Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
-
Chinnasamy Balalakshmi
, Naiyf S. Alharbi
Abstract
The radical proliferation of pathogenic bacteria and their infections causes significant issues for human health and the environment. Today, biopolymers are used to produce different nanoparticles. In the present investigation, the fabricated chitosan/agar-silver nanoparticles (Cht/Ar-AgNPs)-coated papers were tested for antibacterial applications. Agar was used as a reducing agent for the synthesis of AgNPs. Synthesized Ar-AgNPs were examined through optical, phase crystallinity and topological analysis. Cht and Ar-AgNPs solution was mixed with various ratios of 9:1, 8:2, 7:3, 6:4, and 5:5 by weight. In addition to that, the conformity of Cht/Ar-AgNPs-coated papers was characterized by structural, spectral, and morphological analysis. However, Cht/Ar-AgNPs-coated papers were subjected to antibacterial properties. The ratio of (6:4) Cht/Ar-AgNPs-coated paper showed excellent antibacterial agent, and it can be used as extending the food product shelf life.
1 Introduction
Biopolymers are considered an ideal candidate for nanotechnology’s recent advent due to the biocompatibility, reducing/capping ability, and different physicochemical properties, especially nontoxicity to an environment. Depending on the applications, biopolymers are varied. Moreover, they are highly edible as well as biodegradable films in food packaging. The high recyclable natural biomolecules-based packaging materials like fatty acids, amino acids, and polysaccharides are convenient environmental agents than petrochemical-based polymers. Biopolymer films and coatings are used for many applications: it serves as the best food packaging material, minimizes food deterioration, extends food life by serving as solute and gas barriers, and acts as various additives (antioxidants, antimicrobials, coloring agents, and nutrients) [1,2,3,4,5].
The biopolymers and papers-associated materials are providing an eco-friendly approach. Paper has unique characters as well as used for multiple applications: less weight, low cost, superior mechanical properties, low environmental impact, paper sensors, filters, indicators, writing/printing prepossess, packaging, household products, resistance from insufficient grease and oil, low barrier properties [6,7,8,9,10], and due to its lipophilic nature, it is prone to react with fatty molecules and leads to damage in printed papers, and enables usage as synthetic polymers, which leads to recycling issues and causes environmental problems [6,7,8,9,10,11]. Moreover, many researchers pushed towards the advanced and new approaches for developing antimicrobial papers by administering eco-friendly synthesis processes [12,13,14].
Many researchers are likely attracted by agar (Ar) because it is highly renewable, biodegradable, and of low cost. Ar is a mixture of agarose and agaropection polysaccharides: agarose 70% of agar (linear polymer, along with agarobiose repeating unit, a disaccharide making up of d-galactose and 3,6-anhydrous-l-galactopyranose) and agaropection 30% of agar (smaller molecule of a heterogeneous mixture, made up of alternating units of d-galactose and l-galactose highly modified with sulfate and pyruvate, an acidic side-group) [15].
Chitosan (Cht) is a biodegradable polycationic polymer obtained from chitin, a natural polymer that exhibits antibacterial features. It consists of (1,4)-linked 2-amino 2-deoxy-b-d-glucopyranose [16]. Moreover, it has antibacterial and nontoxic characteristics and therefore, been used as individual health properties and metal nanoparticle chitosan material [17]. Cht is an excellent chelating agent consisting of functional NH2 and OH groups [18]. Silver (Ag) ion revealed a strong bacterial inhibiting effect, even a low quantity of Ag nanoparticles (NPs) providing maximum antibactericidal effect [19]. The composite mixture of Cht and AgNPs provided a significant bacterial inhibition effect [20].
