Abstract
Three kinds of acrylamide copolymers were synthesized in this paper, acrylamide (AM) homopolymer, acrylamide/acrylic acid (AA) copolymer and acrylamide/2-acrylamide-2-methylpropanesulfonic acid (AMPS) copolymer by free radical polymerization. The aggregation behaviours of these polymers in salts water solution were studied. The molecular chains of homopolymer were stretched in salt water solutions, since it did not undergo hydrolysis and there were no charges in the backbone and side chains. Therefore, the conformation of homopolymer was hard to be attacked by salt ions. However, the copolymers synthesized from these monomers had large number of anionic groups. These anions were easily attracted by cations from salt solutions forming ionic bonds, which led to changes in molecular conformation. The viscosity of copolymer decreased since the molecular chains were crimped. The viscosity of copolymer decreased distinctly according to the temperature of different salt solutions, as temperature had great impact on the viscosity of copolymer. The interaction mechanism of polymers exhibited great difference in salts solutions, comparing to temperature. High temperature reduced the entanglement between molecular chains, whereas salt ions neutralized the negative charges on the molecular chains causing curling of molecular chains.
1 Introduction
Homopolymer and copolymers of acrylamide, considered as “industry auxiliaries”, are applied in many fields, such as medicine (1,2), materials science (3, 4, 5) and engineering, petroleum engineering (6, 7, 8, 9). Oil industry is one of the largest consumption areas of acrylamide polymers. Acrylamide polymers have consistently been applied in oil fields as chemical agents. For example, acrylamide polymers are used in drilling fluids as filtrate reducer (10,11), eco-friendly flocculants (12,13), thickening agent (14, 15, 16), and so on. With the increase in consumption and the exhaustion of conventional resources of energy, the exploration and exploitation of reservoirs with complex conditions such as high temperature, high salinity and high pressure have also increased greatly. The application of common acrylamide polymers was limited by changes in polymer properties under complex conditions, due to which temperature and salt-resistant acrylamide polymers have attracted wide attention. For example, gellant based on 2-acrylamido-2-methylpropanesulfonic acid, acrylamide and acrylic acid terpolymer can be used in high temperature and high pressure wells after crosslinked with zirconium (17). However, studies have shown that zirconium inhibited the growth of green algae, and the alkaline environment was more likely to promote the toxic effects of zirconium (18). Micro-sized polyacrylamide elastomeric microspheres was used to improve flooding efficiency in formation at 90°C in high salinity of 20,000 mg/L and a broad pH range of 4.0-10.3 (19). Polyacrylamide/clay nanocomposite (PANC) was synthesized and used as a drilling fluid additive in inhibitive water-based drilling fluid system (6). However, it was not suitable for temperatures above 120°C. Salami et al. studied the action mechanism of humic acid-sodium grafted 2-acrylamido-2-methylpropane sulfonate-co-N, N-dimethyl acrylamide-co-acrylic acid as a high temperature fluid loss additive. This multicomponent copolymer can resist the temperature to 150°C (20). The study, however, did not discuss the salt resistance of the polymer. The temperature and salinity resistance of the copolymer, cross-linked poly acrylamide-acrylic acid-2-acrylamide-2-methylpropanesulfonic acid nano-spheres, were superior to those of cross-linked polyacrylamide nano-spheres and cross-linked poly acrylamide-acrylic acid nano-spheres (21). This study showed that the molecular structure of the polymer had a significant effect on its temperature and salt resistance. Numerous studies have shown that applications of acrylamide polymer at high temperature or in salty environment were improved greatly. For example, Rakshith et al. used cetyltrimethylammonium bromide (CTAB) grafted polyacrylamide (PAM) gel, for controlling fluid loss, as it remained stable at 160°C (22). However, still no environmentally friendly ways for use of acrylamide polymer at high temperature and salt conditions have been reported. The main reason is that the effects of temperature and salt on molecular aggregation behaviour are still unclear. With increase in drilling depth and the complexity of stratum, the requirement of acrylamide polymer with high temperature resistance and salt resistance becomes more distinct. Therefore, it is necessary to study the aggregation behaviors of acrylamide polymers at high temperature and high salt conditions.
Acrylamide polymers have a chain-tangled three-dimensional network structure, which can be easily modified by pH, ions, temperature and the other external factors (23,24). The viscosity and the stability of acrylamide polymers is influenced by both physical (25) and chemical factors (26), such as storage time, mechanical stress and photo/sonic radiation, pH, surfactants, metals, salts ion, temperature, concentration of acrylamide, etc. Sensitivity to temperature and salt was particularly high under many performance-influencing conditions of acrylamide polymers. Hence, the applications of acrylamide polymers at high temperature and under high salt concentrations were limited. The gelling time increased with the increase in concentration of salt in the polymer solution (27). The viscosity however decreased with the increase in salt concentration (28, 29, 30, 31, 32) and temperature (33, 34, 35). Recent studies have mainly focused on the changes of viscosity and gelling time of acrylamide polymer at high salinity conditions or at high temperature. However, they did not provide any details on the molecular behaviour of the effect of temperature and salts on acrylamide polymer.
The main aim of this paper was to explore the effects of salt and temperature on the aggregation behaviour of acrylamide polymer. The interaction mechanism of temperature and salts with acrylamide polymer were studied. Apparent viscosity measurement, viscosity average molecular weight determination and Fourier transform infrared spectroscopy were used to analyze the change of molecular chain under the action of salts at room and high temperature.
2 Experimental
2.1 Materials
Acrylamide (AM, 99%), acrylic acid (AA, 98%) and 2-acrylamide-2-methylpropanesulfonic acid (AMPS, 98%) were commercial products obtained from Xilong Chemical Company. Sodium chloride (NaCl, AR), potassium chloride (KCl, AR), calcium chloride (CaCl2, AR), sodium hydroxide (NaOH, AR), potassium peroxydisulphate (K2S2O8, AR), etc. were purchased from Beijing Chemical Reagent Company. All the reagents were used directly without purification.
2.2 Methods
2.2.1 Synthesized of the acrylamide homopolymer
The acrylamide homopolymer (polymer 1) was synthesized by free radical polymerization in aqueous medium. Deionized water (1000 mL) was taken in a flask and then AM (80 g) was added under vigorous stirring. The temperature was raised to 60°C and the reaction was continued at 60°C for 30 min under nitrogen. Then the initiator K2S2O8 (600 mg) was added to the solution gradually and reacted for 3 h (The set-up for polymerization process is shown in Figure 1). The product was allowed to cool to room temperature. The product was then slowly poured into acetone, and the precipitated polymer was isolated and dried at 60°C for 24 h. The homopolymer was dissolved in deionized water, precipitated in acetone and dried again. Dried product was grinded into powder.

