Abstract
Jatropha curcas biodiesel was taken as the research object, studied the single and compound effects of oxidation degree and temperature on kinematic viscosity of biodiesel, and established a mathematical model. The results indicate that the kinematic viscosity of biodiesel decreases gradually with the increase of temperature, and the mathematical model affected by the single factor of temperature is η = e(A + Bt + Ct2) . The kinematic viscosity of biodiesel increases with the increase of oxidation time. The regression equation between kinematic viscosity and conductivity is established as follows: η = A + Bμ. It is found that the influence of temperature on the kinematic viscosity of biodiesel is much greater than that of oxidation, and the higher the temperature, the lower the influence of oxidation on the kinematic viscosity of biodiesel. Through the analysis of the influence weight of two factors and the change rate of kinematic viscosity, it is found that with the increase of temperature, the effect of conductivity on the change rate of kinematic viscosity of biodiesel is positive, but the influence decreases gradually. The relationship between kinematic viscosity, temperature and oxidation
1 Introduction
To cope with the changes of world energy structure, solve the three major problems of oil shortage, environmental pollution and greenhouse effect, and for the reasons of strategic reserves of resources, many countries are actively developing green energy which can be widely used, with little negative effect and little pollution. Biodiesel has been paid much attention to [1,2]. Biodiesel is a kind of renewable liquid fuel [3,4]. It is made from oil crops, engineering microalgae, animal oil and food waste oil by transesterification or thermochemical process.
Biodiesel has the advantages of renewable, biodegradability, non-aromatic and non-sulfur content, low emission [5,6]. Biofuels are carbon neutral fuels, which do not increase carbon dioxide emissions even when burned as fuels. But at the same time, biodiesel mainly consists of saturated fatty acid methyl ester and unsaturated fatty acid methyl ester. Saturated fatty acid methyl ester has high-melting point [7], which makes the low temperature fluidity of biofuels worse. The carbon-carbon double bond and carbon-carbon triple bond in the unsaturated fatty acid methyl ester are easy to oxidize, and the oxidation stability of multiple double bonds is worse because of the synergistic effect [8,9]. Viscosity is one of the key performance indexes of biodiesel, which is related to low temperature fluidity and oxidation stability. High viscosity biodiesel has the disadvantages of poor atomization effect, low combustion efficiency and easy to cause engine wear and tear [10]. In the actual storage and transportation process, unsaturated esters in biodiesel are easily oxidized to form alcohols, aldehydes, organic acids, polymers and precipitates due to the action of oxygen, light, metal ions, etc., resulting in increased biodiesel kinematic viscosity [11]. In low temperature environment, biodiesel is easy to precipitate waxy crystals, which causes blockage of engine pipes and filters, and affects the normal start-up of the engine [12].
In recent years, many models have been proposed to predict the viscosity of biodiesel [13, 14, 15, 16, 17, 18], but multiple factors models about viscosity are scarce. Shu et al. [19] proposed a new topological index, which connected with viscosity, and got two regression equations. By using the regression equation, the viscosity of biodiesel was accurate predicted. Gülüm et al. [20] derived new two-term power models to estimate kinematic viscosities of the blends of biodiesels and commercially available Ultra Force Euro diesel fuel. Compared to the quadratic and Arrhenius type mixing models, these models give the best qualitative and quantitative estimates. Luis [21] developed empirical models to predict both the biodiesel’s density and biodiesel’s viscosity in a wide range of temperature. Juan C. Chavarria-Hernandez et al. [22] proposed three correlations to improve the accuracy in the prediction kinematic viscosity of fatty acid methyl esters (FAMEs) for wide ranges of emperature. Pinzi et al. [23] predicted models of five biodiesel quality properties to predict viscosity, which improve the fittingness of prediction for biodiesel composed by medium chain acids. Yuan et al. [24] combined Grunberg-Nissan equation with a group contribution method as the mixing rule to calculate viscosities of mixtures fatty acid esters in a broad range of both saturated and unsaturated esters.
Up to now, there are no reports about the effect of oxidation degree and temperature on the kinematic viscosity of biodiesel at home and abroad. Therefore, the effects of oxidation degree and temperature on the kinematic viscosity of biodiesel were studied, and the theoretical support was provided for optimizing the low temperature fluidity of biodiesel.
2 Experimental
2.1 Materials and reagents
Preparation process of Jatropha carcass biodiesel: Preliminary biodiesel was prepared by cyclic vapor-phase esterification-transesterification-methanol steam distillation, followed by repeated washing with deionized water (ELGA Lab Water) to remove glycerol and alkaline catalysts. The catalyst is KOH, the dosage is 3%, and volume ratio of raw oil and methanol is 1:3. The reaction time and temperature are 80 min and 85°C, respectively, and the uncertainty values of temperature are ±0.5°C. The water bath is agitated and heated. After refining by vacuum rotary evaporator (R-215, BUCHI), the refined biodiesel can be obtained [25]. Table 1 is the physical and chemical properties of Jatropha carcass biodiesel and the European standard. Table 2 is the composition of fatty acids in Jatropha biodiesel.
