed Central
[85] Su C, Loh KP. Carbocatalysts: graphene oxide and its derivatives. Acc Chem Res. 2013;46(10):2275–85. 10.1021/ar300118v.Suche in Google Scholar PubMed
[86] Lee YH, Kim SG, Tománek D. Catalytic growth of single-wall carbon nanotubes: an ab initio study. Phys Rev Lett. 1997;78:2393–6. 10.1103/PhysRevLett.78.2393.Suche in Google Scholar
[87] Bahuguna A, Kumar A, Krishnan V. Carbon-support-based heterogeneous nanocatalysts: synthesis and applications in organic reactions. Asian J Org Chem. 2019;8(8):1263–305.10.1002/ajoc.201900259Suche in Google Scholar
[88] Jain Y, Kumari M, Laddha H, Gupta R. Ultrasound promoted fabrication of CuO-graphene oxide nanocomposite for facile synthesis of fluorescent coumarin based 1,4-disubsituted 1,2,3-triazoles in aqueous media. Chem Sel. 2019;4(23):7015–26. 10.1002/slct.201901355.Suche in Google Scholar
[89] Wang Y, Wen Z, Zhang H, Cao G, Sun Q, Cao J. CuO nanorods-decorated reduced graphene oxide nanocatalysts for catalytic oxidation of CO. Catalysts. 2016;6(12):214. 10.3390/catal6120214.Suche in Google Scholar
[90] Zhang K, Suh JM, Lee TH, Cha JH, Choi JW, Jang HW, et al. Copper oxide–graphene oxide nanocomposite: efficient catalyst for hydrogenation of nitroaromatics in water. Nano Convergence. 2019;6(1):6. 10.1186/s40580-019-0176-3.Suche in Google Scholar PubMed PubMed Central
[91] Hussain N, Gogoi P, Das MR, Sengupta P, Fedorov VE, Asanov IP, et al. Development of novel efficient 2D nanocomposite catalyst towards the three-component coupling reaction for the synthesis of imidazo[1,2-a]pyridines. Appl Catal A. 2017;542:368–79. 10.1016/j.apcata.2017.05.033.Suche in Google Scholar
[92] Sarkar R, Gupta A, Jamatia R, Pal AK. Reduced graphene oxide supported copper oxide nanocomposites: an efficient heterogeneous and reusable catalyst for the synthesis of ynones, 1,3-diynes and 1,5-benzodiazepines in one-pot under sustainable reaction conditions. Appl Organomet Chem. 2020;34(7):e5646. 10.1002/aoc.5646.Suche in Google Scholar
[93] Choi J, Oh H, Han SW, Ahn S, Noh J, Park JB. Preparation and characterization of graphene oxide supported Cu, Cu2O, and CuO nanocomposites and their high photocatalytic activity for organic dye molecule. Curr Appl Phys. 2017;17(2):137–45. 10.1016/j.cap.2016.11.020.Suche in Google Scholar
[94] Dandia A, Bansal S, Sharma R, Rathore KS, Parewa V. Microwave-assisted nanocatalysis: a CuO NPs/rGO composite as an efficient and recyclable catalyst for the Petasis-borono–Mannich reaction. RSC Adv. 2018;8(53):30280–8. 10.1039/C8RA05203D.Suche in Google Scholar
[95] Gupta A, Jamatia R, Patil RA, Ma YR, Pal AK. Copper oxide/reduced graphene oxide nanocomposite-catalyzed synthesis of flavanones and flavanones with triazole hybrid molecules in one pot: a green and sustainable approach. ACS omega. 2018;3(7):7288–99. 10.1021/acsomega.8b00334.Suche in Google Scholar PubMed PubMed Central
[96] Wu P, Nielsen TE. Petasis three-component reactions for the synthesis of diverse heterocyclic scaffolds. Drug Discovery Today: Technol. 2018;29:27–33. 10.1016/j.ddtec.2018.06.010.Suche in Google Scholar PubMed
[97] Tong M, Bai X, Meng X, Wang J, Wang T, Zhu X, et al. Enantioselective synthesis of α-amino esters through Petasis borono-Mannich multicomponent reaction of potassium trifluoroborate salts. J Chem Res. 2019;43(11–12):557–64. 10.1177/1747519819876822.Suche in Google Scholar
[98] Wu P, Givskov, Nielsen M, TE. Reactivity and synthetic applications of multicomponent petasis reactions. Chem Rev. 2019;119(20):11245–90. 10.1021/acs.chemrev.9b00214.Suche in Google Scholar PubMed PubMed Central
[99] Cabrera M, Simoens M, Falchi G, Lavaggi ML, Piro OE, Castellano EE, et al. Synthetic chalcones, flavanones, and flavones as antitumoral agents: biological evaluation and structure–activity relationships. Bioorg Med Chem. 2007;15(10):3356–67. 10.1016/j.bmc.2007.03.031.Suche in Google Scholar PubMed