Picture of the polymerization process.
2.2.2 Synthesized of the acrylamide copolymer
The AM/AA (polymer 2) and AM/AMPS (polymer 3) copolymers were also synthesized by free radical polymerization in aqueous media. AM (30 g) and AA (10 g) were mixed together in 500 mL deionized water. In a separate flask, AM (56 g) and AMPS (64 g) were mixed in 800 mL of deionized water. To each solution was added sodium hydroxide solution and stirred to adjust the pH to 7-8. The temperature was raised to 60°C and maintained at 60°C for 30 min under nitrogen. Thereafter, the initiator, K2S2O8 (600 mg), was added to each solution and reacted for 3 h. The products were cooled at room temperature, respectively. Then the products were slowly poured into acetone, and the precipitated polymers were isolated and dried at 60°C for 24 h. The polymeric products were dissolved in distilled water, precipitated in acetone and dried again. Dried products were grinded into powder. A portion of AM/AMPS copolymer was hydrolysed by sodium hydroxide to obtain 25% hydrolysed product (polymer 4). The properties of the solution obtained were evaluated. The further treatment was carried out for IR-FT test.
2.2.3 Fluid preparation
Each polymer sample was added to deionized water to make a 1 wt% polymer solution by stirring. It was then divided into 4 portions, to one of which, nothing was added. To the remaining three portions, were added different amounts of NaCl, KCl, CaCl2 separately. The percentages of salts added in this paper were based on the volume of solution.
2.2.4 Rheological property tests
The viscosity of acrylamide polymer was determined with using a MOD.ZNN-D6-type six-speed rotational viscometer (Qingdao Haitongda equipment Limited, China). The liquid was poured into the sample cup up to the marked line and the readings were recorded from 600 rpm to 3 rpm. The rheological parameters, such as the apparent viscosity (AV) which characterized the total viscosity of the drilling fluid as it flows, the plastic viscosity (PV) which characterized the degree of difficulty that destroy the colloidal system after the mud is still, and yield point (YP) of the polymers were determined through measurements of the viscosities at two rotation rate of 600 rpm and 300 rpm at room temperature. Above rheological parameters were calculated from the value of Ø 600 (reading of viscosity at 600 rpm) and Ø 300 (reading of viscosity at 300 rpm) by following formulae (Eq. 1-3):
In this paper, changes of polymer properties were mainly characterized by measuring the changes in apparent viscosity of polymer solution. Aging experiments of polymer fluids were carried out in a GW300-type frequency conversion rolling oven (Qingdao Tongchun Machinery Plant, China) through hot rolling at appointed temperature (80°C). The polymer fluids were placed in the digestion tank and were then taken to roller oven. The rolling time was fixed at 16 h. Fluid property tests were performed before and after the thermal aging experiments.
2.2.5 Testing of viscosity average molecular weight
Test for viscosity average molecular weight were conducted according to the “National Standard of the People’s Republic of China” GB 17514-2008 (36). NaNO3 solution (85 g/L) was prepared after filtration through an acid filter funnel. The sodium nitrate solution was transferred into the Ubbelohde viscometer having inner diameter of 0.55 mm. It was placed in a constant temperature water bath at 30°C and the upper liquid storage ball was submerged in the water. Then the liquid was sucked to the position of the upper liquid storage ball, the liquid was then released, and finally the time (t0) required to pass through the viscometer between the upper- and lower-marks lines was recorded. A colloidal sample containing 0.03 g of solid polymer was dissolved in a little sodium nitrate solution, diluted with a sodium nitrate solution in a 100 mL in a volumetric flask and shaken to homogenous. The polymer solution was added to the Ubbelohde viscometer and the same procedure as that of sodium nitrate solution was followed to obtain value of t1. The viscosity average molecular weight of the polymer was calculated according to Eq. 4 and 5 (36):
where: [ƞ] (dL/g) – the intrinsic viscosity, t1 (s) – the time when the test solution flowed through the upper and lower marking lines of the viscometer, t0 (s) – the time when the sodium nitrate solution flowed through the upper and lower marking lines of the viscometer, M – the viscosity average molecular weight, K and α – the empirical constant related to degree of hydrolysis. When the degree of hydrolysis was 25%, the value of K was 3.20×10-4, and the value of α was 0.707.
2.3 Structure characterization
2.3.1 Fourier transformed infrared spectroscopy (FT-IR) measurements
FT-IR spectral of the polymers was recorded by Spectrum One spectrometer. 2 mg samples and 200 mg KBr were fully mixed. The mixture was put into the mold and kept in 20 MPa pressure by hydraulic press.
2.3.2 Differential scanning calorimeter (DSC)
DSC curves of the three acrylamide polymers were obtained using a synchronous thermal analyser (STA449F3, Germany). The temperature was raised at a rate of 5°C per minute under a nitrogen atmosphere.
2.3.3 Transmission electron microscopy (TEM)
Samples were examined using a F20 transmission electron microscope (JEOL, Japan). A polymer (0.01 wt%) solution with or without salts was prepared by dispersing in water ultrasonically at room temperature for 20 min. One drop of the polymer solution was placed on a carbon membrane. The carbon membrane was dried in infrared light for 20 min before TEM examination.
3 Results and discussion
3.1 Characterization of acrylamide polymers
Figure 2a shows the infrared spectrum of the three acrylamide polymers. The peak at 3438 cm-1 was due to amino group (–NH2), whereas the peak for methylene group (–CH2) appeared at 2952 cm-1. The peak at 1672 cm-1 was characteristic for carbonyl (C=O) group. There was no characteristic peak of C=C on the infrared curve of the polyacrylamide homopolymer (polymer 1), indicating that the polyacrylamide homopolymer has been successfully synthesised. In addition to the peaks for acrylamide groups in the spectrum of polymer 1, the spectra of polymer 2 and polymer 3 showed some characteristic peaks. In polymer 2, the stretching absorption peak for C–O stretching of carboxylic acid (–COOH) appeared at 1307 cm-1. The overlap of C=O and –OH in carboxylic acid with carbonyl and amino groups in amide enhanced the strength of the peak. The spectrum of polymer 3 showed a peak at 1036-1180 cm-1, attributed to the stretching vibration of sulfonate (–SO3). The appearance of carboxylic acid group and sulfonic acid group indicated the successful synthesis of acrylamide copolymers. Figure 2b showed the DSC curves of three acrylamide polymers. Initial degradation at about 100°C corresponded to water loss in the samples. Thereafter, the DSC curve of polymer 1 exhibited intense exothermic peaks in the range of 150°C to 225°C with maximum at 220°C. The peak for polymer 2 appeared a little later, whereas polymer 3 showed no exothermic peaks. The result suggested that the heat resistance of polymer 3 was higher than that of the other two polymers.