AgNPs were used as an effective antimicrobial agent against bacteria, viruses, and fungi [21,22]. Synthesis of AgNPs has been carried out by various methods such as microwave irradiation/sonochemical process [23,24,25], and along with bio-based reduction and capping agent has been proving to develop chemical-free AgNPs [26,27,28,29,30,31]. Previously, researchers have made various attempts to carry out the agar-medicated synthesis of AgNPs and reported that the synthesized Ar/AgNPs composites significantly improve the mechanical and antimicrobial properties. Similarly, the amino acids of tyrosine and tryptophan-mediated synthesized AgNPs had been incorporated into the agar polymer matrix. These Ar/AgNPs nanocomposite films revealed intense antimicrobial activity against Escherichia coli than Listeria monocytogenes [32]. The red seaweed of Gracilaria dura-derived agar extract-mediated synthesized AgNPs were formulated with agar polymer help by the microwave heating process. Synthesized Ar/AgNPs composite films showed a more significant bactericidal effect (99.9%) on Bacillus pumilus – HQ318731 [33]. Rhim et al. [34] reported that the chemically synthesized AgNPs were introduced to the agar polymer by various weight contents. Linearly enhancing the water vapor barrier properties and surface hydrophobicity increases the content of silver without affecting the mechanical strength. However, 1 wt% silver containing the composite film exhibited intense antimicrobial activity against L. monocytogenes and E. coli O157:H7. The same research group has investigated the preparation of AgNPs by a laser ablation method and AgNPs/agar composite films were prepared by the solvent casting method with varying the content of AgNPs. The inclusion of metallic AgNPs somewhat increased water vapor permeability and water contact angle. At the same time, it exhibited potent antimicrobial activity with a higher content at 40 and 80 mg of AgNPs [35].
The present investigation intended at the fabrication of active antibacterial paper using AgNPs asserted bio-based coating material. The AgNPs were synthesized by agar (Ar). The chitosan and Ar-AgNPs solutions were prepared at different ratios, which coated over cellulose paper, and it was assessed by antibacterial activity against human pathogens.
2 Materials and methods
2.1 Chemicals
Silver nitrate was derived from Alfa Aesar, Mumbai (India). Ten percent acetic acid, soluble agar, chitosan (M.W: 3,800–20,000 Daltons: degree of deacetylation: >75.00%) [CAS No. 9012-76-4], nutrient agar, and nutrient broth media were received from HiMedia (India). All the chemicals were derived in a pure form (no further sterilization/purification was required). Plain cellulose paper (100 GSM) and whole investigations were used for double distilled water.
2.2 Bacterial strains
Bacillus subtilis (ATCC 6633), Escherichia coli (MTCC 40), Shigella dysenteriae (ATCC 23513), and Proteus vulgaris (MTCC 7277) strains were acquired from KRIND Institute of Research and Development, Trichy, Tamil Nadu, Southern India.
2.3 Ar-AgNPs synthesis
Initially, Ar-AgNPs solution synthesis added 2.5 g of agar and 100 mL of 10 mM AgNO3 solution, and it was mixed well using a magnetic stirrer at 90°C for 1 h under dark condition [33,34]. Then, the mixture was cooled down at 37°C for back-up.
2.4 Preparation of Cht/Ar-AgNPs coating solution
At first, 2.0 v/v% acetic acid was prepared from 10% acetic acid used to prepare the chitosan solution. The 2.0 v/v% acetic acid was encountered through stirring for 8 h at 90°C to obtain 1.5 wt% of Chitosan solution, which was cooled at 25 ± 1°C and 2.5 pH. Finally, through 10 min vigorous mechanical stirring, the chitosan solution and Ar-AgNPs solutions were mixed at various ratios of 9:1, 8:2, 7:3, 6:4, and 5:5 by weight, respectively.
2.5 Fabrication of Cht/Ar-AgNPs-coated papers
The Cht/Ar-AgNPs cellulose-coated paper was prepared by the bar-coating method. Initially, the uncoated paper was firmed by a stable glass plate, then Cht/Ar-AgNPs coating solution was smeared using a #20 Mayer rod. Cht/Ar-AgNPs cellulose-coated paper allowed the oven for the drying process (90°C for 10 min). Air-tight polyethylene covers are used to ensure coated paper protection against light. Table 1 summarizes the detailed confirmation of uncoated and coated papers at various ratios of chitosan and Ar-AgNPs used in the study.