[100] Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, et al. Flavonoids in cancer and apoptosis. Cancers. 2019;11(1):28. 10.3390/cancers11010028.Suche in Google Scholar PubMed PubMed Central
[101] Frattaruolo L, Carullo G, Brindisi M, Mazzotta S, Bellissimo L, Rago V, et al. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L.(licorice) leaf phytocomplexes: identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants. 2019;8(6):186. 10.3390/antiox8060186.Suche in Google Scholar PubMed PubMed Central
[102] Chen X, Mukwaya E, Wong MS, Zhang Y. A systematic review on biological activities of prenylated flavonoids. Pharm Biol. 2014;52(5):655–60. 10.3109/13880209.2013.853809.Suche in Google Scholar PubMed
[103] Zhang B. Comprehensive review on the anti-bacterial activity of 1, 2, 3-triazole hybrids. Eur J medicinal Chem. 2019;168:357–72. 10.1016/j.ejmech.2019.02.055.Suche in Google Scholar PubMed
[104] Kavala V, Lin C, Kuo CW, Fang H, Yao CF. Iodine catalyzed one-pot synthesis of flavanone and tetrahydropyrimidine derivatives via Mannich type reaction. Tetrahedron. 2012;68(4):1321–9. 10.1016/j.tet.2011.11.022.Suche in Google Scholar
[105] Rostamizadeh S, Zekri N, Tahershamsi L. Nanosilica-supported dual acidic ionic liquid as a heterogeneous and reusable catalyst for the synthesis of flavanones under solvent-free conditions. Chem Heterocycl Compd. 2015;51(6):526–30. 10.1007/s10593-015-1728-z.Suche in Google Scholar
[106] Sakirolla R, Yaeghoobi M, Rahman NA. Synthesis of flavanones, azaflavanones, and thioflavanones catalyzed by PMA-SiO2 as a mild, efficient, and reusable catalyst. Monatshefte für Chem Chem Monthly. 2012;143(5):797–800. 10.1007/s00706-011-0663-7.Suche in Google Scholar
[107] Rocha DH, Vaz PA, Pinto DC, Silva A. Synthesis chalones and their isomerization into flavanones and azaflavanones. Methods Protoc. 2019;2(3):70. 10.3390/mps2030070.Suche in Google Scholar PubMed PubMed Central
[108] Yang C, Li X, Zhang Z, Lv B, Li J, Liu Z, et al. High efficient catalytic oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid under benign conditions with nitrogen-doped graphene encapsulated Cu nanoparticles. J Energy Chem. 2020;50:96–105.10.1016/j.jechem.2020.03.003Suche in Google Scholar
[109] Yoo E, Okata T, Akita T, Kohyama M, Nakamura J, Honma I. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 2009;9(6):2255–9. 10.1021/nl900379c.Suche in Google Scholar PubMed
[110] Yılmaz MS, Kaplan BY, Metin Ö, Gürsel SA. A facile synthesis and assembly of ultrasmall Pt nanoparticles on reduced graphene oxide-carbon black hybrid for enhanced performance in PEMFC. Mater Des. 2018;151:29–36. 10.1016/j.matdes.2018.04.041.Suche in Google Scholar
[111] Lee MH, Wang SY, Chiang WH, Feng H, Huang TY, Yeh MH, et al. Platinum nanoparticles decorated graphene nanoribbon with eco-friendly unzipping process for electrochemical sensors. J Taiwan Inst Chem Eng. 2019;96:566–74. 10.1016/j.jtice.2018.11.012.Suche in Google Scholar
[112] Li F, Guo Y, Wu T, Liu Y, Wang W, Gao J. Platinum nano-catalysts deposited on reduced graphene oxides for alcohol oxidation. Electrochim Acta. 2013;111:614–20. 10.1016/j.electacta.2013.08.058.Suche in Google Scholar
[113] Lee JY, Yung TY, Liu LK. The microwave-assisted ionic liquid nanocomposite synthesis: platinum nanoparticles on graphene and the application on hydrogenation of styrene. Nanoscale Res Lett. 2013;8(1):1–6. 10.1186/1556-276X-8-414.Suche in Google Scholar PubMed PubMed Central
[114] Aday B, Yıldız Y, Ulus R, Eris S, Sen F, Kaya M. One-pot, efficient and green synthesis of acridinedione derivatives using highly monodisperse platinum nanoparticles supported with reduced graphene oxide. N J Chem. 2016;40(1):748–54. 10.1039/C5NJ02098K.Suche in Google Scholar