European standard for biodiesel and physic-chemical performance index of Jatropha biodiesel.
| Project | Quality index | Jatropha biodiesel | Experiment method |
|---|---|---|---|
| Density (15°C), kg/m3 | 860~900 | 862 | ISO 3104 |
| Kinematic viscosity (40°C), mm2/s | 3.5~5.0 | 4.52 | ISO 3104 |
| Flash point, °C | ≥120 | 185 | ISO 3679 |
| Sulfur content (Quality score), % | ≤0.0010 | 0.002 | ISO 20884 |
| 10% Conradson carbon (Quality score), % | ≤0.3 | 0.063 | ISO 10370 |
| Sulphate ash (Quality score), % | ≤0.02 | 0.0013 | ISO 3987 |
| Moisture content (mg/kg) | 500 | 1190 | ISO 12937 |
| Copper corrosion (50°C, 3h), degree | ≤1 | 1a | ISO 2160 |
| Cetane number | ≥51 | 51 | ISO 5165 |
| Oxidation stability (110°C), h | ≥6.0 | 0.76 | EN14112 |
| Acid value, mgKOH/g | ≤0.50 | 2.96 | EN 14104 |
| Free glycerol content (Quality score), % | ≤0.02 | 0.01 | EN 14105 |
| Total glycerol content (Quality score), % | ≤0.25 | 0.17 | EN 14105 |
Composition of fatty acids in Jatropha biodiesel.
| Composition | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 |
|---|---|---|---|---|---|---|---|---|
| Content, % | 0.13 | 13.10 | 0.39 | 7.78 | 38.18 | 39.52 | 0.65 | 0.25 |
2.2 Experiment method
Rancimat instrument (Rancimat 873 biodiesel oxidation stability tester, Switzerland Wantong China Limited) was used to determine the oxidation degree of biodiesel [26,27]. Rancimat assay (EN 14112-2003): the temperature of sample is 110°C, continuously injected into the air, and the air flow rate is 10 L/h. The duration of the test varies according to the conductivity. The unstable secondary oxidation products are carried by the flowing air into another glass bottle filled with ultra-pure water (Classic Villa Biological Laboratory Ultra-Pure Water Instrument, ELGA Lab Water, UK). In this process, the electric conductivity of ultra-pure water changes constantly (the uncertain tie of conductivity is ±0.5 μS/cm). The change of electric conductivity of ultra-pure water is measured by electrodes, and a curve between electric conductivity and time is obtained by plotting the electric conductivity and time. In this paper, conductivity and oxidation time are used as indicators of oxidation degree respectively. An experimental setup for oxidizing biodiesel is shown in Figure 1. A typical curve of conductivity vs time is shown in Figure 2.
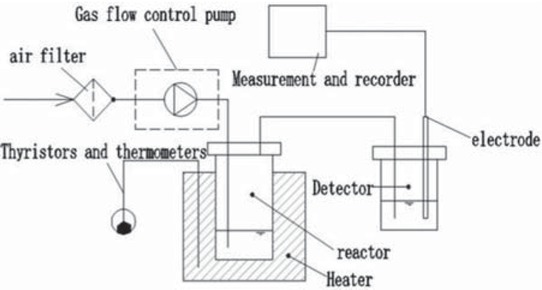
An experimental setup for oxidizing biodiesel.

The curve of conductivity and time.
The viscosity of biodiesel was determined according to standard GB/T265-1988. The experimental apparatus is the viscosity tester of the oil product (Shanghai Changji Geological Instrument Co., Ltd.). The uncertainty value of viscosity is ±3%.
3 Results and discussion
3.1 The effect of temperature on the kinematic viscosity of biodiesel
Biodiesel has the characteristics of high freezing point and poor fluidity at low temperature. At low temperature, the kinematic viscosity of biodiesel will change dramatically, and the kinematic viscosity change law is not obvious [28, 29, 30, 31, 32, 33, 34]. When using biodiesel, to improve the fluidity of biodiesel, improve the atomization effect and combustion efficiency of biodiesel, it is usually used at room temperature or under the condition of preheating. At low temperatures, biodiesel can be used to improve its low temperature fluidity by adding a pour point depressant. The kinematic viscosity of Jatropha curcas biodiesel with different oxidation degree at different temperatures was tested and analyzed by empirical formula. The regression Eq. 1 of kinematic viscosity of biodiesel was proposed by us for optimization.
The η in Eq. 1 is kinematic viscosity (mm2/s) and t is centigrade (°C). Using the optimized empirical formula, the kinematic viscosity and temperature of Jatropha curcas biodiesel with different oxidation degree were treated. The optimized empirical equation of kinematic viscosity and temperature of biodiesel was obtained as shown in Table 3. In this study, the oxidation degree of Jatropha curcas biodiesel was based on the ultra-pure water conductivity (μS/cm) of oxidation stability tester.
Empirical equation for kinematic viscosity and temperature of Jatropha curcas biodiesel.
| Conductivity μS/cm | Empirical equation | R2 |
|---|---|---|
| 0 | η = e(23.45 − 0.12t + 0.0001t2) | 0.9995 |
| 50 | η = e(18.81 − 0.11t + 0.0001t2) | 0.9967 |
| 100 | η = e(20.84 − 0.1t + 0.0001t2) | 0.9999 |
| 150 | η = e(20.26 − 0.11t + 0.0001t2) | 0.9999 |
The curve of regression equation was plotted and analyzed with the experimental data. Figure 3 is a fitting diagram of the empirical equation of kinematic viscosity and temperature of biodiesel with four different conductivity, as well as the measured value of kinematic viscosity.
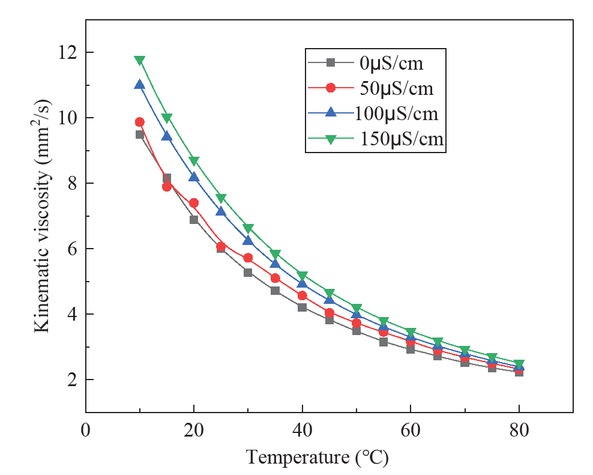
The curve fitting of kinematic viscosity and temperature of biodiesel with different conductivity.
It can be seen from the diagram that the kinematic viscosity of Jatropha curcas biodiesel with different oxidation degree decreases gradually with the increase of temperature, and the change trend is the same as that of biodiesel without oxidation modification. Among them, the kinematic viscosity of Jatropha curcas biodiesel was well distributed on the fitting curve, and the correlation coefficient R2 was above 0.99. The oxidation degree of biodiesel has little influence on biodiesel viscosity variation with temperature, and can still be predicted by Eq. 1.
3.2 Effect of oxidation degree (conductivity/oxidation time) on the kinematic viscosity of biodiesel
The polynomial η = anτn + an-1τn-1 + … + a1τ + a0 fitting equation was used to fit the non-linear curve of kinematic viscosity of Jatropha curcas biodiesel with different oxidation time. The empirical equation was obtained as shown in Table 4. To get the function relationship between kinematic viscosity η and oxidation time τ of Jatropha curcas biodiesel accurately, the experimental data were fitted by 2/3/4 order function.
The empirical equation of kinematic viscosity and oxidation time of Jatropha curcas biodiesel at 25°C temperature.
| Order | Empirical equation | Number | R2 |
|---|---|---|---|
| 2 | η = 4.822 − 0.042τ + 0.0096τ2 | (2) | 0.9461 |
| 3 | η = 4.705 + 0.111τ − 0.031τ2 + 0.001τ3 | (3) | 0.9874 |
| 4 | η = 4.745 − 0.025τ + 0.031τ2 − 0.0044τ3 + 0.0002τ4 | (4) | 0.9987 |
τ – time, h; η – kinematic viscosity, mm2/s.
The regression equation curves and the kinematic viscosity values of the biodiesel were plotted and analyzed. Figure 4 is a fitting diagram of the kinematic viscosity and oxidation time of the biodiesel with three different orders.
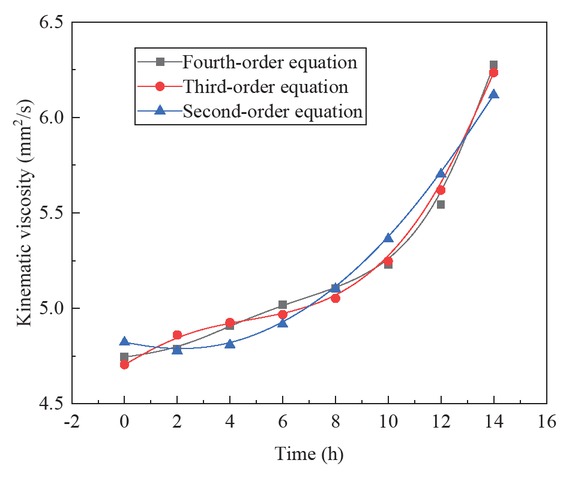
Curve fitting of kinematic viscosity and oxidation time of Jatropha curcas biodiesel.
The kinematic viscosity of biodiesel increases with the increase of oxidation time. Through the fitting curve of 2/3/4 order polynomial, the functional relationship between kinematic viscosity η and oxidation time τ of Jatropha curcas biodiesel can be obtained, and the correlation coefficients R2 are all greater than 0.94. With the increase of the order of polynomials, the correlation coefficient increases more than 0.99. With the increase of the order of polynomial, the sum of the square of the residuals between the measured kinematic viscosity and the curve fitting becomes smaller and smaller. The sum of the square of the residuals of 2/3/4 polynomial fitting is 0.097, 0.023 and 0.00329, respectively.
The fitting formula of kinematic viscosity and conductivity is Eq. 5. The regression equation curves were drawn and the kinematic viscosity values of the biodiesel were analyzed. Figure 5 was a linear fitting diagram of the kinematic viscosity and electrical conductivity of the biodiesel at 20/40/60/80°C.
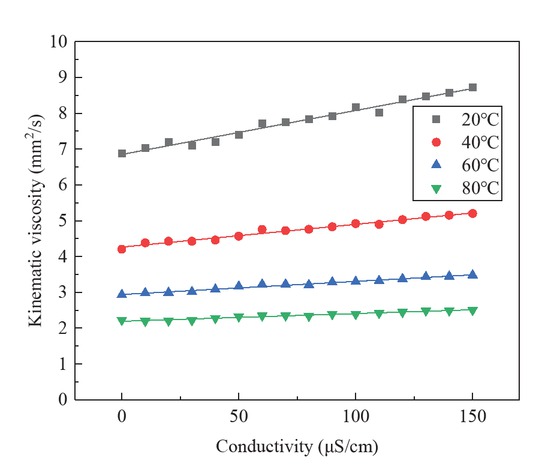
The linear fitting results of kinematic viscosity and conductivity of Jatropha curcas biodiesel.
where η is kinematic viscosity (mm2/s) and μ is conductivity (mm2/s).
Through the comparative analysis of Table 5 and Figure 5, it can be concluded that the kinematic viscosity of Jatropha curcas biodiesel is linearly related to the conductivity, and the correlation coefficients R2 at 20/40/60/80°C are 0.978/0.978/0.979/0.962, respectively. This coefficient is larger than the correlation coefficient of the second-order fitting curve of kinematic viscosity and oxidation time, and less than the correlation coefficient of the third-order fitting curve of kinematic viscosity and oxidation time. However, the relationship between conductivity and kinematic viscosity of Jatropha curcas biodiesel is relatively simple and clear.
The empirical equation for kinematic viscosity and conductivity of biodiesel.
| Temperature, °C | Empirical equation | R2 |
|---|---|---|
| 20 | η = 6.85 + 0.0123μ | 0.9787 |
| 40 | η = 4.265 + 0.0063μ | 0.9786 |
| 60 | η = 2.94 + 0.0037μ | 0.9798 |
| 80 | η = 2.193 + 0.0021μ | 0.9625 |
3.3 The effect of oxidation degree and temperature on kinematic viscosity of biodiesel
The results indicate that the regression equation is only obtained under the condition that a single dependent variable (temperature/oxidation degree) affects the kinematic viscosity of Jatropha curcas biodiesel. In practical engineering applications, the kinematic viscosity of biodiesel is affected by multiple dependent variables. To obtain the functional relationship between the kinematic viscosity η of the Jatropha curcas biodiesel and the conductivity μ and the temperature t, the kinematic viscosity, conductivity and temperature of Jatropha curcas biodiesel were fitted by Eq. 6 and 7 two regression equations. The empirical equation is shown in Table 6.
The empirical equations for kinematic viscosity, oxidation degree and temperature of biodiesel.
| Serial number | Empirical equation | R2 |
|---|---|---|
| (6)’ | 0.9984 | |
| (7)’ | 0.9916 |
μ – conductivity, μS/cm; t – temperature, °C; η – kinematic viscosity, mm2/s.
The regression equation curve was plotted and the kinematic viscosity of the biodiesel was analyzed. Figure 6 is a non-linear surface fitting diagram of the three: the empirical equation of kinematic viscosity, conductivity and temperature of biodiesel and the experimental measurement of kinematic viscosity of biodiesel.