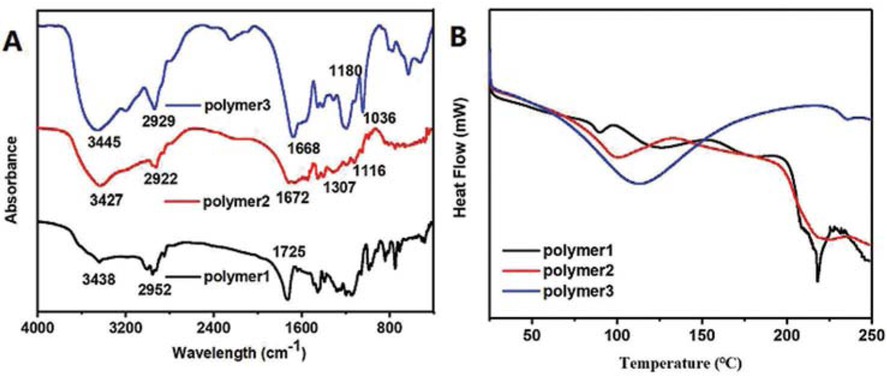
Infrared spectrum and DSC curves of acrylamide polymers: (a) FT-IR, (b) DSC.
3.2 Effect of salts on molecular structure of acrylamide polymer
The viscosity of polyacrylamide polymer was mainly dependent on its molecular weight, molecular structure, molecular conformation and molecular aggregate. Changes in molecular structure and molecular weight were due to either breaking or combination of molecular chains through covalent bond. Obviously, the addition of salts only led to changes in molecular aggregation and molecular conformation through ionic bonds and hydrogen bonds. The changes in apparent viscosity of polyacrylamide homopolymer after the addition of three kinds of salt ions can been seen in Figure 3. The apparent viscosity of polyacrylamide homopolymer increased initially and then declined slightly on addition of NaCl. It reached its minimum value when 10 wt% NaCl was added. With further addition of NaCl, the apparent viscosity increased gradually. On addition of KCl, the apparent viscosity was stable. It was evident that the apparent viscosity declined slightly on addition of 1 wt% KCl. Thereafter, the curve was almost stable. The changes in apparent viscosity of polyacrylamide homopolymer in CaCl2 solution were similar to the changes observed in case of NaCl and KCl solutions. The apparent viscosity of polyacrylamide homopolymer declined when 1 wt% CaCl2 was added. With further addition, the curve was stable. The experimental results showed that the apparent viscosity of polyacrylamide homopolymer changed slightly in salt solution, indicating that the salt ions had only a slight effect on the polyacrylamide homopolymer. There were no anionic groups present on the molecular chain of the polyacrylamide homopolymer, which could be combined with the cations in salt solutions. Therefore, acrylic acid having carboxyl groups was chosen for copolymerization with acrylamide.
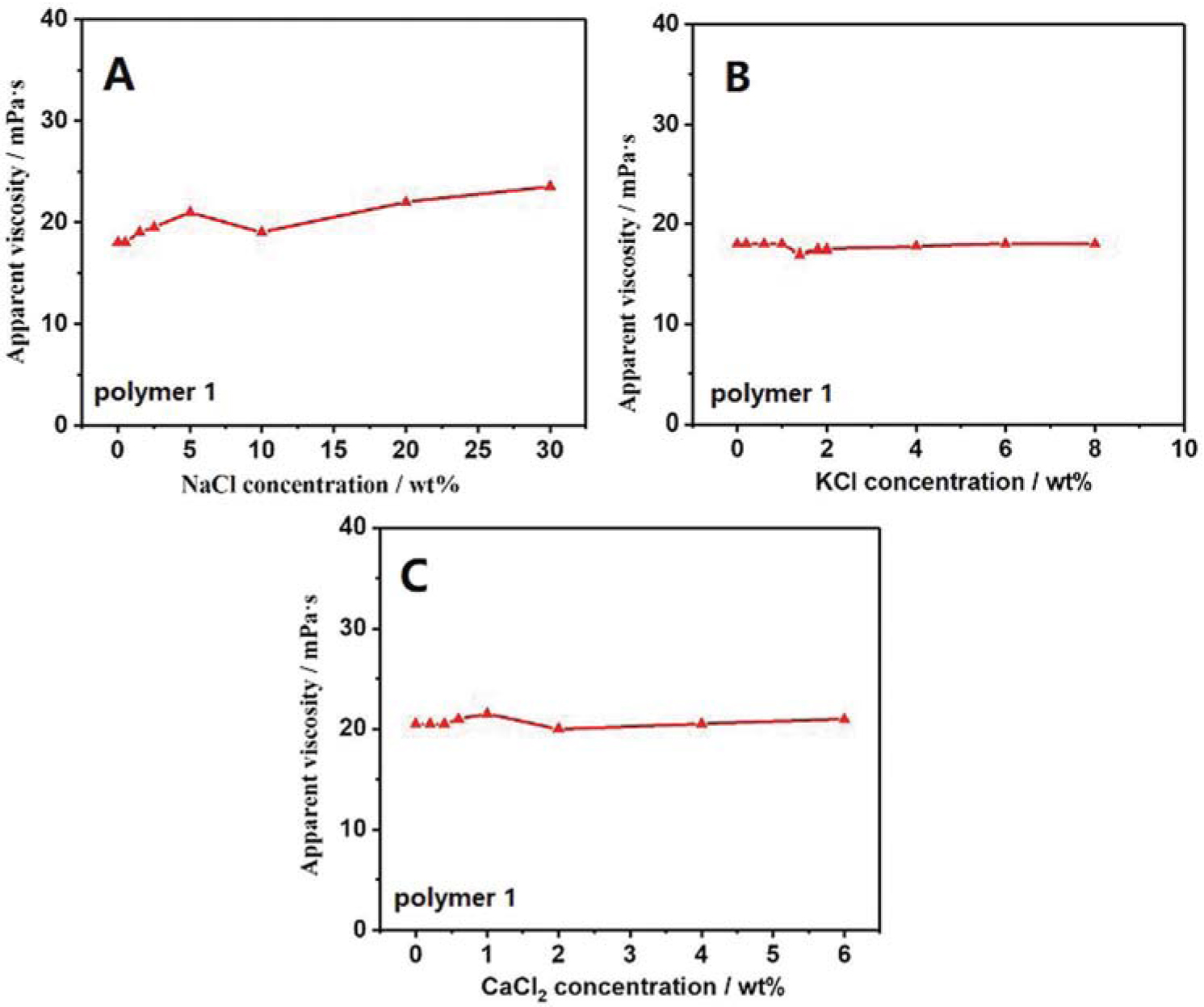
Changes in apparent viscosities of polyacrylamide homopolymer with varying salt concentration: (a) NaCl, (b) KCl, (c) CaCl2. Polyacrylamide homopolymer concentration: 1 wt%.
The changes in apparent viscosity of acrylamide/acrylic acid copolymer after the addition of three kinds of salt ions were shown in Figure 4. There were many carboxylic acid ions present in the molecular chains of the acrylamide/acrylic acid copolymer. From Figure 4 it was clear that the apparent viscosity of copolymer declined sharply on the addition of salt ions. In Figure 4a the apparent viscosity of acrylamide/acrylic acid copolymer was obviously decreased when NaCl solution was added. After the addition of 3 wt% NaCl solution into copolymer solution the change of apparent viscosity of copolymer showed a stable trend. The curves in Figures 4b and 4c showed similar trends. On addition of 2 wt% KCl solution, the decreasing trend of apparent viscosity showed down and stable, whereas the decrease in case of CaCl2 addition was more rapid. The apparent viscosity showed a stable trend on addition of 1 wt% CaCl2 solution. Acrylic acid has negatively charged carboxylic groups. The conformation of the copolymer changed when these anions were attracted by cations. The original molecular chains of copolymer were stretched. However, when carboxylic acid groups were attracted by cations, the molecular chain crimped together since the cations effectively shielded the electrolyte effect. This increased the flexibility of the molecule and reduce the hydrodynamic radius (37). So, the apparent viscosity of copolymer reduced. Similarly, another copolymer was synthesised to confirm this phenomenon. The acrylamide/2-acrylamide-2-methylpropanesulfonic acid (AMPS) copolymer was synthesized under the same conditions. Changes in apparent viscosity of AM/AMPS copolymer after the addition of three kinds of salt ions were shown in Figure 5. The copolymer contained many sulfonic acid groups and the 2-acrylamide-2-methylpropanesulfonic acid monomer also had a long side chain. Figures 4 and 5 showed similar changes. The apparent viscosity of copolymer declined sharply when salt ions were added. After adding 5 wt% NaCl solution, the apparent viscosity of copolymer reached its lowest value. Thereafter, unlike KCl and CaCl2, with increasing concentration of NaCl, the apparent viscosity of the AM/AMPS copolymer increased slowly. With continuous increase in concentration of NaCl, excessive number of Na+ was hydrated, which increased the polarity of the polymer solution. This weakened the hydration layer on the surface of the AM/AMPS copolymer molecule, which caused an increase in the hydrodynamic volume of the molecular chain and the apparent viscosity of the AM/AMPS copolymer increased slowly. However, the hydration of K+ and Ca2+ ions was weaker than that of Na+, so the apparent viscosity did not show any increase in Figures 4b and 5c The value of apparent viscosity continued to reduce slowly after adding 2 wt% KCl solution. This trend of AM/AMPS copolymer was similar to AM/AA copolymer. After the amount of added CaCl2 reached 1 wt%, the apparent viscosity of copolymer showed a stable trend. These experimental results further proved that the polymer with anions was easily attracted by cations. As a consequence, the negative charges on the polymeric chain were neutralised, the electrostatic repulsion was weakened, and then the molecular chain was curled which finally resulted in the sharp decrease in apparent viscosity of the copolymer.

Changes in apparent viscosity of acrylamide/acrylic acid copolymer with varying salt concentrations: (a) NaCl, (b) KCl, (c) CaCl2. Copolymer concentration: 1 wt%.
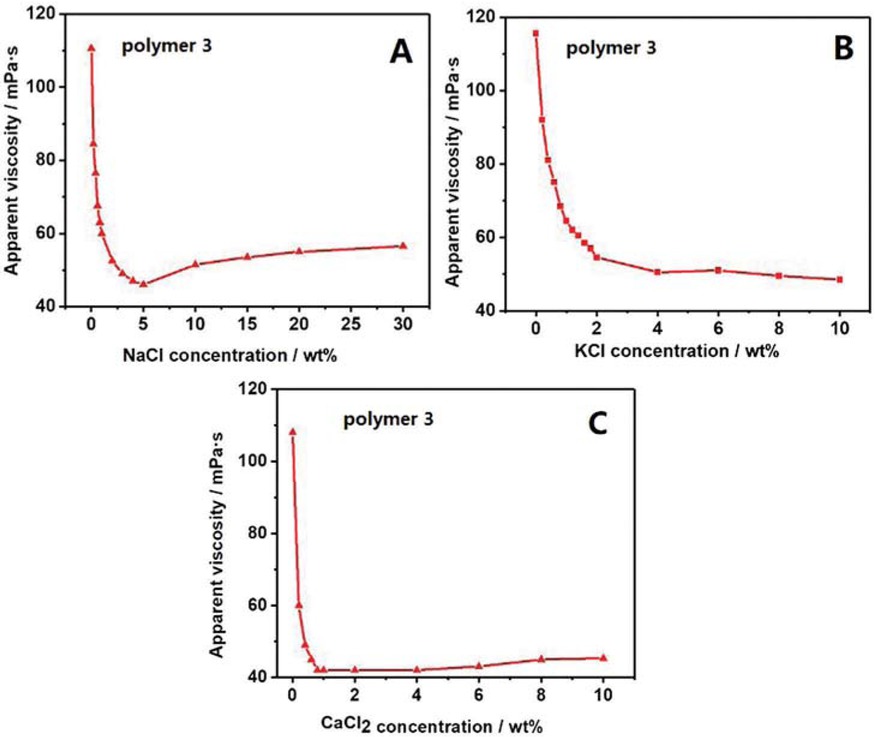
Changes in apparent viscosity of AM/AMPS copolymer with different salt concentrations: (a) NaCl, (b) KCl, (c) CaCl2. Copolymer concentration: 1 wt%.
The commonly used method to measure the viscosity average molecular weight is “wood viscosity method” which uses the Ubbelohde viscometer. The principle is based on the viscosity of polymer solution, which is measured in terms of viscosity average molecular weight. Therefore, the change in the molecular weight, measured by the Wood viscosity method, can reflect the change in viscosity. It is well known that there was no chemical reaction between the salts and the polymer, and therefore, the actual molecular weight of polymer did not undergo and change. However, in Figure 6, the effect of different concentrations of three salt solutions on the viscosity average molecular weight of the AM/AMPS copolymer showed that the viscosity average molecular weight of the AM/AMPS copolymer after the addition of different salts was somewhat reduced in all cases. The molecular weight of AM/AMPS copolymer was about 2.036×106. On addition of 5 wt% NaCl, 10 wt% KCl and 2 wt% CaCl2 solutions, the molecular weights changed to 1.597×106 1.731×106, and 1.434×106, respectively. Since no chemical change occurred and the value of the viscosity average molecular weight was affected by the viscosity of the copolymer, the decreased in the viscosity average molecular weight of the copolymer at this time could only be attributed to the decreased in the apparent viscosity of the copolymer. This result was fully consistent with the previous test results of apparent viscosity and it further demonstrated that the salts caused a change in the conformation of the polymer molecule.

Molecular weight changes of the AM/AMPS copolymer with different concentrations of the three salt solutions. Polymer 3 was AM/AMPS copolymer.
3.3 Effect of temperature on molecular structure of acrylamide polymer
It is well known that temperature is a major factor in drilling operations. Three kinds of polyacrylamide polymer were synthesized and the effect of temperature on molecular structure of these polymers was studied. The apparent viscosity changes of polymer were still considered as the evaluation criterion. Figure 7 showed the changes in apparent viscosity of polyacrylamide homopolymer before and after rolled at 80°C with different salt solutions. The apparent viscosity of polyacrylamide homopolymer reduced sharply after rolling. It showed that the molecular conformation changed significantly. At this time, the polymer with different salt solutions showed better temperature resistance.

Changes in apparent viscosity of polyacrylamide homopolymer with different salts after hot rolling at 80°C. Polyacrylamide homopolymer (polymer1) concentration: 1 wt%.
To further study the effect of temperature, the changes in apparent viscosity of AM/AA copolymer were measured as shown in Figure 8. The apparent viscosity of the copolymer declined sharply after rolling, which indicated that the introduce of carboxyl groups could not improve the temperature resistance of polymer. However, the addition of different salt ions improved temperature resistance clearly distinctly. This result was similar to that of the property of polyacrylamide homopolymer. Compared to the carboxyl group, sulfonic acid group exhibited the better resistance to temperature.
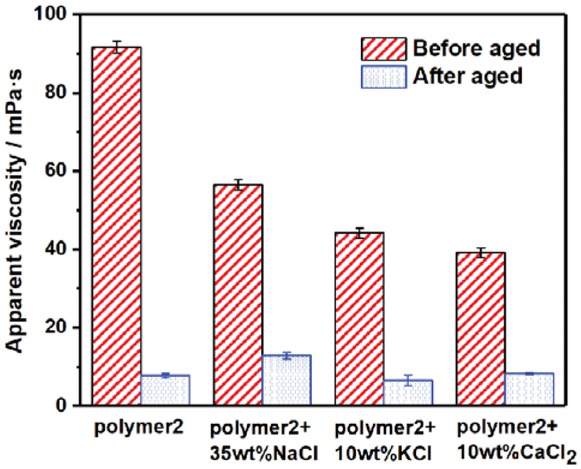
Changes in apparent viscosity of acrylamide/acrylic acid copolymer with different salts after hot rolling at 80°C. Concentration of acrylamide/acrylic acid copolymer (polymer 2): 1 wt%.
The changes in apparent viscosity of AM/AMPS copolymer were observed in Figure 9a The trend of AM/AMPS copolymer was different than those of AM/AA copolymer and acrylamide homopolymer. The sulfonic acid copolymer without salts had a slightly higher apparent viscosity than the carboxyl copolymer after aging. This result suggested that the temperature resistance of sulfonic acid group was better than that of carboxyl group. However, after the addition of salt ions, the apparent viscosity of AM/AMPS copolymer declined more than that of AM/AA copolymer. This experiment was repeated several times and the same experimental results were obtained.

Changes in apparent viscosity of AM/AMPS copolymer and hydrolysed AM/AMPS copolymer with different salts after hot rolling at 80°C. (a) Concentration of AM/AMPS copolymer (polymer 3): 1 wt%; (b) Concentration of hydrolysed AM/AMPS copolymer (polymer 4): 1 wt%.
Sulfonic acid was a strong anion which interacts strongly with cations. Such an interaction causes changes in molecular conformation of copolymer. Studies by Xin et al. have shown that an increase in temperature and alkalinity could accelerate the decreasing trend of apparent viscosity in case of hydrolyzed polyacrylamide (38). Alkaline conditions promoted the crosslinking between salt and polymer (39). Hence, the AM/AMPS copolymer was hydrolyzed at certain alkaline condition, the more carboxyl group would be produced. Changes in apparent viscosity of hydrolyzed AM/AMPS copolymer with different salt ions was shown in Figure 9b The apparent viscosity of hydrolyzed AM/AMPS copolymer without salts increased, indicating the interaction between molecular chains. However, the apparent viscosity of copolymer still declined clearly after hot rolled at 80°C. The rate of declined before and after hydrolysis was similar. After the addition of salt ions, the apparent viscosity of hydrolysed copolymer decreased sharply further without any significant improvement. At 80°C, the entanglements between the molecular chains of the polymer were lowered, which weakened the intermolecular interaction force, resulting in a decrease in the viscosity of the polymer. The results of viscosity average molecular weight of AM/AMPS copolymer were shown in Figure 10. The viscosity average molecular weight of AM/AMPS copolymer with and without salt ions decreased sharply after rolling at 80°C. It was precisely due to the decreased chain entanglements of polymer molecules at 80°C that the forces between the molecular chains were lowered, so the apparent viscosity of the polymer decreased. At the same time, the salt ions could combine with the exposed anions such as carboxyl acid groups and sulfonic acid groups, resulting in curling of molecular chains and further reduction in apparent viscosity of the polymer. Under the dual action of temperature and salt ions, the apparent viscosity of the polymer declined sharply.

Changes in molecular weight of AM/AMPS copolymer (polymer 3) with different salts after hot rolling at 80°C.
3.4 Mechanism discussion
The sulfonic acid groups improved temperature resistance of the polymer slightly. Hence, the AM/AMPS copolymer was chosen to discuss the mechanism responsible for the comprehensive properties of three kinds polymer mentioned above. The polymer samples containing different salts, before and after aging, were dried and ground into powder. The eight samples were analysed by infrared spectroscopy, and the findings are as follows.
Figure 11a showed the infrared spectra of AM/AMPS copolymers with different salts. At room temperature, the peak for amino (–NH2) group of the salt-free AM/AMPS copolymer appeared at 3447 cm-1. This peak was shifted to 3433 cm-1, 3439 cm-1, 3426 cm-1, in presence of NaCl, KCl and CaCl2, respectively. Comparison of changes in amino absorption peaks showed that the position of the peak for amino group shifted to lower wavelengths after the addition of salts. This small change suggested of changes occurring in the molecular conformation of the polymer after adding salt ions. The strong interaction between the copolymer molecules and the salt ions caused the molecular chains to curl due to weakening of electrostatic repulsion, resulting in a reduce of apparent viscosity. Temperature affected the apparent viscosity of the polymer more as mentioned above. The infrared spectra of AM/AMPS copolymer before and after aging at 80°C was measured and showed in Figure 11b The absorption peaks of amino (–NH2) vibrations changed from 3447 cm-1 to 3472 cm-1, whereas the absorption peaks of methylene (–CH2–) vibrations changed from 2936 cm-1 to 2984 cm-1, after ageing. Comparing the changes in amino (–NH2), methylene (–CH2–) peaks before and after aging, it was evident that the manner in which temperature affected was different from that of salt ions. To further explore the mechanism, the infrared spectra of AM/AMPS copolymer after aging at 80°C with different salt ions were recorded and the curves were shown in Figure 10c After aging, the salt-free copolymer showed an absorption peak at 3472 cm-1 for amino (–NH2). The absorption peaks of amino groups (–NH2) containing NaCl, KCl and CaCl2 salts appeared at 3452 cm-1, 3453 cm-1 and 3399 cm-1, respectively. Compared to the amino peak before aging, the change was more obvious when temperature was applied. The effect of temperature on the copolymer was greater than that of salts, due to which the peak was shifted to higher frequency as shown in the Figure 11b whereas in case of salts the peak was shifter to lower frequencies as shown in Figure 11a However, due to the influence of temperature and salts, the peak for amino group shifted to higher frequency, but its offset degree was lower than that due to temperature. Infrared spectroscopy results showed that both temperature and salts have an effect on the molecular conformation of the polymer, but their mechanisms were different.
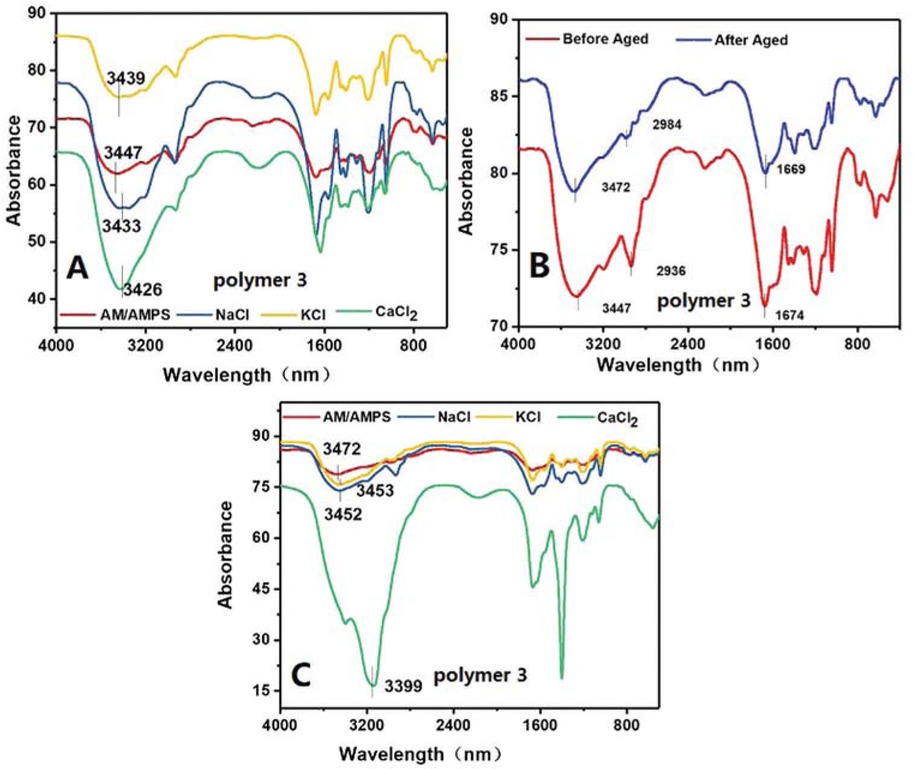
Infrared spectra of the AM/AMPS copolymer (a) with and without salt ions; (b) before and after aging at 80°C; (c) containing different salts after aging at 80°C.
To investigate the changes in micro-morphologies, TEM images of AM/AMPS copolymer on different condition was obtained and showed in Figure 12. The molecular chain of AM/AMPS copolymer appeared stretched and entangled with the other molecular chains (Figure 12a) However, after the addition of CaCl2 ions, the molecular chains crimped and were aggregated together (Figure 12b) Then the AM/AMPS copolymer with CaCl2 ions was rolled at 80°C for 16 h. In this case, molecular chains were not entangled with each other and the spacing between the molecular chains was increased. Therefore, the polymeric chains were separated from each other and could not form the network structure, due to which their apparent viscosity decreased. These observations further demonstrated the above mechanism and were found to be consistent with an earlier study (31) on the effect of divalent ions on the structure of acrylamide polymers.

TEM images of AM/AMPS copolymer with CaCl2 before and after aging at 80°C: (a) AM/AMPS copolymer, (b) AM/AMPS copolymer with CaCl2, (c) AM/AMPS copolymer with CaCl2 after aging at 80°C.
The infrared spectra and TEM images of polymer 1 and polymer 2 after adding CaCl2 and aging were shown in Figure 13. From the infrared spectra and TEM images, it can be seen that the interaction mechanism between polyacrylamide homopolymer and AM/AMPS copolymer with salt ions and temperature was consistent with that of AM/AMPS copolymer. The molecular structure of acrylamide polymers was not changed by temperature and salt at 80°C. However, the molecular conformation of acrylamide polymers underwent changed, owing to changes in molecular structures, resulting in decrease in apparent viscosity.

Infrared spectrum and TEM images of polyacrylamide homopolymer and AM/AA copolymer. (a1) The infrared spectrum of polyacrylamide homopolymer at different conditions; (a2) Infrared spectrum of AM/AA copolymer under different conditions; (b) TEM images of polyacrylamide homopolymer under different conditions; (c) TEM images of AM/AA copolymer under different conditions.
4 Conclusion
The effects of salts on acrylamide homopolymer and acrylamide copolymer and the mechanism of interaction between salts and acrylamide polymer were studied from results of apparent viscosity, molecular weight and infrared spectroscopy. At room temperature, the salts did not react with the unhydrolyzed acrylamide homopolymer, since it existed in molecular form in aqueous medium. Hence, there was little influence of salts on the apparent viscosity of the unhydrolyzed acrylamide homopolymer. On the other hand, the acrylamide copolymer had less resistance to salts due to the carboxyl group on AA and the sulfonic acid groups on AMPS. Hence, their apparent viscosities decreased sharply on addition of salt ions. The ordinary acrylamide polymer had long chains whose apparent viscosity was high, due to stretched molecular chains and large hydrodynamic volume. However, salt ions neutralized the negative charges on the molecular chains of the copolymer and the electrostatic repulsions of the molecular chains itself were weakened. This resulted in curling of the molecular chains and a decrease in viscosity of the polymer. Temperature affected in a different manner, which caused a decrease in the entanglement between different molecular chains in the polymer. The intermolecular forces were weakened so that the viscosity of the polymer was reduced.
The effect of salts and temperature on polyacrylamide polymer was studied in detail. Meanwhile, there still remain some problems. How to improve the resistant-salt and resistant temperature was not clear. On the other side, a series of polymer need be synthesized. The next work is in progress.
Acknowledgments
We would like to thank for the financial support from China Postdoctoral Fund (H29216), Natural Science Foundation of China (J218076) and National Key R&D Program of China (2016YFE0202200) for this work.
References
1 Liu H., Shi D., Duan F., Yang Z., Chen M., Liu S., Fabrication of polyacrylamide inverse opal photonic crystal Films based on co-deposition. Mater. Lett., 2015, 150, 5-8.10.1016/j.matlet.2015.02.123Suche in Google Scholar
2 Zhang W., Feng J., Chen T., Wang G., Yang T., Huang R., et al., Novel Potentiometric Sensor Based on Molecular Imprinted Polyacrylamide for the Determination of Shikimic Acid in Herbal Medicine. Anal. Lett., 2018, 51(18), 2920-2932.10.1080/00032719.2018.1455203Suche in Google Scholar
3 Freddi G., Tsukada M., Beretta S., Structure and physical properties of silk fibroin/polyacrylamide blend films. J. Appl. Polym. Sci., 2015, 71(10), 1563-1571.10.1002/(SICI)1097-4628(19990307)71:10<1563::AID-APP4>3.0.CO;2-ESuche in Google Scholar
4 Smirnov M.A., Sokolova M.P., Bobrova N.V., Toikka A.M., Morganti P., Lahderanta E., Synergistic effect of chitin nanofibers and polyacrylamide on electrochemical performance of their ternary composite with polypyrrole. J. Energ. Chem., 2018, 27(3), 843-853.10.1016/j.jechem.2017.06.002Suche in Google Scholar
5 Vijayalekshmi V., Khastgir D., Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J. Membrane Sci., 2017, 523, 45-59.10.1016/j.memsci.2016.09.058Suche in Google Scholar
6 Jain R., Mahto V., Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Petrol. Sci. Eng., 2015, 133(Supplement C), 612-621.10.1016/j.petrol.2015.07.009Suche in Google Scholar
7 Amin S.P., Marmur E.S., Goldberg D.J., Complications from Injectable Polyacrylamide Gel, a New Nonbiodegradable Soft Tissue Filler. Dermatol. Surg., 2004, 30(12 Pt 2), 1507.10.1111/j.1524-4725.2004.30551.xSuche in Google Scholar
8 Zhang X.M., Jiang G.C., Dong T.F., Wang L., Li X.L., Wang G.S., An amphoteric polymer as a shale borehole stabilizer in water-based drilling fluids. J. Petrol. Sci. Eng., 2018, 170, 112-120.10.1016/j.petrol.2018.06.051Suche in Google Scholar
9 Chang X.F., Sun J.S., Xu Z., Lv K.H., Dai Z.W., Zhang F., et al., Synthesis of a novel environment-friendly filtration reducer and its application in water-based drilling fluids. Colloid. Surface. A, 2019, 568, 284-293.10.1016/j.colsurfa.2019.01.055Suche in Google Scholar
10 Ma J., An Y., Yu P., Core-shell structure acrylamide copolymer grafted on nano-silica surface as an anti-calcium and anti-temperature fluid loss agent. J. Mater. Sci., 2019, 54(7), 5927-5941.10.1007/s10853-018-03239-0Suche in Google Scholar
11 Cao J., Meng L., Yang Y., Zhu Y., Wang X., Yao C., et al., Novel Acrylamide/2-Acrylamide-2-methylpropanesulfonic Acid/4-Vinylpyridine Terpolymer as an Anti-calcium Contamination Fluid-Loss Additive for Water-Based Drilling Fluids. Energ. Fuel., 2017, 31(11), 11963-11970.10.1021/acs.energyfuels.7b02354Suche in Google Scholar
12 Zou J., Zhu H., Wang F.H., Sui H.Y., Fan J.T., Preparation of a new inorganic-organic composite flocculant used in solid-liquid separation for waste drilling fluid. Chem. Eng. J., 2011, 171(1), 350-356.10.1016/j.cej.2011.03.100Suche in Google Scholar
13 Liu H.Y., Yang X.G., Zhang Y., Zhu H.C., Yao J.M., Flocculation characteristics of polyacrylamide grafted cellulose from Phyllostachys heterocycla: An efficient and eco-friendly flocculant. Water Res., 2014, 59, 165-171.10.1016/j.watres.2014.04.022Suche in Google Scholar PubMed
14 Khakpour H., Abdollahi M., Synthesis, characterization, rheological properties and hydrophobic nano-association of acrylamide/styrene and acrylamide/sodium styrene sulfonate/styrene co- and terpolymers. J. Polym. Res., 2016, 23(8).10.1007/s10965-016-1064-8Suche in Google Scholar
15 Huo J.-H., Peng Z.-G., Ye Z.-B., Feng Q., Zheng Y., Zhang J., et al., Investigation of synthesized polymer on the rheological and filtration performance of water-based drilling fluid system. J. Petrol. Sci. Eng., 2018, 165, 655-663.10.1016/j.petrol.2018.03.003Suche in Google Scholar
16 Chu Q., Lin L., Synthesis and properties of an improved agent with restricted viscosity and shearing strength in water-based drilling fluid. J. Petrol. Sci. Eng., 2019, 173, 1254-1263.10.1016/j.petrol.2018.10.074Suche in Google Scholar
17 Funkhouser G.P., Holtsclaw J., Blevins J., Hydraulic Fracturing under Extreme HPHT Conditions: Successful Application of a New Synthetic Fluid in South Texas Gas Wells. SPE Deep Gas Conference and Exhibition. Society of Petroleum Engineers, Manama, Bahrain, 2010, 1-8.10.2118/132173-MSSuche in Google Scholar
18 Martins A.S.B., Fresco P., Pereira M.J., Toxicity of zirconium on growth of two green algae. Fresen. Environ. Bull., 2007, 16(8), 869-874.Suche in Google Scholar
19 Yao C., Lei G., Cathles L.M., Steenhuis T.S., Research and Application of Micron-Size Polyacrylamide Elastic Microspheres (MPEMs) as a Smart Sweep Improvement and Profile Modification Agent. SPE Middle East Oil & Gas Show and Conference, 2016.10.2118/172636-MSSuche in Google Scholar
20 Salami O.T., Plank J., Synthesis, effectiveness, and working mechanism of humic acid‐{sodium 2‐acrylamido‐2‐methyl-propane sulfonate-co-N,N-dimethyl acrylamide-co-acrylic acid} graft copolymer as high-temperature fluid loss additive in oil well cementing. J. Appl. Polym. Sci., 2012, 126(4), 1449-1460.10.1002/app.36725Suche in Google Scholar
21 Zhou M., Nie X., Zhou L., Hou L., Zhao J., Yang Y., Study of crosslinked copolymer nanospheres with temperature resistance, salinity resistance, and deep profile control. J. Appl. Polym. Sci., 2017, 134(40).10.1002/app.45131Suche in Google Scholar
22 Shettigar R.R., Misra N.M., Patel K., CTAB grafted PAM gel and its application in drilling fluid. J. Petrol. Sci. Eng., 2018, 160, 129-135.10.1016/j.petrol.2017.10.040Suche in Google Scholar
23 Ferrer G.J., Larreal B.J., Microscopic observations of polyacrylamide structures. J. Petrol. Technol., 1973, 25, 80-81.10.2118/3919-PASuche in Google Scholar
24 Saadatabadi A.R., Nourani M., Emadi M.A., Rheological Behaviour and Hydrodynamic Diameter of High Molecular Weight, Partially Hydrolyzed Poly(acrylamide) in High Salinity and Temperature Conditions. Iran. Polym. J., 2010, 19(2), 105-113.Suche in Google Scholar
25 Feng S., Yuan D., Dong J., Ma S., Sui X., Wu H., et al., Effect of Physical Factors on Viscosity of Polyacrylamide Solution Prepared by Oilfield Produced Water. Appl. Mech. Mater., Changsha, China, 2013, 601-604.10.4028/www.scientific.net/AMM.268-270.601Suche in Google Scholar
26 Shupe R.D., Chemical Stability of Polyacrylamide Polymers. J. Petrol. Technol., 1981, 33(8), 1513-1529.10.2118/9299-PASuche in Google Scholar
27 El Karsani K.S.M., Al-Muntasheri G.A., Sultan A.S., Hussein I.A., Impact of salts on polyacrylamide hydrolysis and gelation: New insights. J. Appl. Polym. Sci., 2014, 131(23).10.1002/app.41185Suche in Google Scholar
28 Feng S., Yuan D., Sui X., Dong J., Ma S., Wu H., et al., Effect of Specific Ions in Preparing Water on Viscosity of Polyacrylamide Solution. Appl. Mech. Mater., Changsha, China, 2013, 442-446.10.4028/www.scientific.net/AMM.268-270.442Suche in Google Scholar
29 Mungan N., Shear Viscosities of Ionic Polyacrylamide Solutions. SPE J., 1972, 12(6), 469-473.10.2118/3521-PASuche in Google Scholar
30 Pelton R.H., Allen L.H., The effects of some electrolytes on flocculation with a cationic polyacrylamide. Colloid. Polym. Sci., 1983, 261(6), 485-492.10.1007/BF01419832Suche in Google Scholar
31 Seright R.S., Skjevrak I., Effect of Dissolved Iron and Oxygen on Stability of Hydrolyzed Polyacrylamide Polymers. SPE J., 2015, 20(3), 433-441.10.2118/169030-PASuche in Google Scholar
32 Sui X., Wang B., Wu H., Dong J., Feng S., Morphology of polyacrylamide in solution: effect of water quality on viscosity. Polym. Advan. Technol., 2015, 26(11), 1326-1330.10.1002/pat.3681Suche in Google Scholar
33 Gao C., Viscosity of partially hydrolyzed polyacrylamide under shearing and heat. J. Petrol. Explor. Prod. Technol., 2013, 3(3), 203-206.10.1007/s13202-013-0051-4Suche in Google Scholar
34 Gao C., Empirical correlations for viscosity of partially hydrolyzed Polyacrylamide. J. Petrol. Explor. Prod. Technol., 2013, 4(2), 209-213.10.1007/s13202-013-0064-zSuche in Google Scholar
35 Swiecinski F., Reed P., Andrews W., The Thermal Stability of Polyacrylamides in EOR Applications. SPE Improved Oil Recovery Conference. Society of Petroleum Engineers, Tulsa, Oklahoma, 2016, 1-17.10.2118/179558-MSSuche in Google Scholar
36 China NSotPsRo, Water treatment chemicals-Polyacrylamide. China Petroleum and Chemical Industry Society, 2008.Suche in Google Scholar
37 Zhang Q., Zhou J.-S., Zhai Y.-A., Liu F.-Q., Gao G., Effect of salt solutions on chain structure of partially hydrolyzed polyacrylamide. J. Cent. South Univ., 2008, 15(1), 80-83.10.1007/s11771-008-0319-xSuche in Google Scholar
38 Xin H.P., Yin S., Ao D., Wang X.J., Li R.Y., Zhang J., et al., Degradation Behaviour of Hydrolyzed Polyacrylamide in Solution Induced by Autoxidation of Pyrogallol. Asian J. Chem., 2014, 26(11), 3155-3160.10.14233/ajchem.2014.15856Suche in Google Scholar
39 Krul L.P., Nareiko E.I., Matusevich Y.I., Yakimtsova L.B., Matusevich V., Seeber W., Water super absorbents based on copolymers of acrylamide with sodium acrylate. Polym. Bull., 2000, 45(2), 159-165.10.1007/PL00006832Suche in Google Scholar
© 2019 Ma et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