[115] Moallem SA, Dehghani N, Mehri S, Shahsavand S, Alibolandi M, Hadizadeh F. Synthesis of novel 1, 8-acridinediones derivatives: investigation of MDR reversibility on breast cancer cell lines T47D and tamoxifen-resistant T47D. Res Pharm Sci. 2015;10(3):214. PMCID: PMC4621628.Suche in Google Scholar
[116] Gündüz MG, İşli F, El-Khouly A, Yıldırım Ş, Fincan GS, Şimşek R, et al. Microwave-assisted synthesis and myorelaxant activity of 9-indolyl-1, 8-acridinedione derivatives. Eur J Med Chem. 2014;75:258–66. 10.1016/j.ejmech.2014.01.059.Suche in Google Scholar PubMed
[117] Ulus R, Yeşildağ İ, Elmastaş M, Kaya M. Rapid synthesis of novel 1,8-dioxoacridine carboxylic acid derivatives by microwave irradiation and their free radical scavenging activity. Med Chem Res. 2015;24(10):3752–9. 10.1007/s00044-015-1417-6.Suche in Google Scholar
[118] Singh TP, Singh OM. Recent progress in biological activities of indole and indole alkaloids. Mini Rev Med Chem. 2018;18(1):9–25. 10.2174/1389557517666170807123201.Suche in Google Scholar PubMed
[119] Jensen T, Pedersen H, Bang-Andersen B, Madsen R, Jørgensen M. Palladium-catalyzed aryl amination–heck cyclization cascade: a one-flask approach to 3-substituted indoles. Angew Chem Int Ed. 2008;47(5):888–90. 10.1002/anie.200703763.Suche in Google Scholar PubMed
[120] Dobish MC, Johnston JN. Chiral Brønsted base-promoted nitroalkane alkylation: enantioselective synthesis of sec-alkyl-3-substituted indoles. Org Lett. 2010;12(24):5744–7. 10.1021/ol1025712.Suche in Google Scholar PubMed PubMed Central
[121] Peddibhotla S. 3-Substituted-3-hydroxy-2-oxindole, an emerging new scaffold for drug discovery with potential anti-cancer and other biological activities. Curr Bioact Compd. 2009;5(1):20–38. 10.2174/157340709787580900.Suche in Google Scholar
[122] Rajesh UC, Wang J, Prescott S, Tsuzuki T, Rawat DS. RGO/ZnO nanocomposite: an efficient, sustainable, heterogeneous, amphiphilic catalyst for synthesis of 3-substituted indoles in water. ACS Sustainable Chem Eng. 2015;3(1):9–18. 10.1021/sc500594w.Suche in Google Scholar
[123] Wang J, Tsuzuki T, Tang B, Sun L, Dai XJ, Rajmohan GD, et al. Recyclable textiles functionalized with reduced graphene oxide@ ZnO for removal of oil spills and dye pollutants. Australian J Chem. 2014;67(1):71–7. 10.1071/CH13323.Suche in Google Scholar
[124] Khatun R, Biswas S, Islam S, Biswas IH, Riyajuddin S, Ghosh K, et al. Modified graphene oxide based zinc composite: an efficient catalyst for N-formylation and carbamate formation reactions through CO2 fixation. ChemCatChem. 2019;11(4):1303–12. 10.1002/cctc.201801963.Suche in Google Scholar
[125] Ruiz-Botella S, Peris E. Immobilization of pyrene-adorned N-heterocyclic carbene complexes of rhodium (i) on reduced graphene oxide and study of their catalytic activity. ChemCatChem. 2018;10(8):1874–81. 10.1002/cctc.201701277.Suche in Google Scholar
[126] Rodriguez-Perez L, Herranz MÁ, Martín N. The chemistry of pristine graphene. Chem Commun. 2013;49(36):3721–35. 10.1039/C3CC38950B.Suche in Google Scholar
[127] Le Goff A, Reuillard B, Cosnier S. A pyrene-substituted tris (bipyridine) osmium (ii) complex as a versatile redox probe for characterizing and functionalizing carbon nanotube-and graphene-based electrodes. Langmuir. 2013;29(27):8736–42. 10.1021/la401712u.Suche in Google Scholar PubMed
[128] Ventura-Espinosa D, Vicent C, Baya M, Mata JA. Ruthenium molecular complexes immobilized on graphene as active catalysts for the synthesis of carboxylic acids from alcohol dehydrogenation. Catal Sci Technol. 2016;6(22):8024–35. 10.1039/C6CY01455K.Suche in Google Scholar
