Abstract
The present study explores the potential of Valeriana jatamansi shoot extract for Ag-metal bio-reduction and its antimicrobial activity. Among the different ratios of AgNO3 and extract tested, 1:5 (1 mL AgNO3 and 5 mL extract) gave maximum SPR peak at 411.0 nm during UV-Vis spectrophotometric analysis, indicating the synthesis of maximum amount of AgNPs in solution. XRD analysis reported the crystalline nature of AgNPs with 13.32 nm nanocrystallite size. FTIR studies suggested the involvement of carboxylic acid (–[C–O–O–H]) and methane (–CH–) functional groups of different compounds in AgNPs reduction and fabrication. Average size of synthesized uniform shaped nanospheres was 32 nm by SEM image analysis. The produced AgNPs (1.5 mg/disc) showed growth inhibition of 71.46, 65.97, 61.5, 55.32, and 54.83% against Pseudomonas aeruginosa, Escherichia coli, Candida albicans, Xanthomonas campestris, and Staphylococcus aureus. While the least growth inhibition of 48.55% was recorded for Klebsiella pneumonia, suggesting it as the least-susceptible microbe among all the tested microbial species. P. aeruginosa was found to be most sensitive of all tested microbes, while E. coli, C. albicans, and X. campestris reported moderate susceptibility to AgNPs.
1 Introduction
Nanobiotechnology is the intersection of biology and nanotechnology. The development and the use of nanotools like nanoparticles in a vast field to achieve numerous goals have attracted scientists and researchers to this field. Drug delivery through nanoparticles (NPs) is an emerging field with promising results. NPs can be synthesized by several ways including chemical, physical, and biological approaches. Biologically synthesized nanoparticles are efficient, sustainable, hazardous chemical-free, and low-cost alternatives of nanoparticles derived from chemical and physical methods [1]. During the past few years, various materials and metals have been used for nanoparticle production [2,3,4] from bacteria, fungi, algae, viruses, and numerous plants [5,6,7,8,9,10]. Metallic NPs have shown enhanced antimicrobial, antioxidant, and many other properties due to the increased surface area, smaller particle size, various shapes, and altered characteristics [4,11,12].
Valeriana jatamansi (family Valerianaceae), an indigenous medicinal plant of Himalaya region, is a hairy, perennial dwarf and rhizomatous wild herb. It can be found growing at an altitude of 1,200–3,000 m. Its bitter and acrid thick roots covered with root fibers are used as carminative and laxative. The plant is regarded as nerve tonic, ophthalmic, tranquilizer, aphrodisiac, antispasmodic, expectorant, and sedative. It is also used as tonic in hysteria, cholera, snakebite, scorpion sting, and asthma traditionally [13]. It consists of an array of various biologically active components such as terpenoids, flavonoids, sesquiterpenes, and lignans having health-promoting benefits. In Asia, it has been used as an insect repellent, antioxidant, antidepressant, antimicrobial, and cytotoxic agent. Valepotriates and valerenic acid derived from this species is used for the preparation of drugs. Valepotriates/iridoids are predominant bioactive components present in V. jatamansi that are used for the treatment of bacterial and fungal infections, cancer, inflammation, oxidation, liver diseases, and neurological-related disorders. Various parts of V. jatamansi such as roots (dried), leaves (crushed), and rhizomes (dried) have been used in severe headaches, perfume formulations, asthma, and intermittent fever [14]. Phytochemically this plant is explored very little, and different classes of compounds such as iridoids comprising jatamanins A–M, lignin, and (+)9′-isovaleroxyl lariciresinol have been reported from the whole plant of Valeriana jatamansi [15]. Ester iridoids isolated from family Valerianaceae are documented for cytotoxic, sedative, antifungal, and antitumor properties [15]. Therefore, this present study was designed, owing to medicinal potential of V. jatamansi, to investigate the green synthesis of silver nanoparticles from V. jatamansi shoot extract and its antimicrobial activity.
2 Materials and methods
2.1 Plant collection and extract preparation
Shoots of V. jatamansi were collected and shade dried after thorough washing with distilled water. Dried plant material was finely grinded and soaked in methanol for 10 days to get methanolic crude extract. The extract was dried in a rotary evaporator to completely eliminate methanol from the extract.
2.2 Green synthesis of AgNPs
For the production of biologically synthesized AgNPs, methanolic crude extract of V. jatamansi, 50 mg extract dissolved in 100 mL deionized water, was employed to reduce 0.1 mM AgNO3 solution. Different ratios of both solutions were mixed to assess the maximum and stable AgNPs yielding ratios. All the solutions were analyzed by UV-visible (UV-Vis) spectrophotometer, and the solution containing maximum amount of AgNPs was further processed for AgNPs characterization.
2.3 AgNPs characterization
UV-Vis spectrophotometric analysis was carried out to monitor the synthesis of AgNPs by observing a characteristic surface plasmon resonance (SPR) peak in the wavelength range of 400–500 nm. X-ray diffraction (XRD) investigation was used to determine nature and nanocrystallite size of AgNPs. Fourier transform infrared (FTIR) spectroscopy was employed to identify the possible functional groups involved in bioreduction of Ag-metal. The size and the shape of AgNPs were determined by scanning electron microscope (SEM) studies.
2.4 AgNPs stability studies
Salt (NaCl – 1 mM, 0.5 M, and 1 M) and temperature (20–40°C and 80–100°C) stress were applied to AgNPs to study their effects, and samples were analyzed by UV-Vis spectrophotometric analysis.
2.5 Antimicrobial potential
Antimicrobial potential of AgNPs was assessed against different clinical isolates of Klebsiella pneumonia, Bacillus subtilis, and ATCC strains of Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Xanthomonas campestris, and Candida albicans by following the protocol described by Bakht et al. [16]. Nutrient broth (NB) media (3.25 g/250 mL) and nutrient agar (NA) media (7 g/250 mL) were prepared as per requirement and autoclaved. After pouring media in plates and solidification of media, fresh cultures of microbes standardized with 0.5 McFarland standards were spread on media. AgNPs (0.5, 1.0, and 1.5 mg disc−1) and control ciprofloxacin (50 μg per 6 μL) were applied on 6 mm diameter Whatman filter paper discs. These assay plates were incubated at 37°C temperature for 24 h. The zone of inhibition was measured in millimeters for each sample, and percent (%) inhibition was calculated as follows:
The experiment was repeated in triplicate, and results are reported as mean with standard deviation.
3 Results and discussion
3.1 Effect of different reaction mixtures on stability of prepared AgNPs
AgNO3 solution and V. jatamansi shoot methanolic extract were mixed in different ratios to evaluate the synthesis of AgNPs. AgNPs synthesis in samples was initially traced visually and then confirmed by UV-Vis spectrophotometric analysis. Change in color of the solution from colorless to dark yellow or brown indicated the formation of AgNPs. Figure 1 shows a comparison of UV-Vis spectrums of all the tested ratios. Highest sharp peak was recorded for sample containing 1:5 ratios (1 mL AgNO3 and 5 mL methanolic extract solution), indicating the formation of higher amounts of AgNPs. Samples of other ratios reported very less or no AgNPs synthesis. Furthermore, it was generally observed in some samples that plant extract solution when used in higher ratios than AgNO3 solution resulted in intense colored solution and indicated the synthesis of relatively higher amounts of silver NPs in sample. The highest surface plasmon resonance peak was recorded at 411.0 nm wavelength with 1.783 maximum absorption for the sample containing 1:5 ratios and suggested 1:5 ratios as optimum concentration of reactants to yield AgNPs (Figure 2).