Different ratios of chitosan and agar-AgNPs-coated cellulose paper and their respective coating weight (g)
| Sample code | Chitosan (1.5%) | Agar-AgNPs solution (agar = 2.5%, AgNO3 = 10 mM) |
|---|---|---|
| Plain | — | — |
| Cht | 10 | 0 |
| Cht:Ar-AgNPs (9:1) | 9 | 1 |
| Cht:Ar-AgNPs (8:2) | 8 | 2 |
| Cht:Ar-AgNPs (7:3) | 7 | 3 |
| Cht:Ar-AgNPs (6:4) | 6 | 4 |
| Cht:Ar-AgNPs (5:5) | 5 | 5 |
2.6 Characterization of Ar-AgNPs analysis
Various spectral and microscopic methods analyzed the synthesized Ar-AgNPs solution: UV-Vis spectrometer (Shimadzu, UV-1800 spectrophotometer operating absorbance measurements with a resolution of 1 nm, with a 200–800 nm wavelength range). Shape and size of the synthesized Ar-AgNPs solution were characterized using transmission electron microscopy (Tecnai F20 model) at accelerating voltage of 200 kV. The crystalline phase of Ar-AgNPs solution, plain paper, and coated papers was analyzed by XRD spectrometer (PANalyticalX’Pert Pro, wavelength: 1.5418 Å, diffraction patterns were collected at 25°C, over an angular range of 10–80° with a step size of 0.05° and a step time of 10.16 s per increment). The functional groups of plain paper and coated papers were analyzed by FT-IR spectrometer Thermo Nicolet 380, from 500 to 4,000 cm−1, and the surface topology analyzed through FE-SEM microscopy (Hitachi S-4500).
2.7 Antibacterial activity
The antibacterial activity examined Gram-positive bacteria of B. subtilis and three Gram-negative bacteria of E. coli, S. dysenteriae, and P. vulgaris strains. The aforementioned bacterial strains were grown in nutrient broth at overnight culture. 20 mL of autoclaved nutrient agar culture media was poured into agar plates. The respective bacteria were evenly spread over the respective nutrient agar media with a cotton swab. The 1 × 1 cm of plain and coated papers were placed on the medium and plain paper considered as control. All the plates were maintained at 38°C for 24 h. After completing the incubation period, the inhibition zone was calculated by each coated paper and photographed. Further, this experiment was repeated three times [36].
2.8 Statistical analysis
Data from antibacterial experiments were expressed as mean ± SE and we employed ANOVA followed by Tukey’s HSD test (P ≤ 0.05) using the SPSS software package 16.0 version.
3 Results
3.1 Optical, structural, and morphological analysis of Ar-AgNPs
During AgNPs synthesis, in the reduction process, the colorless agar with AgNO3 mixture changed to brown color, and it is the necessary conformity of AgNPs synthesis. The UV-visible spectrum showed an absorption hump at 403 nm by the synthesized agar-mediated AgNPs (Figure 1). This absorbance peak indicates a reduction of Ag+ to Ag0. It confirms the formation of AgNPs owing to the surface plasmon resonance and similar observation reported by previous biopolymer reports [37]. The XRD patterns of Ar-AgNPs appeared 2θ value at 38.13°, 44.27°, 64.47°, and 77.30°, which indexed to the planes (111), (200), (220), and (311), respectively (Figure 2), and it revealed the crystalline phase of face-centered cubic structure. Further, Figure 3a and b prove the TEM images of Ar-AgNPs reveal the spherical shape, size of particles ranging from 5 to ∼20 nm, and the mean value of 10 nm; Figure 3c depicted the selected area diffraction patterns well-correlated with the XRD indexed planes.

UV-Vis spectrum of synthesized agar-mediated silver nanoparticles (Ar-AgNPs).

XRD patterns of synthesized Ar-AgNPs.

(a and b) TEM images; (c) SAED pattern of synthesized Ar-AgNPs.
3.2 XRD analysis of coated papers
The plain chitosan and chitosan combined agar-mediated silver nanoparticles-coated paper were considered by XRD analysis (Figure 4a). Two sharp characteristic intense peaks were observed at 2θ = 16.36° and 22.56° in the plain cellulose paper. Further, chitosan-coated cellulose paper revealed peaks at 2θ = 15.90°, 22.71°, 29.86°, and 39.80°. However, the first peak position slightly shifted towards front side. The second peak showed high crystalline nature as compared to the pure cellulose paper peak. Besides, a newly raised peak at 2θ = 29.86° confirmed chitosan on the cellulose paper surfaces. The even ratio of Cht: Ar-AgNPs-coated cellulose paper peaks was located at 2θ = 15.62°, 22.71°, 29.51°, 39.57°, and 43.21°, respectively. Whereas, the peak position at 15.62° and 22.71° crystalline decreased due to the composition of Cht: Ar-AgNPs coated on the cellulose paper surface as compared to the chitosan-coated paper. Interestingly, the newly appeared two peaks located at 39.57° and 43.21° correspond to the AgNPs’ presence on the cellulose paper surface (Figure 4b).

(a) XRD pattern of plain paper, chitosan-coated paper, and chitosan with agar-mediated AgNPs-coated paper; (b) asterisk symbol exhibited AgNPs peak at 2θ values.