[129] Ruiz-Botella S, Peris E. Unveiling the importance of π-stacking in borrowing-hydrogen processes catalysed by iridium complexes with pyrene tags. Chemistry–A Eur J. 2015;21(43):15263–71. 10.1002/chem.201502948.Suche in Google Scholar PubMed
[130] Peris E. Polyaromatic N-heterocyclic carbene ligands and π-stacking. Catalytic consequences. Chem Commun. 2016;52(34):5777–87. 10.1039/C6CC02017H.Suche in Google Scholar PubMed
[131] Sabater S, Mata JA, Peris E. Immobilization of pyrene-tagged palladium and ruthenium complexes onto reduced graphene oxide: an efficient and highly recyclable catalyst for hydrodefluorination. Organometallics. 2015;34(7):1186–90. 10.1021/om501040x.Suche in Google Scholar
[132] Sabater S, Mata JA, Peris E. Catalyst enhancement and recyclability by immobilization of metal complexes onto graphene surface by noncovalent interactions. ACS Catal. 2014;4(6):2038–47. 10.1021/cs5003959.Suche in Google Scholar
[133] Gutiérrez-Blanco A, Peris E, Poyatos M. Pyrene-connected tetraimidazolylidene complexes of iridium and rhodium. Structural features and catalytic applications. Organometallics. 2018;37(21):4070–6. 10.1021/acs.organomet.8b00633.Suche in Google Scholar
[134] Smith CA, Narouz MR, Lummis PA, Singh I, Nazemi A, Li CH, et al. N-heterocyclic carbenes in materials chemistry. Chem Rev. 2019;119(8):4986–5056. 10.1021/acs.chemrev.8b00514.Suche in Google Scholar PubMed
[135] Zhang W, Wang S, Ji J, Li Y, Zhang G, Zhang F, et al. Primary and tertiary amines bifunctional graphene oxide for cooperative catalysis. Nanoscale. 2013;5(13):6030–3. 10.1039/c3nr01323e.Suche in Google Scholar PubMed
[136] Movahed SK, Esmatpoursalmani R, Bazgir A. N-heterocyclic carbene palladium complex supported on ionic liquid-modified graphene oxide as an efficient and recyclable catalyst for Suzuki reaction. RSC Adv. 2014;4(28):14586–91. 10.1039/C3RA46056H.Suche in Google Scholar
[137] Shang N, Gao S, Feng C, Zhang H, Wang C, Wang Z. Graphene oxide supported N-heterocyclic carbene-palladium as a novel catalyst for the Suzuki–Miyaura reaction. RSC Adv. 2013;3(44):21863–8. 10.1039/C3RA44620D.Suche in Google Scholar
[138] Sánchez-Page B, Jiménez MV, Pérez-Torrente JJ, Passarelli V, Blasco J, Subias G, et al. Hybrid catalysts comprised of graphene modified with rhodium-based N-heterocyclic carbenes for alkyne hydrosilylation. ACS Appl Nano Mater. 2020;3(2):1640–55. 10.1021/acsanm.9b02398.Suche in Google Scholar
[139] Ji J, Zhang G, Chen H, Wang S, Zhang G, Zhang F, et al. Sulfonated graphene as water-tolerant solid acid catalyst. Chem Sci. 2011;2(3):484–7. 10.1039/C0SC00484G.Suche in Google Scholar
[140] Liu F, Sun J, Zhu L, Meng X, Qi C, Xiao FS. Sulfated graphene as an efficient solid catalyst for acid-catalyzed liquid reactions. J Mater Chem. 2012;22(12):5495–502. 10.1039/C2JM16608A.Suche in Google Scholar
[141] Yang Z, Huang R, Qi W, Tong L, Su R, He Z. Hydrolysis of cellulose by sulfonated magnetic reduced graphene oxide. Chem Eng J. 2015;280:90–98. 10.1016/j.cej.2015.05.091.Suche in Google Scholar
[142] Antunes MM, Russo PA, Wiper PV, Veiga JM, Pillinger M, Mafra L, et al. Sulfonated graphene oxide as effective catalyst for conversion of 5-(hydroxymethyl)-2-furfural into biofuels. ChemSusChem. 2014;7(3):804–12. 10.1002/cssc.201301149.Suche in Google Scholar PubMed
[143] Naeimi H, Golestanzadeh M. Microwave-assisted synthesis of 6,6′-(aryl(alkyl)methylene) bis (2,4-dialkylphenol) antioxidants catalyzed by multi-sulfonated reduced graphene oxide nanosheets in water. N J Chem. 2015;39(4):2697–710. 10.1039/C4NJ02340D.Suche in Google Scholar
[144] Zhou J, Wang Y, Guo X, Mao J, Zhang S. Etherification of glycerol with isobutene on sulfonated graphene: reaction and separation. Green Chem. 2014;16(11):4669–79. 10.1039/C4GC01044B.Suche in Google Scholar
[145] Pitasse-Santos P, Sueth-Santiago V, Lima ME. 1, 2, 4-and 1, 3, 4-oxadiazoles as scaffolds in the development of antiparasitic agents. J Braz Chem Soc. 2018;29(3):435–56. 10.21577/0103-5053.20170208.Suche in Google Scholar
[146] Wang PY, Shao WB, Xue HT, Fang HS, Zhou J, Wu ZB, et al. Synthesis of novel 1, 3, 4-oxadiazole derivatives containing diamides as promising antibacterial and antiviral agents. Res Chem Intermed. 2017;43(11):6115–30. 10.1007/s11164-017-2980-x.Suche in Google Scholar
[147] Zhang G, Yu Y, Zhao Y, Xie X, Ding C. Iron (III)/TEMPO-catalyzed synthesis of 2, 5-disubstituted 1, 3, 4-oxadiazoles by oxidative cyclization under mild conditions. Synlett. 2017;28(11):1373–7. 10.1055/s-0036-1588747.Suche in Google Scholar
[148] Fan Y, He Y, Liu X, Hu T, Ma H, Yang X, et al. Iodine-mediated domino oxidative cyclization: one-pot synthesis of 1, 3, 4-oxadiazoles via oxidative cleavage of C (sp2)–H or C (sp)–H bond. J Org Chem. 2016;81(15):6820–5. 10.1021/acs.joc.6b01135.Suche in Google Scholar PubMed
[149] Brahmayya M, Dai SA, Suen SY. Synthesis of 5-substituted-3 H-[1, 3, 4]-oxadiazol-2-one derivatives: a carbon dioxide route (CDR). RSC Adv. 2015;5(80):65351–7. 10.1039/C5RA08910G.Suche in Google Scholar
[150] Brahmayya M, Dai SA, Suen SY. Sulfonated reduced graphene oxide catalyzed cyclization of hydrazides and carbon dioxide to 1, 3, 4-oxadiazoles under sonication. Sci Rep. 2017;7(1):1–3. 10.1038/s41598-017-04143-4.Suche in Google Scholar PubMed PubMed Central
[151] Behravesh S, Fareghi-Alamdari R, Badri R. Sulfonated reduced graphene oxide (RGO-SO3H): As an efficient nanocatalyst for one-pot synthesis of 2-amino-3-cyano-7-hydroxy-4H-chromenes derivatives in water. Polycycl Aromatic Compd. 2018;38(1):51–65. 10.1080/10406638.2016.1149080.Suche in Google Scholar
[152] Mirza-Aghayan M, Tavana MM, Boukherroub R. Sulfonated reduced graphene oxide as a highly efficient catalyst for direct amidation of carboxylic acids with amines using ultrasonic irradiation. Ultrason Sonochem. 2016;29:371–9. 10.1016/j.ultsonch.2015.10.009.Suche in Google Scholar PubMed
[153] Verma C, Ebenso EE. Ionic liquid-mediated functionalization of graphene-based materials for versatile applications: a review. Graphene Technol. 2019;4(1-2):1–5. 10.1007/s41127-018-0023-z.Suche in Google Scholar
[154] Fraga TJ, Carvalho MN, Ghislandi MG, Motta Sobrinho MA. Functionalized graphene-based materials as innovative adsorbents of organic pollutants: a concise overview. Braz J Chem Eng. 2019;36(1):1–31. 10.1590/0104-6632.20190361s20180283.Suche in Google Scholar
[155] Yin B, Zhang X, Zhang X, Wang J, Wen Y, Jia H, et al. Ionic liquid functionalized graphene oxide for enhancement of styrene-butadiene rubber nanocomposites. Polym Adv Technol. 2017;28(3):293–302. 10.1002/pat.3886.Suche in Google Scholar
[156] Lin B, Yuan W, Xu F, Chen Q, Zhu H, Li X, et al. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl Surf Sci. 2018;455:295–301. 10.1016/j.apsusc.2018.05.205.Suche in Google Scholar
[157] Liu N, Chen X, Ma Z. Ionic liquid functionalized graphene/Au nanocomposites and its application for electrochemical immunosensor. Biosens Bioelectron. 2013;48:33–8. 10.1016/j.bios.2013.03.080.Suche in Google Scholar PubMed
[158] Shi X, Cai C. Imidazolium-based ionic liquid functionalized reduced graphene oxide supported palladium as a reusable catalyst for Suzuki–Miyaura reactions. N J Chem. 2018;42(4):2364–7. 10.1039/C7NJ04312K.Suche in Google Scholar
[159] Tang W, Huang Z, Wang B. Synthesis of ionic liquid functionalized graphene oxides and their tribological property under water lubrication. Fullerenes Nanotubes Carbon Nanostruct. 2018;26(3):175–83. 10.1080/1536383X.2017.1422246.Suche in Google Scholar
[160] Fraga TJ, Carvalho MN, Ghislandi MG, Motta Sobrinho MA. Functionalized grahene-based materials as innovative adsorbents of organic pollutants: a concise overview. Braz J Chem Eng. 2019;36(1):1–31. 10.1590/0104-6632.20190361s20180283.Suche in Google Scholar
[161] Nakhate AV, Yadav GD. Graphene-oxide-supported SO3H-functionalized imidazolium-based ionic liquid: efficient and recyclable heterogeneous catalyst for alcoholysis and aminolysis reactions. Chemistry Select. 2018;3(16):4547–56. 10.1002/slct.201703064.Suche in Google Scholar
[162] Garkoti C, Shabir J, Gupta P, Sharma M, Mozumdar S. Heterogenization of amine-functionalized ionic liquids using graphene oxide as a support material: a highly efficient catalyst for the synthesis of 3-substituted indoles via Yonemitsu-type reaction. N J Chem. 2017;41(24):15545–54. 10.1039/C7NJ03450D.Suche in Google Scholar
[163] Hanoon HD, Kowsari E, Abdouss M, Zandi H, Ghasemi MH. Efficient preparation of acidic ionic liquid-functionalized reduced graphene oxide and its catalytic performance in synthesis of benzimidazole derivatives. Res Chem Intermed. 2017;43(3):1751–66. 10.1007/s11164-016-2727-0.Suche in Google Scholar
[164] Shaharyar M, Mazumder A. Benzimidazoles: a biologically active compounds. Arab J Chem. 2017;10:S157–S173. 10.1016/j.arabjc.2012.07.017Suche in Google Scholar
[165] Dreyer DR, Jia HP, Bielawski CW. Graphene oxide: a convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew Chem. 2010;122(38):6965–8. 10.1002/anie.201002160.Suche in Google Scholar PubMed
[166] Basu B, Kundu S, Sengupta D. Graphene oxide as a carbocatalyst: the first example of a one-pot sequential dehydration–hydrothiolation of secondary aryl alcohols. RSC Adv. 2013;3(44):22130–4. 10.1039/C3RA44712J.Suche in Google Scholar
[167] Patel KP, Gayakwad EM, Shankarling GS. Graphene oxide: a convenient metal-free carbocatalyst for facilitating amidation of esters with amines. N J Chem. 2020;44(6):2661–8. 10.1039/C9NJ05283F.Suche in Google Scholar
[168] Karthik M, Suresh P. Graphene oxide as a carbocatalyst for sustainable ipso-hydroxylation of arylboronic acids: a simple and straightforward strategy to access phenols. ACS Sustainable Chem Eng. 2019;7(9):9028–34. 10.1021/acssuschemeng.9b01361.Suche in Google Scholar
[169] Eskandari K, Karami B. Graphene oxide nanosheets-catalyzed synthesis of novel benzylbarbiturocoumarin derivatives under green conditions. Monatshefte Chem Chem Monthly. 2016;147(12):2119–26. 10.1007/s00706-016-1724-8.Suche in Google Scholar
[170] Kausar N, Roy I, Chattopadhyay D, Das AR. Synthesis of 2, 3-dihydroquinazo- linones and quinazolin-4 (3 H)-ones catalyzed by graphene oxide nanosheets in an aqueous medium:“on-water” synthesis accompanied by carbocatalysis and selective C–C bond cleavage. RSC Adv. 2016;6(27):22320–30. 10.1039/C6RA00388E.Suche in Google Scholar
[171] Siddiqui TA, Ghule BG, Shaikh S, Shinde PV, Gunturu KC, Zubaidha PK, et al. Metal-free heterogeneous and mesoporous biogenic graphene-oxide nanoparticle-catalyzed synthesis of bioactive benzylpyrazolyl coumarin derivatives. RSC Adv. 2018;8(31):17373–9. 10.1039/C7RA12550J.Suche in Google Scholar
[172] Chen CY, Guo XY, Lu GQ, Pedersen CM, Qiao Y, Hou XL, et al. Graphene oxide: a novel acid catalyst for the synthesis of 2, 5-dimethyl-N-phenyl pyrrole by the Paal–Knorr condensation. N Carbon Mater. 2017;32(2):160–7. 10.1016/S1872-5805(17)60013-6.Suche in Google Scholar
[173] Reddy MS, Kumar NS, Chowhan LR. Heterogeneous graphene oxide as recyclable catalyst for azomethine ylide mediated 1, 3 dipolar cycloaddition reaction in aqueous medium. RSC Adv. 2018;8(62):35587–93. 10.1039/C8RA06714G.Suche in Google Scholar PubMed PubMed Central
[174] Kundu S, Basu B. Graphene oxide (GO)-catalyzed multi-component reactions: green synthesis of library of pharmacophore 3-sulfenylimidazo [1, 2-a] pyridines. RSC Adv. 2015;5(62):50178–85. 10.1039/C5RA04983K.Suche in Google Scholar