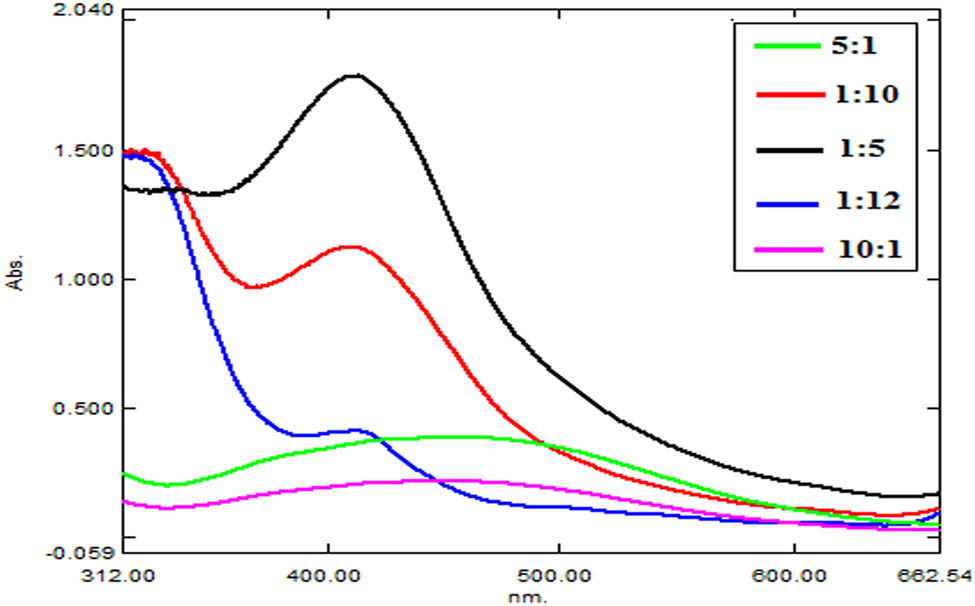
Comparison of UV-Vis spectra of V. jatamansi shoot AgNPs synthesized in different samples.

UV-Vis spectrum of V. jatamansi shoot AgNPs in sample containing 1:5 (1 mL of AgNO3:5 mL extract) showing AgNPs SPR peak at 411.05 nm.
Crude methanolic extract of V. jatamansi shoot was used for the bio-reduction of Ag and AgNPs production from AgNO3. Extract and AgNO3 solution were mixed in different ratios, and the NP synthesis was monitored during continuous stirring. Initial observation of AgNP production was made based on the color change of solution. On synthesis of nanoparticles, solution changed its color, and the resultant dense colored solution (dark yellow or brown) indicated the synthesis of AgNPs. This color change agrees with the findings of Bharathi et al. [17]. These researchers reported brown color of solution on AgNPs synthesis from Diospyros montana extract. Final confirmation of Ag nanoparticle production was carried out by the UV-Vis spectrophotometric analysis. AgNPs absorb light and give characteristic absorption peak in 400–500 nm wavelength range. Peak intensity refers to AgNPs concentration. Highest SPR peak at 411.0 nm wavelength represented maximum silver NPs synthesis in solution containing 1:5 ratios (1 mL AgNO3 and 5 mL methanolic extract solution) among all samples. Our UV-Vis spectrophotometric data coincide with the study by Sreekanth et al. [18] who reported the synthesis of AgNPs from Nelumbo nucifera extract and observed its SPR peak at 412.0 nm. During our studies, an increase in the extract concentration with respect to AgNO3 yields larger amount of AgNPs. Similar observation was also made by Umoren et al. [19] who revealed that higher extract concentration increased the possibility of stable and well-defined AgNPs synthesis.
3.2 Effect of salt concentration and temperature on stability of prepared AgNPs
Synthesized silver NPs were checked for their stability at different temperatures and salt stresses. Different temperature ranges (20–40°C and 80–100°C) and different NaCl (sodium chloride) salt concentration (1 M, 0.5 M, and 1 mM) were applied to the samples separately. On comparison of the UV-Vis spectra of the samples, a decrease in stability of the AgNPs was observed with an increase in the temperature and salt concentration. AgNPs heated at 20–40°C were comparatively stable than the AgNPs heated up to 100°C (Figure 3). Almost complete degradation of the AgNPs was observed at 100°C. Different NaCl stresses affected the synthesized AgNPs (Figure 4). With a gradual increase in salt concentration, stability of AgNPs decreased. AgNPs showed less stability at 1 M NaCl, comparatively moderate stability at 0.5 M, and highest stability at 1 mM NaCl among all the tested samples. A decrease in sharpness and height of the SPR peak suggested degradation of AgNPs in sample in higher saline conditions.