3.3 Functional group analysis of coated papers
The FT-IR spectral analysis of plain chitosan and chitosan combined agar-mediated silver nanoparticles-coated papers exhibited several characteristic peaks at 3,321–3,342, 2,899–2,920, 1,631–1,652, 1,547–1,552, 1,403–1,423, 1,140–1,161, and 1,014–1,025 cm−1. It shows the functional groups OH– stretching, C–H bending, C═O stretching, N–H bending, CH3 wagging, anti-symmetric stretching of (C–O–C) bridge, and C–O stretching (Table 2 and Figure 5). Cellulose, chitosan, and agar biopolymers approximately had similar functional groups structure. Notably, the chitosan-coated paper showed characteristic peaks at 3,321, 1,403, and 1,014 cm−1 somewhat shifted to lower frequencies than the plain cellulose paper. Moreover, the Cht/Ar-AgNPs-coated paper showed characteristic peaks at 3,342, 2,909, and 1,161 cm−1 shifted to higher frequencies than cellulose and chitosan-coated paper. Also, the newly raised peak at 549 cm−1 corresponded to metal (Ag) peak. This metal peak confirmed the presence of AgNPs on the cellulose paper surface.
FT-IR analysis of the plain paper, chitosan-coated paper, and chitosan with agar-mediated AgNPs-coated paper
| Wavenumber (cm−1) | |||
|---|---|---|---|
| Plain paper | Cht-coated paper | Cht + Ar-AgNPs-coated paper | Assignments |
| 3,332 | 3,321 | 3,342 | O–H stretching |
| 2,899 | 2,920 | 2,909 | C–H bending |
| 1,631 | 1,641 | 1,652 | C═O stretching |
| 1,547 | 1,557 | 1,552 | N–H bending |
| 1,424 | 1,403 | 1,424 | CH3 wagging |
| 1,140 | 1,150 | 1,161 | Anti-symmetric C–O–C stretching |
| 1,025 | 1,014 | 1,024 | C–O stretching |
| — | — | 549 | Ag–metal |

FTIR spectra of plain paper, chitosan-coated paper, and chitosan with agar-mediated AgNPs-coated paper.
3.4 Scanning electron microscope
The SEM analysis evaluated the AgNPs dispersion on the paper. Due to the interwoven fibers, plain cellulose paper showed a rough and uneven surface, and the chitosan-coated paper appeared evenly spread and of smooth surface. Besides, Cht/Ar-AgNPs’ well-distributed coating surface exhibited spherical shape AgNPs (Figure 6a–c).

SEM images: (a) plain paper, (b) chitosan-coated paper, and (c) chitosan with agar-mediated AgNPs-coated paper.
3.5 Antibacterial properties
Safety and maintaining food quality have a vital role in antibacterial food packaging applications [38]. The antibacterial activity of coated paper was measured by a disk diffusion method, as shown in Figure 7. Obtained results, 6:4 (Cht/Ar-AgNPs) ratio coated paper, showed excellent antibacterial activity in Gram-positive (B. subtilis) and Gram-negative (E. coli, S. dysenteriae, and P. vulgaris) bacterial strains (Figure 8). This ratio promotes better electromotive interaction with the negatively charged bacterial cells to positively charged AgNPs. As per the Swiss norm 1,95,920-ASTM E2149-0, the antibacterial activity showed the result in over than one mm, as considered for the superior bactericidal agent [23,39]. The inhibition zones’ appearance around the Cht/Ar-AgNPs-coated papers was showed on the antibacterial mechanism of AgNPs. Generally, AgNPs enhanced the reactive oxygen species (ROS) generation. Hitherto, the antibacterial activity of AgNPs’ different mechanisms has been reported [40,41]. The best antibacterial activity mechanism was achieved by the electrostatic interactions of negatively charged bacterial cell walls and positively charged AgNPs [41]. At first, the bacterial cell lose the cell wall integrity. Consequently, bacterial electrolytes (Proteinaceous constituents and K+ ions) leakage retards the cell division. It causes osmatic imbalances and inhibits bacterial growth. Besides, it enhanced the ROS generations, superoxide (˙O2−), hydroxyl ion (˙OH−), hydroxyl radical (˙OH), and hydrogen peroxide (H2O2) [42,43], which leads to oxidative cellular damage. On the other hand, when nanoparticles were in contact with the cell surface, they migrate into the intercellular matrix and bind with sulfur-containing amino acids like cysteine and methionine. These S–H bondings with the AgNPs act as a sulfa drug related to folic acid synthesis metabolism. Besides, it had damaged the DNA replication, protein synthesis, intracellular signal communication, intracellular organelle of mesosome, adenosine triphosphate synthesis, and the regularity of electron transport chain. Finally, bacteria lose survivability. However, nanoparticles’ antibacterial activity depended upon the dose of the treatment and species specificity [44,45,46,47,48].