[175] Bavadi M, Niknam K. Synthesis of functionalized dihydro-2-oxopyrroles using graphene oxide as heterogeneous catalyst. Mol diversity. 2018;22(3):561–73. 10.1007/s11030-017-9809-9.Suche in Google Scholar PubMed
[176] Roy B, Ghosh S, Ghosh P, Basu B. Graphene oxide (GO) or reduced graphene oxide (rGO): efficient catalysts for one-pot metal-free synthesis of quinoxalines from 2-nitroaniline. Tetrahedron Lett. 2015;56(48):6762–7. 10.1016/j.tetlet.2015.10.065.Suche in Google Scholar
[177] Cheng G, Sa W, Cao C, Guo L, Hao H, Liu Z, et al. Quinoxaline 1, 4-di-N-oxides: biological activities and mechanisms of actions. Front Pharmacol. 2016;7:64. 10.3389/fphar.2016.00064.Suche in Google Scholar PubMed PubMed Central
[178] Ebajo VD, Santos CR, Alea GV, Lin YA, Chen CH. Regenerable acidity of graphene oxide in promoting multicomponent organic synthesis. Sci Rep. 2019;9(1):1–2. 10.1038/s41598-019-51833-2.Suche in Google Scholar PubMed PubMed Central
[179] Sengupta D, Ghosh S, Basu B. Advances and prospects of graphene oxide (GO) as heterogeneous’ carbocatalyst’. Curr Org Chem. 2017;21(9):834–54. 10.2174/1385272820666161021102757.Suche in Google Scholar
[180] Majumdar B, Sarma D, Sarma TK. Carbocatalytic activity of graphene oxide in organic synthesis. Graphene Oxide Appl Opportunities. 2018;25. 10.5772/intechopen.77361.Suche in Google Scholar
[181] Yoo E, Okata T, Akita T, Kohyama M, Nakamura J, Honma I. Enhanced electrocatalytic activity of Pt sub nanoclusters on graphene nanosheet surface. Nano Lett. 2009;9:2255–9. 10.1021/nl900397t.Suche in Google Scholar PubMed
[182] Yan Y, Wang X, Lou DX, Xia BY. Recent progress on graphene-based hybrid electrocatalysts. Mater Horiz. 2014;1(4):379–99. 10.1039/C4MH00040D.Suche in Google Scholar
[183] Huang C, Li C, Shi G. Graphene based catalysts. Energy Environ Sci. 2012;5(10):8848–68. 10.1039/C2EE22238H.Suche in Google Scholar
[184] Machado BF, Serp P. Graphene-based materials for catalysis. Catal Sci Technol. 2012;2(1):54–75. 10.1039/C1CY00361E.Suche in Google Scholar
[185] Ke Q, Wang J. Graphene-based materials for supercapacitor electrodes–a review. J Materiomics. 2016;2(1):37–54. 10.1016/j.jmat.2016.01.001.Suche in Google Scholar
[186] Wang J, Gong C, Wen S, Liu H, Qin C, Xiong C, et al. Proton exchange membrane based on chitosan and solvent-free carbon nanotube fluids for fuel cells applications. Carbohydr Polym. 2018;186:200–7. 10.1016/j.carbpol.2018.01.032.Suche in Google Scholar PubMed
[187] Ioroi T, Siroma Z, Yamazaki SI, Yasuda K. Electrocatalysts for PEM fuel cells. Adv Energy Mater. 2019;9(23):1801284. 10.1002/aenm.201801284.Suche in Google Scholar
[188] Guo S, Sun S. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J Am Chem Soc. 2012;134(5):2492–5. 10.1021/ja2104334.Suche in Google Scholar PubMed
[189] Ma R, Lin G, Zhou Y, Liu Q, Zhang T, Shan G, et al. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput Mater. 2019;5(1):1–5. 10.1038/s41524-019-0210-3.Suche in Google Scholar
[190] Lu Z, Li S, Liu C, He C, Yang X, Ma D, et al. Sulfur doped graphene as a promising metal-free electrocatalyst for oxygen reduction reaction: a DFT-D study. RSC Adv. 2017;7(33):20398–405. 10.1039/C7RA00632BSuche in Google Scholar
[191] Zhang C, Mahmood N, Yin H, Liu F, Hou Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv Mater. 2013;25(35):4932–7. 10.1002/adma.201301870.Suche in Google Scholar PubMed
[192] Marinoiu A, Raceanu M, Carcadea E, Varlam M, Balan D, Ion-Ebrasu D, et al. Iodine-doped graphene for enhanced electrocatalytic oxygen reduction reaction in proton exchange membrane fuel cell applications. J Electrochem Energy Convers Storage. 2017;14(3). 10.1115/1.4036684.Suche in Google Scholar
[193] Jeon IY, Choi HJ, Choi M, Seo JM, Jung SM, Kim MJ, et al. Facile, scalable synthesis of edge-halogenated graphene nanoplatelets as efficient metal-free eletrocatalysts for oxygen reduction reaction. Sci Rep. 2013;3:1810. 10.1038/srep01810.Suche in Google Scholar PubMed PubMed Central
[194] Yang W, Fellinger TP, Antonietti M. Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases. J Am Chem Soc. 2011;133(2):206–9. 10.1021/ja108039j.Suche in Google Scholar PubMed
[195] Iqbal MZ, Rehman AU, Siddique S. Prospects and challenges of graphene based fuel cells. J Energy Chem. 2019;39:217–34.10.1016/j.jechem.2019.02.009Suche in Google Scholar
[196] Ji X, Zhang X, Zhang X. Three-dimensional graphene-based nanomaterials as electrocatalysts for oxygen reduction reaction. J Nanomater. 2015;2015:357196. 10.1155/2015/357196.Suche in Google Scholar
[197] Kulesza PJ, Zak JK, Rutkowska IA, Dembinska B, Zoladek S, Miecznikowski K, et al. Elucidation of role of graphene in catalytic designs for electroreduction of oxygen. Curr Opin Electrochem. 2018;9:257–64. arXiv:1805.03152v1.10.1016/j.coelec.2018.05.012Suche in Google Scholar
[198] Ma R, Lin G, Zhou Y, Liu Q, Zhang T, Shan G, et al. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput Mater. 2019;5(1):1–5. 10.1038/s41524-019-0210-3.Suche in Google Scholar
[199] Kumar S, Gonen S, Friedman A, Elbaz L, Nessim GD. Doping and reduction of graphene oxide using chitosan-derived volatile N-heterocyclic compounds for metal-free oxygen reduction reaction. Carbon. 2017;120:419–26. 10.1016/j.carbon.2017.05.071.Suche in Google Scholar
[200] Ali A, Shen PK. Nonprecious metal's graphene-supported electrocatalysts for hydrogen evolution reaction: fundamentals to applications. Carbon Energy. 2020;2(1):99–121. 10.1002/cey2.26.Suche in Google Scholar
[201] Huang YG, Fan HL, Chen ZK, Gu CB, Sun MX, Wang HQ, et al. The effect of graphene for the hydrogen evolution reaction in alkaline medium. Int J Hydrog Energy. 2016;41(6):3786–93. 10.1016/j.ijhydene.2015.12.113.Suche in Google Scholar
[202] Rashid M, Al Mesfer MK, Naseem H, Danish M. Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int J Adv. 2015;4:80–93.Suche in Google Scholar
[203] Sammes N. Fuel cell technology: reaching towards commercialization in engineering materials and processes series. London, UK: Springer; 2006. ISBN 978-1-84628-207-2.10.1007/1-84628-207-1Suche in Google Scholar
[204] Eftekhari A. Electrocatalysts for hydrogen evolution reaction. Int J Hydrog Energ. 2017;42:11053–77. 10.1016/j.ijhydene.2017.02.125.Suche in Google Scholar
[205] Luis-Sunga M, Regent L, Pastor E, García G. Non-precious metal graphene-based catalysts for hydrogen evolution reaction. Electrochem. 2020;1(2):75–86. 10.3390/electrochem1020008.Suche in Google Scholar
[206] Park CM, Kim YM, Kim KH, Wang D, Su C, Yoon Y. Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: a mini review. Chemosphere. 2019;221:392–402. 10.1016/j.chemosphere.2019.01.063.Suche in Google Scholar PubMed PubMed Central
[207] Wang Y, Jiang C, Le Y, Cheng B, Yu J. Hierarchical honeycomb-like Pt/NiFe-LDH/rGO nanocomposite with excellent formaldehyde decomposition activity. Chem Eng J. 2019;365:378–88. 10.1016/j.cej.2019.01.187.Suche in Google Scholar
[208] Kumar V, Lee YS, Shin JW, Kim KH, Kukkar D, Tsang YF. Potential applications of graphene-based nanomaterials as adsorbent for removal of volatile organic compounds. Environ Int. 2020;135:105356. 10.1016/j.envint.2019.105356.Suche in Google Scholar PubMed
[209] Peng B, Cui J, Wang Y, Liu J, Zheng H, Jin L, et al. CeO2− x/C/rGO nanocomposites derived from Ce-MOF and graphene oxide as a robust platform for highly sensitive uric acid detection. Nanoscale. 2018;10(4):1939–45. 10.1039/C7NR08858B.Suche in Google Scholar
© 2020 Harshita Sachdeva, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Artikel in diesem Heft
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis