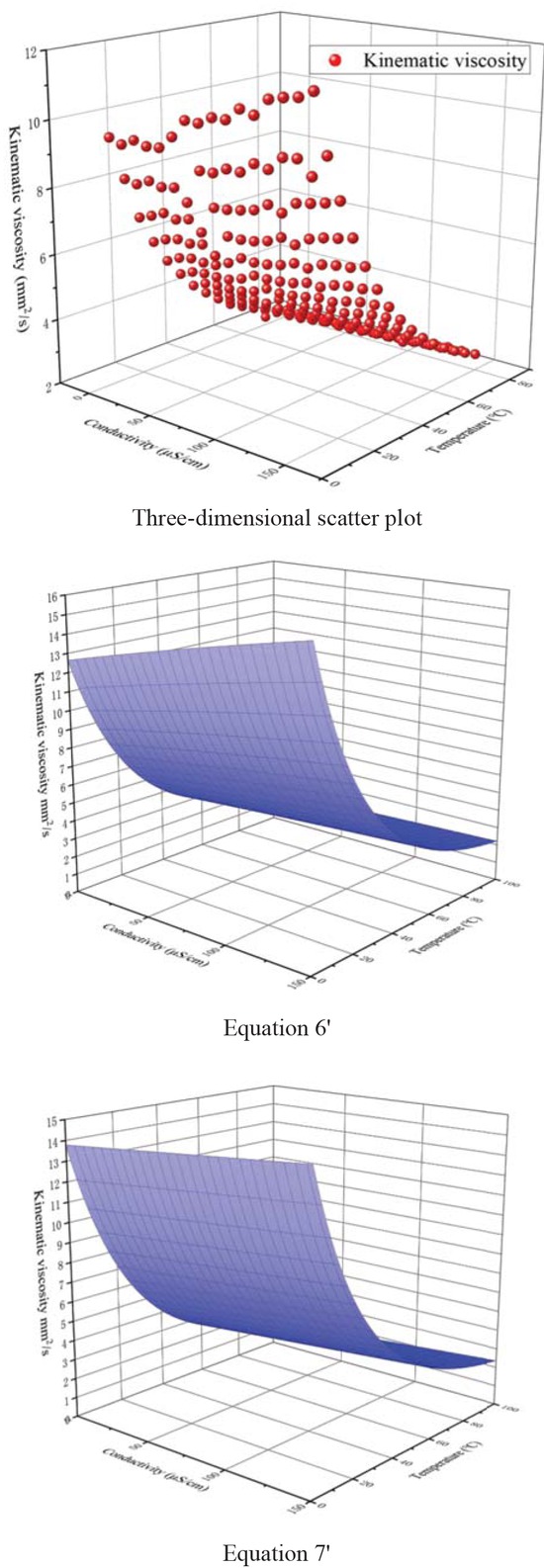
The nonlinear surface fitting of kinematic viscosity, conductivity and temperature of Jatropha curcas biodiesel.
Through comparative analysis of Table 6 and Figure 6, it is found that: The fitting correlation coefficients R2 of surface Eq. 6’ and surface Eq. 7’ are 0.998 and 0.991 respectively for three-dimensional scatter plots. The difference between Eq. 6’ and Eq. 7’ is that Eq. 6’ considers the internal relationship between temperature and oxidation rate in the oxidation process of biodiesel. When the temperature rises, the chain oxidation reaction accelerates and the oxidation degree increases slightly, which influences the kinematic viscosity of biodiesel. Eq. 7’ uses a simple polynomial accumulation of two factors acting alone, ignoring the effect of temperature on oxidation rate. The structure of surface Eq. 6’ is complex, with two polynomial and two roots. The structure of surface Eq. 7’ is only the sum of a polynomial and exponential, and relatively simple. In the analysis of specific problems, if the correlation requirements are high, the use of Eq. 6 analysis; for the calculation speed and equation simple requirements are high, the use of Eq. 7 analysis.
Further analysis of surface Eq. 7’ shows that neglecting the binomial coefficient of 0.0001 (0), the coefficient of temperature t is −0.35, the coefficient of conductivity μ is 0.007, and the absolute value of temperature t coefficient is five times that of conductivity coefficient. In the single factor test, when the conductivity is 0/10/20/30/40/50/60/70/80/90/100 /110 μS/cm, when the temperature rises from 10°C to 80°C, the decrease of kinematic viscosity Δη is about 7.16~9.28 mm2/s. When the temperature is 10/15/20/25/ 30/35/40/45/50/55/60/65/70/75/80°C, and the conductivity increases from 0 μS/cm to 150 μS/cm, the increase of kinematic viscosity Δη is about 0.28~2.30 mm2/s. When the temperature is 80°C, the increase of kinematic viscosity caused by oxidation is only 0.28 mm2/s. The results indicate that the influence of temperature on the kinematic viscosity of biodiesel is greater than that of oxidation degree. The higher the temperature is, the lower the influence of oxidation degree on the kinematic viscosity of biodiesel is.
3.4 The comparative analysis of temperature and oxidation degree on the change rate of kinematic viscosity
To further analyze the influence of temperature and oxidation degree on the kinematic viscosity change rate of biodiesel, partial derivatives of
The partial derivative curve of the fitting equation is obtained by drawing the partial derivative Eq. 9. As shown in Figure 7.
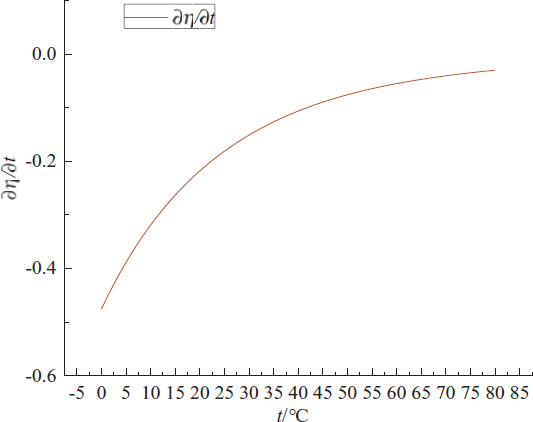
The partial derivative curve of
By comparing the Eq. 8 and Figure 7, it is found that the slope of the line projected by the tangent of t = t0 intersecting line at point (μ0,t0,f(μ0,t0)) on the μ0η plane is 0.007. Because the intersection line between the fitting surface and t = t0 is a straight line, 0.007 is the slope of the straight line. Its physical meaning is that the change rate of kinematic viscosity of Jatropha curcas biodiesel is +0.007 mm2/s with the increase of conductivity in the range of experimental temperature and oxidation degree. The partial derivative of
For fitting Eq. 6’,
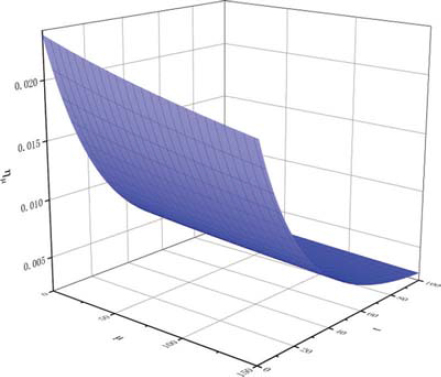
The partial derivative function diagram of
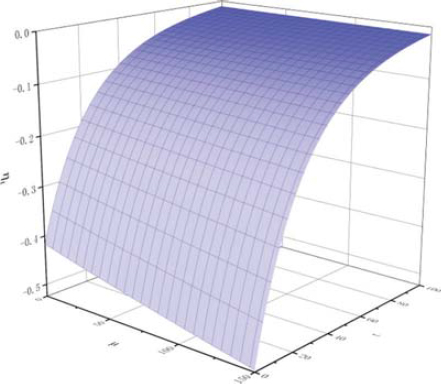
The partial derivative function diagram of
Comparing with Figures 8 and 9, it can be found that the slope of projection on μ0η plane decreases with the increase of conductivity, that is, with the increase of temperature, the effect of conductivity on the change rate of kinematic viscosity of biodiesel is positive, but the influence decreases gradually.
The results of partial derivative analysis of Eq. 7’ are quite different from those of Table 4. The results of partial derivative analysis of Eq. 6’ have the same trend as those of straight line slope in Table 4. The results of partial derivative analysis show that the fitting Eq. 6’ reflects the experimental data more authentically, and proves that the functional relationship between kinematic viscosity of biodiesel, temperature and oxidation degree is not a simple first-order dependent variable fitting equation, but a second-order dependent variable fitting equation.
4 Conclusions
The kinematic viscosity of biodiesel gradually decreases with increasing temperature. The kinematic viscosity of biodiesel before and after oxidation has the same change trend with temperature. The correlation coefficient is above 0.99 by Eq. 1 regression equation.
The kinematic viscosity of biodiesel increased with the increase of oxidation time. The correlation coefficient of kinematic viscosity η and oxidation time τ of Jatropha curcas biodiesel was generally greater than 0.94.
The kinematic viscosity of Jatropha curcas biodiesel was linearly correlated with electrical conductivity, and the data were processed by Eq. 5 regression equation. When the temperature is 20/40/60/80°C, the fitting linear correlation coefficient of kinematic viscosity and conductivity is 0.978/0.978/0.979/0.962, respectively.
By comparing the two influencing factors of kinematic viscosity of biodiesel, it is found that the influence of temperature is greater than that of oxidation degree. The higher the temperature is, the lower the influence of oxidation degree on kinematic viscosity of biodiesel is. It can be fitted by two regression equations Eq. 6 and 7, and the correlation coefficient is over 0.99.
With the increase of temperature, the effect of conductivity on the kinematic viscosity of biodiesel is positive, but the influence decreases gradually. The functional relationship between kinematic viscosity of biodiesel, temperature and oxidation degree is a second-order dependent variable fitting equation, and the fitting equation Eq. 6’ is closer to the actual situation.
References
[1] Silitonga A.S., Atabani A.E., Mahlia T.M.I., A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sust. Energ. Rev., 2011, 15(8), 3733-3756.10.1016/j.rser.2011.07.011Search in Google Scholar
[2] Yan Y., Li X., Wang G., Gui X., Li G., Su F., et al., Biotechnological preparation of biodiesel and its high-valued derivatives: A review. Appl. Energ., 2014, 113, 1614-1631.10.1016/j.apenergy.2013.09.029Search in Google Scholar
[3] Leung D.Y.C., Koo B.C. P., Guo Y., Degradation of biodiesel under different storage conditions. Bioresource Technol., 2006, 97 (2), 250-256.10.1016/j.biortech.2005.02.006Search in Google Scholar PubMed
[4] Song J.H., Xu Y.B., Shi Q.Q., Oxidation degradation characteristics of three kinds of biodiesels. Chin. Oil. Fat., 2017, 42(6), 115-120.Search in Google Scholar
[5] Olusegun D.S., Mert G., Mechanical and corrosion properties of brass exposed to waste sunflower oil biodiesel-diesel fuel blends. Chem. Eng. Commun., 2019. 206(5), 682-694.10.1080/00986445.2018.1519508Search in Google Scholar
[6] Gülüm M., Yesilyurt M.K., Bilgin A., The performance assessment of cubic spline interpolation and response surface methodology in the mathematical modeling to optimize biodiesel production from waste cooking oil. Fuel, 2019, 8(5), 682-694.10.1016/j.fuel.2019.115778Search in Google Scholar
[7] Xue Y., Yang. C., Xu G. W., Zhao Z., Lian X., Sheng H., et al., The influence of polymethyl acrylate as a pour point depressant for biodiesel. Energ. Source., 2017, 39(1), 17-22.10.1080/15567036.2016.1201550Search in Google Scholar
[8] Madihalli C., Sudhakar H., Doble M., Mannosylerythritol lipid-α as a pour point depressant for enhancing the low-temperature fluidity of biodiesel and hydrocarbon fuels. Energ. Fuel., 2016, 30(5), 4118-4125.10.1021/acs.energyfuels.6b00315Search in Google Scholar
[9] Shan C.S., Li F.S., Bao G.R., Determination and optimization of oxidation stability of eight kinds of biodiesels at room temperature. Chin. Oil. Fat., 2017, 42(10), 106-109.Search in Google Scholar
[10] Das M., Sarkar M., Datta A., Santra A.K., Study on viscosity and surface tension properties of biodiesel-diesel blends and their effects on spray parameters for CI engines. Fuel, 2018, 220, 769-779.10.1016/j.fuel.2018.02.021Search in Google Scholar
[11] Li F.S., Ni Z.H., Du W., Composition analysis of biodiesel before and after oxidation. Chin. Oil Fat., 2015, 40(1), 64-68.Search in Google Scholar
[12] Du W., Bao G.R., Wang H., Improvement of low-temperature fluidity of Jatropha curcas L biodiesel. J. Yunnan Univ. Nat. Sci., 2011, 33(5), 578-582.Search in Google Scholar
[13] Aminian A., Zare Nezhad B., Accurate predicting the viscosity of biodiesels and blends using soft computing models. Renew. Energ., 2018, 120, 488-500.10.1016/j.renene.2017.12.038Search in Google Scholar
[14] Geacai S., Iulian O., Nita I., Measurement, correlation and prediction of biodiesel blends viscosity. Fuel, 2015, 143, 268-274.10.1016/j.fuel.2014.11.041Search in Google Scholar
[15] Do Carmo F.R., Sousa Jr. P.M., Santiago-Aguiar R.S., de Sant’Ana H.B., Development of a new model for biodiesel viscosity prediction based on the principle of corresponding state. Fuel, 2012, 92(1), 250-257.10.1016/j.fuel.2011.08.012Search in Google Scholar
[16] Gülüm M., Bilgin A., Density, flash point and heating value variations of corn oil biodiesel-diesel fuel blends. Fuel Process. Technol., 2015, 134, 456-464.10.1016/j.fuproc.2015.02.026Search in Google Scholar
[17] Phankosol S., Sudaprasert K., Lilitchan S., Aryusuk K., Krisnangkura K., An empirical equation for estimation of kinematic viscosity of Fatty acid methyl esters and biodiesel. J. Am. Oil Chem. Soc., 2015, 92, 1051-1061.10.1007/s11746-015-2667-7Search in Google Scholar
[18] Freitas S.V.D., Pratas M.J., Ceriani R., Lima A.S., Coutinho J.A.P., Evaluation of predictive models for the viscosity of biodiesel. Energ. Fuel., 2011, 25, 352-358.10.1021/ef101299dSearch in Google Scholar
[19] Shu Q., Yang B., Yang J., Qing S., Predicting the viscosity of biodiesel fuels based on the mixture topological index method. Fuel, 2007, 86, 1849-1854.10.1016/j.fuel.2006.12.021Search in Google Scholar
[20] Gülüm M., Bilgin A., Two-term power models for estimating kinematic viscosities of different biodiesel-diesel fuel blends. Fuel Process. Technol., 2016, 149, 121-130.10.1016/j.fuproc.2016.04.013Search in Google Scholar
[21] Luis F.R.V., Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sust. Energ. Rev., 2013, 19, 652-665.10.1016/j.rser.2012.11.022Search in Google Scholar
[22] Chavarria-Hernandez J.C., Pacheco-Catalán D.E., Predicting the kinematic viscosity of FAMEs and biodiesel: Empirical models. Fuel, 2014, 124, 212-220.10.1016/j.fuel.2014.01.105Search in Google Scholar
[23] Pinzi S., Leiva D., Arzamendi G., Gandia L.M., Dorado M.P., Multiple response optimization of vegetable oils fatty acid composition to improve biodiesel physical properties. Bioresource Technol., 2011, 102, 7280-7288.10.1016/j.biortech.2011.05.005Search in Google Scholar PubMed
[24] Yuan W., Hansen A.C., Zhang Q., Predicting the temperature dependent viscosity of biodiesel fuels. Fuel, 2009, 88, 1120-1126.10.1016/j.fuel.2008.11.011Search in Google Scholar
[25] Wang D., Su Y.Y., Wang H., Bao G.R., Study on continuous preparation of biodiesel from rapeseed oil. Chin. Oil. Fat., 2009, 34(2), 46-48.Search in Google Scholar
[26] Ferrari R.A., Oliveira V.S., Scabio A., Oxidative stability of biodiesel from soybean oil fatty acid ethylesters. Sci. Agr., 2005, 62(6), 3-11.10.1590/S0103-90162005000300014Search in Google Scholar
[27] Sendzikiene E., Makareviciene V., Oxidation stability of biodiesel fuel produced from fatty wastes. Pol. J. Environ. Stud., 2005, 14(3), 335-339.Search in Google Scholar
[28] Aminian A., Bahman Z.N., Accurate predicting the viscosity of biodiesels and blends using soft computing models. Renew. Energ., 2018, 120, 488-500.10.1016/j.renene.2017.12.038Search in Google Scholar
[29] Knothe G., Steidley K.R., Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel, 2005, 84(9), 1059-1065.10.1016/j.fuel.2005.01.016Search in Google Scholar
[30] Tat M.E., Van Gerpen J.H., The kinematic viscosity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc., 1999, 76(12), 1511-1513.10.1007/s11746-999-0194-0Search in Google Scholar
[31] Knothe G., Steidley K.R., Kinematic viscosity of biodiesel components (fatty acid alkyl esters) and related compounds at low temperatures. Fuel, 2007, 86(16), 2560-2567.10.1016/j.fuel.2007.02.006Search in Google Scholar
[32] Phankosol S., Sudaprasert K., Lilitchan S., An empirical equation for estimation of kinematic viscosity of fatty acid methyl esters and biodiesel. J. Am. Oil Chem. Soc., 2015, 92(7), 1051-1061.10.1007/s11746-015-2667-7Search in Google Scholar
[33] Corach J., Colman M., Sorichetti P.A., Kinematic viscosity of soybean biodiesel and diesel fossil fuel blends: Estimation from permittivity and temperature. Fuel, 2017, 207, 488-492.10.1016/j.fuel.2017.06.102Search in Google Scholar
[34] Zhang W.B., Yuan W.Q, Zhang X.M., Predicting the dynamic and kinematic viscosities of biodiesel-diesel blends using mid- and near-infrared spectroscopy. Appl. Energ., 2012, 98, 122-127.10.1016/j.apenergy.2012.03.013Search in Google Scholar
© 2020 Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis