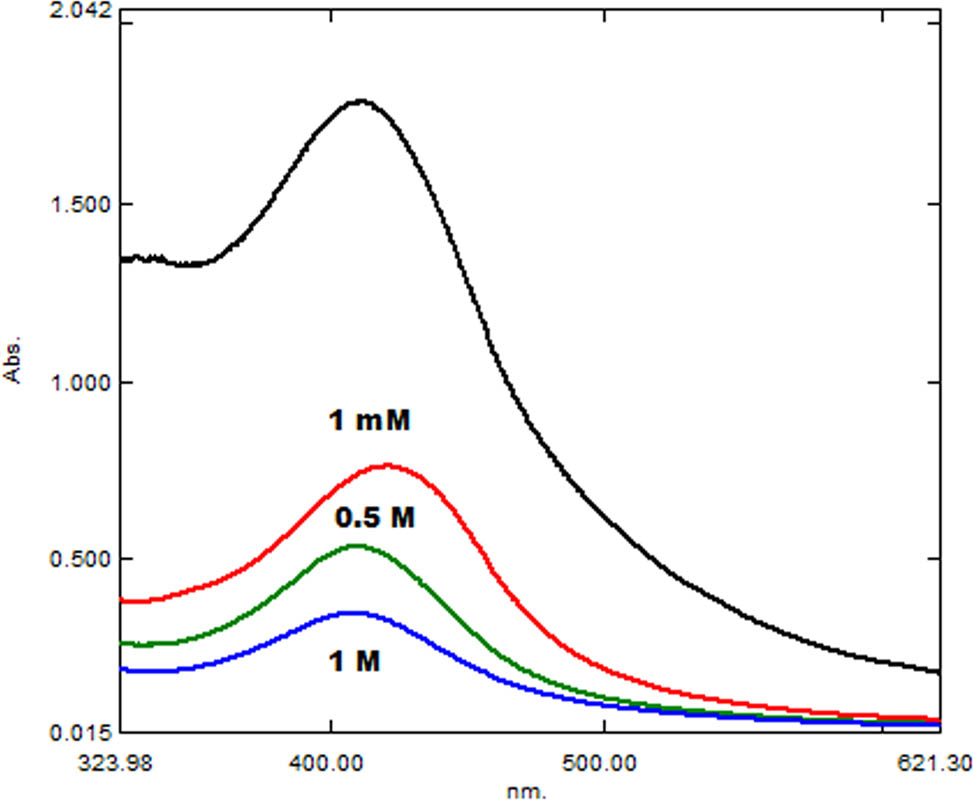
Comparison of UV-Vis spectra of AgNPs stability at different NaCl salt concentrations.

Comparison of UV-Vis spectra of AgNPs stability at different temperatures.
The stability of AgNPs at high temperature and salt conditions was assessed by heating samples at different temperature ranges (20–40°C and 80–100°C) and salt concentrations (1 M, 0.5 M, and 1 mM). AgNPs were comparatively more stable at lower temperatures and salt concentrations. A change in sharpness and height of AgNPs SPR peak represented degradation of NPs in samples isolated at higher salt and temperature levels. Mittal et al. [20] also reported a decrease in UV-Vis light absorbance at higher temperatures referring to AgNPs degradation in solution at higher temperatures [20].
3.3 AgNPs characterization
XRD patterns of V. jatamansi shoot AgNPs suggested the crystalline nature of AgNPs (Figure 5). Bragg’s reflection indexing of (311), (232), (220), (202), (200), (141), and (111) corresponding to, respectively, 77.38°, 66.14°, 64.54°, 50.21°, 44.39°, 40.58°, and 38.05° two theta values suggested fcc (face centered cubic) structure of Ag (silver). The average size of the synthesized Ag nanocrystallite was calculated as 13.32 nm by determining the full width half maximum (FWHM) of most intense peaks (202), (141), and (111) and applying sheerer equation. Comparison of FTIR spectrums of V. jatamansi shoot extract and its AgNPs indicated vanishing of the same absorption bands at 923.84 and 1264.06 cm−1 wave numbers initially present in the extract, thus suggesting the involvement of carboxylic acid (–[C–O–O–H]) and methane (–CH–) functional groups of different compounds in bioreduction of Ag-metal, respectively (Figure 6). A closer insight reported small shift (±1 to ±100) of wave numbers in other absorption bands and confirmed the synthesis of AgNPs in solution. FT-IR profiling shows that peaks at 3,324 and 3,287 cm−1 may be attributed to O–H vibrations of hydroxyl functional groups. However, bands at 2,929 and 2,918 cm−1 might be assigned to C–H vibrations of alkanes. Peaks in region of 1,598 and 1,575 cm−1 correspond to C–O stretching vibrations. Two distinct bands noticed at 1,376 and 1,380 cm−1 are characteristic to C–N stretching vibrations aromatic amino groups. Likewise, a peak recorded at 1,264 cm−1 may be because of C–O stretching of flavonoids. Two bands observed in the region of 1,035 and 1,032 cm−1 may correspond to N–C bond stretching of aliphatic amine groups.

XRD patterns of V. jatamansi shoot AgNPs.

Comparative FTIR spectra of pure extract and AgNPs.
SEM analysis indicated the average size of synthesized uniformed shaped nanospheres as 32 nm (Figure 7). Same XRD patterns are reported by Singh et al. [21] during their studies on AgNPs synthesis from Argemone mexicana extracts. Comparison of FTIR spectra of extract and AgNPs indicated the possible involvement of carboxylic acid (–[C–O–O–H]) and methane (–CH–) functional groups of different compounds in Ag metal reduction. Ganaie et al. [22] also reported the involvement of same functional groups in reduction of metal to synthesize NPs. Interpretation of SEM images confirmed the uniform shape of synthesized nanospheres and size as 32 nm. Same size of AgNPs synthesized from Mulberry leaves through SEM in the range of 20–40 nm was investigated by Awwad and Salem [23].

SEM photograph of V. jatamansi shoot AgNPs.
3.4 Antimicrobial properties of prepared AgNPs
Effect of AgNPs synthesized from methanolic crude extract of V. jatamansi shoot on the growth of seven different microbes is shown in Figure 8. Reduction in microbial growth increased with the increase in AgNP concentration. P. aeruginosa was the most sensitive of all the tested microbes and showed 71.46% growth inhibition at 1.5 mg disc−1 concentration. Moderate growth inhibition of 65.97, 61.5, 55.32, and 54.83% was recorded for E. coli, C. albicans, X. campestris, and S. aureus at highest tested concentration, respectively. Least growth inhibition of 48.55% recorded for K. pneumonia suggested it as the least-susceptible microbe among all the tested microbial species.

Antimicrobial potential of AgNPs, with standard deviation, against tested microbial strains.
AgNPs were found efficient in inhibiting the microbial growth. Highest zone of inhibition by AgNPs was measured for P. aeruginosa followed by E. coli and C. albicans. The least activity of AgNPs was recorded against K. pneumonia among all the test microbes. Our findings are supported by the results of Jeeva et al. [24]. During their study, Jeeva et al. [24] found P. aeruginosa as the most susceptible, while K. pneumonia as less susceptible to AgNPs among all the tested microbes.
4 Conclusion
Synthesis of AgNPs from V. jatamansi shoot extract and AgNO3 during the present study supports the efficient reduction of silver and synthesis of stable, spherical, and crystalline AgNPs at this much lower concentration of extract and silver salt. Moreover, prepared AgNPs were active against the tested bacterial and fungal strains and inhibited their growth.
Conflict of interest: One of the authors (Abdur Rauf) is a member of the Editorial Board of Green Processing and Synthesis.
References
[1] Rajan R, Chandran K, Harper SL, Yun SI, Kalaichelvan PT. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind Crop Prod. 2015;70:356–73.10.1016/j.indcrop.2015.03.015Search in Google Scholar
[2] Ahmad Z, Sharma S, Khuller GK. Inhalable alginate nanoparticles as anti-tubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents. 2005;26:298–303.10.1016/j.ijantimicag.2005.07.012Search in Google Scholar
[3] Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnol. 2007;18:105104–15.10.1088/0957-4484/18/10/105104Search in Google Scholar
[4] Jhaa AK, Prasad K, Kulkarni AR. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf B Biointerfaces. 2009;71:226–9.10.1016/j.colsurfb.2009.02.007Search in Google Scholar
[5] Dubey SP, Lahtinen M, Sillanpaa M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp. 2010;364:34–41.10.1016/j.colsurfa.2010.04.023Search in Google Scholar
[6] Merin DD, Prakash S, Bhimba BV. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac Trop Med. 2010;3:797–9.10.1016/S1995-7645(10)60191-5Search in Google Scholar
[7] Bankar A, Joshi B, Kumar AR, Zinjardea S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf B Biointerfaces. 2010;80:45–50.10.1016/j.colsurfb.2010.05.029Search in Google Scholar PubMed
[8] Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces. 2010;76:50–6.10.1016/j.colsurfb.2009.10.008Search in Google Scholar PubMed
[9] Bai HJ, Yang BS, Chai CJ, Yang GE, Jia WL, Yi ZB. Green synthesis of silver nanoparticles using Rhodobacter sphaeroides. World J Microbiol Biotechnol. 2011;27:2723–8.10.1007/s11274-011-0747-xSearch in Google Scholar
[10] Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C, et al. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2011;108:693–702.10.1007/s00436-010-2115-4Search in Google Scholar PubMed
[11] Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502.10.1016/j.jcis.2004.03.003Search in Google Scholar PubMed
[12] Ali DM, Thajuddina N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces. 2011;85:360–5.10.1016/j.colsurfb.2011.03.009Search in Google Scholar PubMed
[13] Ahmad M, Khan MA, Rashid U, Zafar M, Arshad M, Sultana S. Quality assurance of herbal drug valerian by chemotaxonomic markers. Afri J Biotechnol. 2009;8:1148–54.Search in Google Scholar
[14] Jugran AK, Rawat S, Bhatt ID, Rawal RS. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother Res. 2019;33:482–503.10.1002/ptr.6245Search in Google Scholar PubMed
[15] Lin S, Chen T, Liu X-H, Shen Y-H, Li H-L, Shan L, et al. Iridoids and Lignans from Valeriana jatamansi. J Nat Products. 2010;73(4):632–8.10.1021/np900795cSearch in Google Scholar PubMed
[16] Bakht J, Ali H, Khan MA, Khan A, Saeed M, Shafi M, et al. Antimicrobial activities of different solvents extracted samples of Linum usitatissimum by disc diffusion method. Afri J Biotechnol. 2011;10:19825–35.Search in Google Scholar
[17] Bharathi D, Josebin MD, Vasantharaj S, Bhuvaneshwari V. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J Nanostruct Chem. 2018;8:83–92.10.1007/s40097-018-0256-7Search in Google Scholar
[18] Sreekanth TVM, Ravikumar S, Eom IY. Green synthesized silver nanoparticles using Nelumbo nucifera root extract for efficient protein binding, antioxidant and cytotoxicity activities. J Photochem Photobiol B, Biol. 2014;141:100–5.10.1016/j.jphotobiol.2014.10.002Search in Google Scholar PubMed
[19] Umoren SA, Obot IB, Gasem ZM. Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J Env Sci. 2014;5:907–14.Search in Google Scholar
[20] Mittal AK, Kaler A, Banerjee UC. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed Eng. 2012;4:118–24.10.5101/nbe.v4i3.p118-124Search in Google Scholar
[21] Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma HN. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct. 2010;5:483–9.Search in Google Scholar
[22] Ganaie SU, Abbasi T, Abbasi SA. Rapid and green synthesis of bimetallic Au-Ag nanoparticles using an otherwise worthless weed Antigonon leptopus. J Exp Nanosci. 2016;11:395–417.10.1080/17458080.2015.1070311Search in Google Scholar
[23] Awwad AM, Salem NM. Green synthesis of silver nanoparticles by Mulberry leaves extract. Nanosci Nanotechnol. 2012;2:125–8.10.5923/j.nn.20120204.06Search in Google Scholar
[24] Jeeva K, Thiyagarajana M, Elangovanb V, Geethac N, Venkatachalama P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crop Prod. 2014;52:714–20.10.1016/j.indcrop.2013.11.037Search in Google Scholar
© 2020 Madiha Iqbal et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis