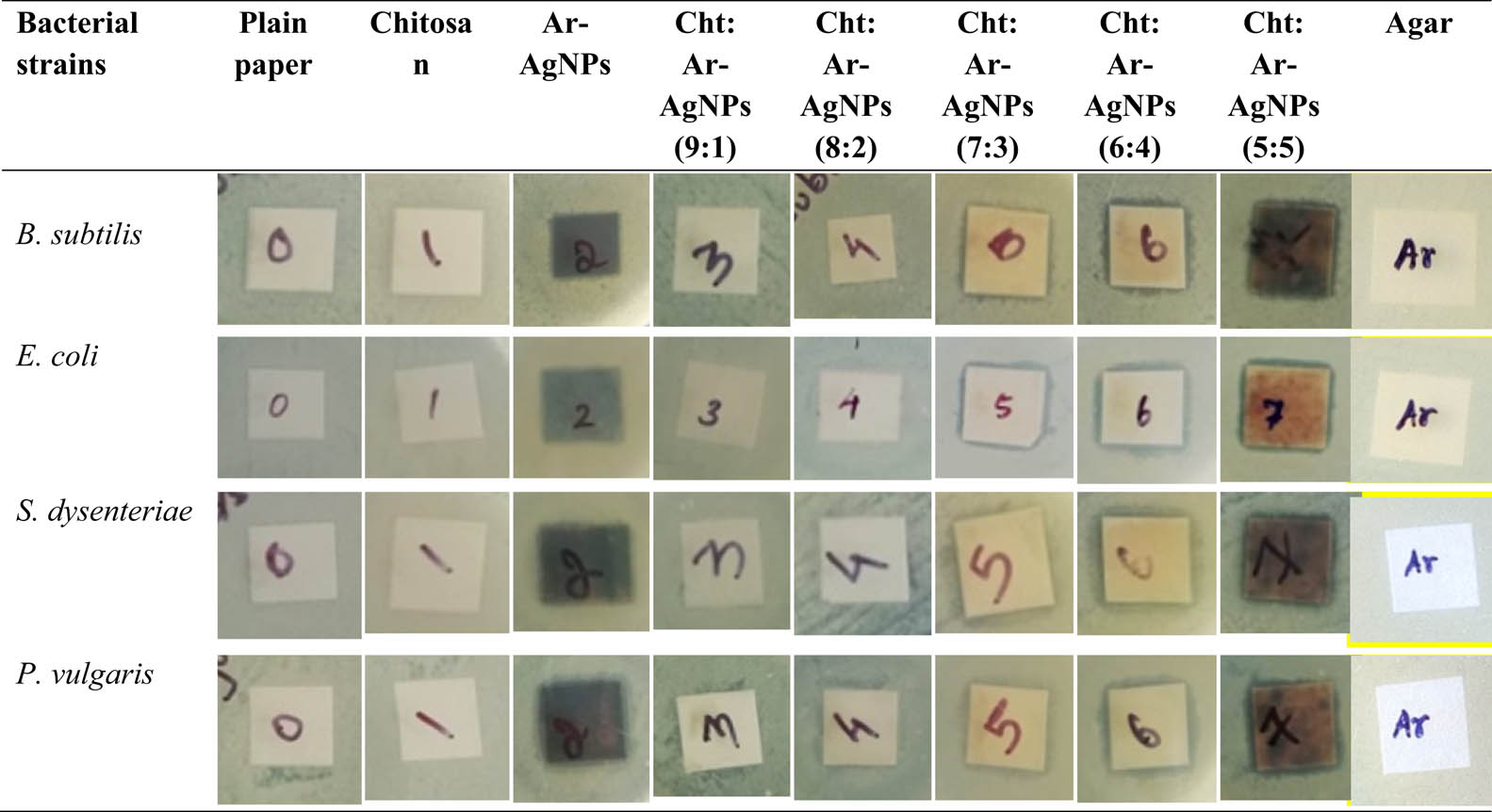
Antibacterial activity of plain paper, chitosan, agar, Ar-AgNPs-coated paper, and coated papers with different ratios of chitosan and Ar-AgNPs.

Effect of antibacterial activity of plain paper, chitosan, agar, Ar-AgNPs-coated paper, and coated papers with different ratios of chitosan and Ar-AgNPs.
5 Conclusion
This study was performed to develop the functional coated papers and to apply for antibacterial application. AgNPs are successfully synthesized by a single-step method. Synthesized Ar-AgNPs revealed a face-centered cubic crystalline structure and the spherical morphology with a mean value of 10 nm. Different ratios of chitosan and Ar-AgNPs solution-coated cellulose papers were investigated against the antibacterial activity. Overall obtained results, the optimum composition level of Cht:Ar-AgNPs 6:4 (weight ratio), showed excellent antibacterial activity. Appreciably, the facile-synthesized AgNPs can promote a potential candidate for food packaging application in future market.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/70), King Saud University, Riyadh, Saudi Arabia.
Credit author statement: Chinnasamy Balalakshmi: Conceptualization, Methodology, Software; Naiyf S. Alharbi: Resources, Funding acquisition, Validation; Shine Kadaikunnan: Funding acquisition, Formal analysis; Kasi Gopinath: Conceptualization, Writing – Original Draft; Ayyakannu Arumugam: Formal analysis; Marimuthu Govindarajan: Writing – Review and Editing.
References
[1] Guilbert S, Gontard N, Gorris LG. Prolongation of the shelf-life of perishable food products using biodegradable films and coatings. LWT-Food Sci Technol. 1996;29(1–2):10–7.10.1006/fstl.1996.0002Search in Google Scholar
[2] Miller KS, Krochta JM. Oxygen and aroma barrier properties of edible films: a review. Trends Food Sci Tech. 1997;8(7):228–37.10.1016/S0924-2244(97)01051-0Search in Google Scholar
[3] Debeaufort F, Quezada-Gallo JA, Voilley A. Edible films and coatings: tomorrow’s packagings: a review. Crit Rev Food Sci. 1998;38(4):299–313.10.1080/10408699891274219Search in Google Scholar PubMed
[4] Padmanabhan S, Baldwin RL. Tests for helix‐stabilizing interactions between various nonpolar side chains in alanine-based peptides. Protein Sci. 1994;3(11):1992–7.10.1002/pro.5560031111Search in Google Scholar PubMed PubMed Central
[5] Shahidi F, Han XQ. Encapsulation of food ingredients. Cri Rev Food Sci. 1993;33(6):501–47.10.1080/10408399309527645Search in Google Scholar PubMed
[6] Rastogi VK, Samyn P. Bio-based coatings for paper applications. Coatings. 2015;5(4):887–930.10.3390/coatings5040887Search in Google Scholar
[7] Mahato K, Srivastava A, Chandra P. Paper based diagnostics for personalized health care: emerging technologies and commercial aspects. Biosens Bioelectron. 2017;96:246–59.10.1016/j.bios.2017.05.001Search in Google Scholar PubMed
[8] Schoonover DV, Gibson HW. Facile removal of tosyl chloride from tosylates using cellulosic materials, eg, filter paper. Tetrahedron Lett. 2017;58(3):242–4.10.1016/j.tetlet.2016.12.014Search in Google Scholar
[9] Reshetnyak EA, Ostrovskaya VM, Goloviznina KV, Kamneva NN. Influence of tetraalkylammonium halides on analytical properties of universal acid–base indicator paper. J Mol Liq. 2017;248:610–5.10.1016/j.molliq.2017.10.019Search in Google Scholar
[10] Piselli A, Garbagnoli P, Alfieri I, Lorenzi A, Del Curto B. Natural-based coatings for food paper packaging. Int J Eng Res Appl. 2014;55–78.Search in Google Scholar
[11] Khwaldia K, Arab-Tehrany E, Desobry S. Biopolymer coatings on paper packaging materials. Compr Rev Food Sci F. 2010;9(1):82–91.10.1111/j.1541-4337.2009.00095.xSearch in Google Scholar PubMed
[12] Ma Y, Liu P, Si C, Liu Z. Chitosan nanoparticles: preparation and application in antibacterial paper. J Macromol Sci B. 2010;49(5):994–1001.10.1080/00222341003609542Search in Google Scholar
[13] Gottesman R, Shukla S, Perkas N, Solovyov LA, Nitzan Y, Gedanken A. Sonochemical coating of paper by microbiocidal silver nanoparticles. Langmuir. 2011;27(2):720–6.10.1021/la103401zSearch in Google Scholar PubMed
[14] Brobbey KJ, Haapanen J, Gunell M, Mäkelä JM, Eerola E, Toivakka M, et al. One-step flame synthesis of silver nanoparticles for roll-to-roll production of antibacterial paper. Appl Surf Sci. 2017;420:558–65.10.1016/j.apsusc.2017.05.143Search in Google Scholar
[15] Bertasa M, Dodero A, Alloisio M, Vicini S, Riedo C, Sansonetti A, et al. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur Polym J. 2020;2020(123):109442.10.1016/j.eurpolymj.2019.109442Search in Google Scholar
[16] Thamilarasan V, Sethuraman V, Gopinath K, Balalakshmi C, Govindarajan M, Mothana RA, et al. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: potential as new insecticides, antimicrobials and plant growth promoters. J Clust Sci. 2018;29(2):375–84.10.1007/s10876-018-1342-1Search in Google Scholar
[17] Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–65.10.1021/bm034130mSearch in Google Scholar PubMed
[18] Varma AJ, Deshpande SV, Kennedy JF. Metal complexation by chitosan and its derivatives: a review. Carbohyd Polym. 2004;55(1):77–93.10.1016/j.carbpol.2003.08.005Search in Google Scholar
[19] Dror-Ehre A, Mamane H, Belenkova T, Markovich G, Adin A. Silver nanoparticle – E. coli colloidal interaction in water and effect on E. coli survival. J Colloid Interface Sci. 2009;339(2):521–6.10.1016/j.jcis.2009.07.052Search in Google Scholar PubMed
[20] Tankhiwale R, Bajpai SK. Silver-nanoparticle-loaded chitosan lactate films with fair antibacterial properties. J Appl Polym Sci. 2010;115(3):1894–900.10.1002/app.31168Search in Google Scholar
[21] Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–1.10.1021/ja029267jSearch in Google Scholar PubMed
[22] Darroudi M, Zak AK, Muhamad MR, Huang NM, Hakimi M. Green synthesis of colloidal silver nanoparticles by sonochemical method. Mater Lett. 2012;66(1):117–20.10.1016/j.matlet.2011.08.016Search in Google Scholar
[23] Raghavendra GM, Jung J, Seo J. Step-reduced synthesis of starch-silver nanoparticles. Int J Biol Macromol. 2016;86:126–8.10.1016/j.ijbiomac.2016.01.057Search in Google Scholar PubMed
[24] Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A. Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology. 2008;19(24):245705.10.1088/0957-4484/19/24/245705Search in Google Scholar PubMed
[25] Ismail M, Gul S, Khan MI, Khan MA, Asiri AM, Khan SB. Medicago polymorpha-mediated antibacterial silver nanoparticles in the reduction of methyl orange. Green Process Synth. 2019;8(1):118–27.10.1515/gps-2018-0030Search in Google Scholar
[26] Mohammadlou M, Jafarizadeh-Malmiri H, Maghsoudi H. Hydrothermal green synthesis of silver nanoparticles using Pelargonium/Geranium leaf extract and evaluation of their antifungal activity. Green Process Synth. 2017;6(1):31–42.10.1515/gps-2016-0075Search in Google Scholar
[27] Munir H, Shahid M, Anjum F, Akhtar MN, Badawy SM, El-Ghorab A. Application of Acacia modesta and Dalbergia sissoo gums as green matrix for silver nanoparticle binding. Green Process Synth. 2016;5(1):101–6.10.1515/gps-2015-0064Search in Google Scholar
[28] Chartarrayawadee W, Charoensin P, Saenma J, Rin T, Khamai P, Nasomjai P, et al. Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity. Green Process Synth. 2020;9(1):107–18.10.1515/gps-2020-0012Search in Google Scholar
[29] Ngoc UTP, Nguyen DH. Synergistic antifungal effect of fungicide and chitosan-silver nanoparticles on Neoscytalidium dimidiatum. Green Process Synth. 2018;7(2):132–8.10.1515/gps-2016-0206Search in Google Scholar
[30] Rose GK, Soni R, Rishi P, Soni SK. Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens. Green Process Synth. 2019;8(1):144–56.10.1515/gps-2018-0042Search in Google Scholar
[31] Sivagnanam SP, Getachew AT, Choi JH, Park YB, Woo HC, Chun BS. Green synthesis of silver nanoparticles from deoiled brown algal extract via Box-Behnken based design and their antimicrobial and sensing properties. Green Process Synth. 2017;6(2):147–60.Search in Google Scholar
[32] Shankar S, Rhim JW. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohyd Polym. 2015;130:353–63.10.1016/j.carbpol.2015.05.018Search in Google Scholar PubMed
[33] Shukla MK, Singh RP, Reddy CRK, Jha B. Synthesis and characterization of agar-based silver nanoparticles and nanocomposite film with antibacterial applications. Bioresour Technol. 2012;107:295–300.10.1016/j.biortech.2011.11.092Search in Google Scholar PubMed
[34] Rhim JW, Wang LF, Hong SI. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocolloid. 2013;33(2):327–35.10.1016/j.foodhyd.2013.04.002Search in Google Scholar
[35] Rhim JW, Wang LF, Lee Y, Hong SI. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: laser ablation method. Carbohyd Polym. 2014;103:456–65.10.1016/j.carbpol.2013.12.075Search in Google Scholar PubMed
[36] Jung J, Kasi G, Seo J. Development of functional antimicrobial papers using chitosan/starch-silver nanoparticles. Int J Biol Macromol. 2018;112:530–6.10.1016/j.ijbiomac.2018.01.155Search in Google Scholar PubMed
[37] Shameli K, Bin Ahmad M, Jazayeri SD, Jazayeri SD, Sedaghat, S, Jahangirian H, et al. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int J Mol Sci. 2012;13(6):6639–50.10.3390/ijms13066639Search in Google Scholar PubMed PubMed Central
[38] Espitia PJP, Soares NDFF, dos Reis Coimbra JS, de Andrade NJ, Cruz RS, Medeiros EAA. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Tech. 2012;5(5):1447–64.10.1007/s11947-012-0797-6Search in Google Scholar
[39] Vimala K, Mohan YM, Sivudu KS, Varaprasad K, Ravindra S, Reddy NN, et al. Fabrication of porous chitosan films impregnated with silver nanoparticles: a facile approach for superior antibacterial application. Colloids Surf B. 2010;76(1):248–58.10.1016/j.colsurfb.2009.10.044Search in Google Scholar PubMed
[40] Abdal Dayem A, Hossain M, Lee S, Kim K, Saha S, Yang GM, et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci. 2017;18(1):120.10.3390/ijms18010120Search in Google Scholar PubMed PubMed Central
[41] Gopinath K, Devi NP, Govindarajan M, Bhakyaraj K, Kumaraguru S, Arumugam A. One-Pot green synthesis of silver nanoparticles using the orchid leaf extracts of Anoectochilus elatus: growth inhibition activity on seven microbial pathogens. J Clust Sci. 2017;28(3):1541–50.10.1007/s10876-017-1164-6Search in Google Scholar
[42] Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275(1):177–82.10.1016/j.jcis.2004.02.012Search in Google Scholar PubMed
[43] Jung J, Raghavendra GM, Kim D, Seo J. Improving properties of Hanji by coating chitosan–silver nanoparticle solution. Int J Biol Macromol. 2016;93:933–9.10.1016/j.ijbiomac.2016.09.067Search in Google Scholar PubMed
[44] Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(1):32.10.1186/2228-5326-2-32Search in Google Scholar
[45] Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9(1):30.10.1186/1477-3155-9-30Search in Google Scholar PubMed PubMed Central
[46] Gopinath K, Gowri S, Arumugam A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J Nanostruct Chem. 2013;3(1):68.10.1186/2193-8865-3-68Search in Google Scholar
[47] Al-Ansari M, Alkubaisi N, Gopinath K, Karthika V, Arumugam A, Govindarajan M. Facile and cost-effective Ag nanoparticles fabricated by Lilium lancifolium leaf extract: antibacterial and antibiofilm potential. J Clust Sci. 2019;30(4):1081–9.10.1007/s10876-019-01569-wSearch in Google Scholar
[48] Alharbi NS, Govindarajan M, Kadaikunnan S, Khaled JM, Almanaa TN, Alyahya SA, et al. Nanosilver crystals capped with Bauhinia acuminata phytochemicals as new antimicrobials and mosquito larvicides. J Trace Elem Med Bio. 2018;50:146–53.10.1016/j.jtemb.2018.06.016Search in Google Scholar PubMed
© 2020 Chinnasamy Balalakshmi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis

