Graphical Abstract
Abstract
In this paper, a millimeter-sized temporary plugging agent particles based on polyacrylamide were synthesized by combining microfluidics technology with consolidation mechanism of suspension polymerization, which using W/O emulsion template and with droplet as micro-reaction vessel. The effect of various variables on the gel particle properties was studied so as to determine the best proportion and optimize the preparation process. Fourier transform infrared (FTIR) spectroscopy, Digital imaging and SEM were used to characterize the composition and morphology of the PAM gel particle. The results indicated that the obtained PAM gel particle has regular shape and good transparency. And it is concluded that when the molar ratio of acrylic acid (AA) and acrylamie (AM) monomers is 5:5, w(K2S2O8) = 0.8%, w(Al(NO3)3) = 1.0%, neutralization degree was 60%, the prepared PAM gel particles has the best performance. The preparation process is simple while the cost is low, so it could be used as a promising temporary plugging agent in future.
1 Introduction
The use of polyacrylamide-based hydrogel particles (PHPs) consisting of proper cross-linked polymer networks as temporary plugging agents have gained increasing attention in oil recovery applications (1, 2, 3, 4, 5) due to their
special swelling and dissolving properties, as well as the low price of chemicals needed, health and environmental concerns. In addition, a striking feature (6,7) of the PHPs lies in that their desired performances toward specific needs under different application conditions can be achieved by controlling their structures. The studies shows that; the solution containing polyacrylamide hydrogel particles is injected into the desired area, leaving sufficient time for curing to form a gel that can be manipulated and easily removed after treatment. And the larger particles of hydrogel can match the pore throat size of reservoir rock, which is helpful to improve the blocking behavior and achieve overall profile control (8).
The researches revealed (9) that the larger particles matched with sub-micron pore throat size of the reservoir rocks can contribute to improve the plugging behavior, and to realize the whole profile control. So the preparation methods and basic properties of large PHPs are of great significance to establish the new profile control and displacement method of PHPs with pore throat size.
Generally, there are various methods for preparing general gel particles, such as emulsion polymerization (10), dispersion polymerization (11), precipitation polymerization, etc., but most of the scope of the prepared gel particles range from 10 nm to 10 μm. The gel particles have a good sealing effect on the low-permeability zone and the small-hole throat in the early stage of collection, but have a poor sealing effect on high water content, high permeability, large-hole throat zone, low practicability and currently for millimeters (12). But there are few studies on the preparation methods and processes of the grade polymer gel particles.
In recent years, emerging microfluidics techniques provide some of the most promising approaches to production and functionalization monodisperse microparticles (13, 14, 15). Droplet microfluidics techniques have enabled the preparation of highly uniform droplets with a wide range of sizes from a few hundred nanometers to a few millimeters. In addition, the consolidation mechanism used in the suspension polymerization
(16) method can directly apply to the droplet template to prepare monodisperse spherical particles, and microfluidics technology (17) can be used to further functionalize the microspheres. This method can further realize the controllability of microspheres in morphology and particle size.
In this paper, we demonstrated a facile fabrication scheme for chemically functional and large size hydrogel microspheres of PHPs with controlled structures via combined droplet microfluidics technology with consolidation mechanism of suspension polymerization. For this, with acrylamide (AM), acrylic acid (AA) as functional monomer, aluminum nitrate (Al(NO3)3) as crosslinking agent and potassium persulfate (K2S2O8) as the initiator, with the help of the droplet templates, the droplet was solidified by free radical polymerization in the droplet as a micro-reaction vessel, thus isotropic polyacrylamide high-strength temporary plugging agent particles were prepared. Our simple fabrication approach based on droplet microfluidics techniques allows consistent fabrication of larger PHPs with controlled structures and mechanical integrity. Morphology and of the particles (i.e., uniform or core-shell) is controlled without any delicate controls, complex devices, or multistep procedures. The swelling gelation behavior of PHPs was comprehensively investigated by simulating reservoir formation conditions, and the effects of various variables on the performance of PHPs were studied to determine the optimal ratio and optimize the preparation process.
2 Experiments and methods
2.1 Materials
Ethanol (AR, Anhui Ante Food co. LTD), acrylamide (AR, Tianjin Guangfu Fine Chemical Research Institute), acrylic acid (AR, Tianjin Damao Chemical Reagent Factory), NaOH (AR, Tianjin New Fine Chemical Development Center), auminum nitrate (AR, Tianjin Bast Chemical co. LTD), potassium persulfate (AR, Tianjin Zhiyuan Chemical Reagent co. LTD), tetrahydrofuran (AR, Tianjin Deen Chemical Reagent co. LTD), liquid paraffin (AR, Shanghai Yongsheng Reagent Factory), sodium chloride (AR, Jining Baiyi Chemical co. LTD), calcium chloride (AR, Tianjin Fuyu Fine Chemical co. LTD), magnesium nitrate (AR, Tianjin Hengxing Chemical Reagent Manufacturing), distilled water.
The apparatus used was as follows; IFS66V/S infrared spectrometer (Bruker, Germany), Snb-1 digital display viscometer (Shanghai Fangrui Instrument Co. LTD.) and self-made temporary plugging agent particle strength tester.
2.2 Synthesis
2.2.1 Synthesis of the PAM gel particle temporary plugging agent
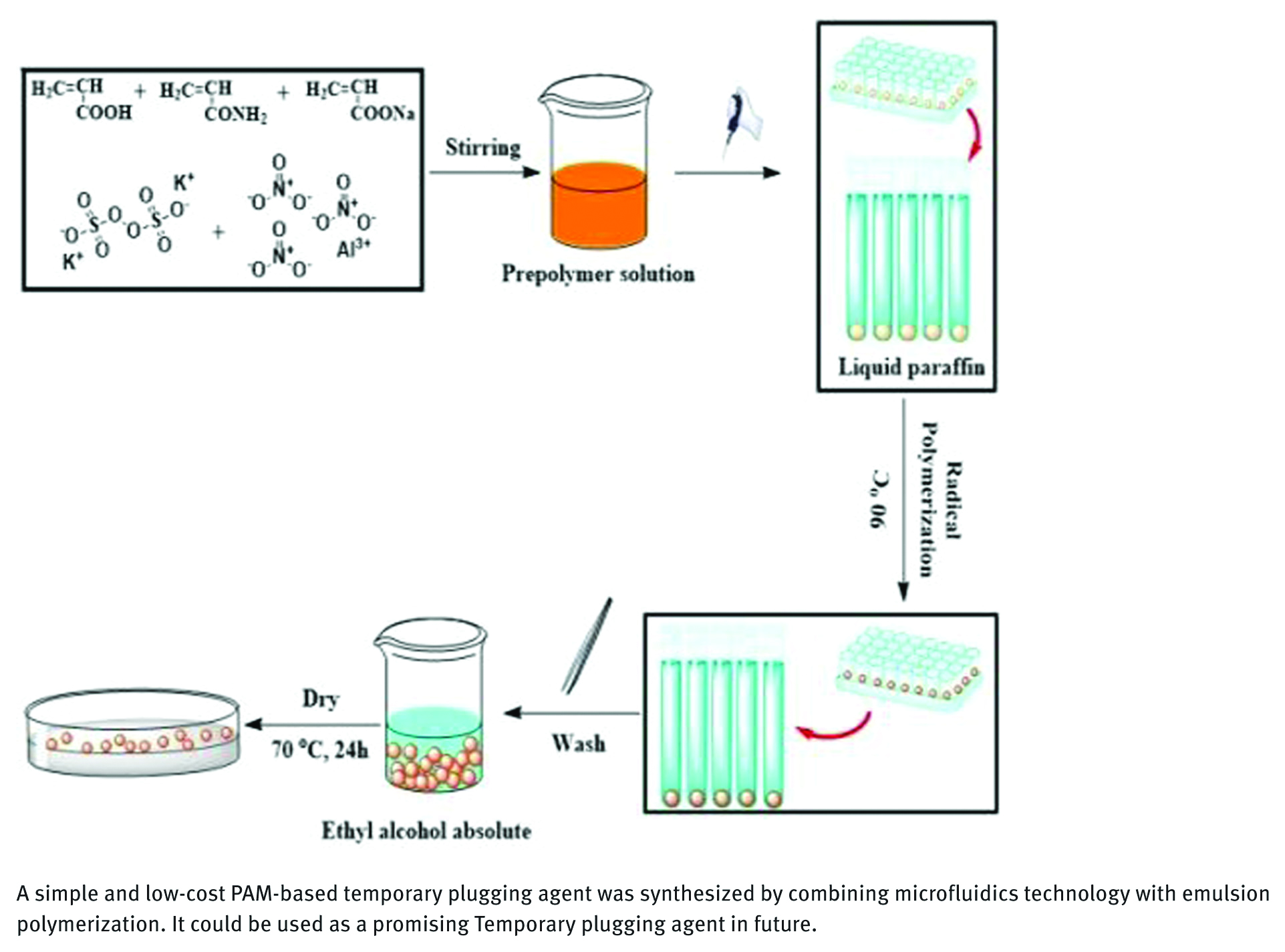
The preparation process of the PAM gel particles is shown in Figure 1. Firstly, a certain amount of monomer acrylic acid, acrylamide and 2 mL distilled water were added into a beaker equipped with magnetic stirring, and then a certain amount of NaOH was slowly added into the beaker under the condition of ice water bath to obtain an acrylic acid aqueous solution with a certain degree of neutralization. Secondly, a certain amount of crosslinker aluminum nitrate (Al(NO3)3), a certain amount of initiator potassium persulfate (K2S2O8) and 1 mL distilled water were added to the beaker and ultrasonic 30 min. then the prepared solution was dripped into a 5 mL centrifuge tube containing liquid paraffin (no bubbles when dripping). The centrifuge tube was placed in a constant temperature water-bath at 90°C for 10 min, and the prepared temporary plugging agent gel was washed with ethanol for several times. Then dried at 70°C and set aside. The dosages of each variables system were shown in Table 1.

Procedure for preparation of PAM gel particle temporary plugging agent.
Proportion of different variable systems (m for mass ratio, n for mole ratio).
| number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| m(AA):m(H2O) | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
| n(AA):n(AM) | 3:7 | 3.5:6.5 | 4:6 | 4.5:5.5 | 5:5 | 5.5:4.5 | 6:4 | 6.5:3.5 | 7:3 | - |
| m(Al(NO3)2) | ||||||||||
| m(AA+AM) | 0.1% | 0.5% | 1.0% | 1.5% | 2.0% | 2.5% | 3.0% | 3.5% | 4.0% | - |
| m(K2S2O8) | ||||||||||
| m(AA+AM) | 0.2% | 0.4% | 0.6% | 0.8% | 1.0% | 1.2% | 1.4% | 1.6% | 1.8% | 2.0% |
| neutralization | 0 | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
3 Characterization
3.1 FTIR spectra
The IR spectra were recorded on a Vectoer-22 Bruker Fourier transform infrared (FT-IR) spectrometer using a KBr pellet, over the range 4000-500 cm-1 from 64 scans at 4 cm-1 resolution.
3.2 SEM images
The SEM images of cross section of PAM gel particles were examined using a Philips scanning electron microscope (SEM) at an accelerating voltage of 20 kV. The samples were coated with a thin layer of gold before the measurements.
3.3 Determination of density
Apparent density: particles of a certain mass in each group of samples are randomly selected as units. Flow gauge calipers are used to measure the diameter of each particle. The maximum and minimum values are removed. The average was denoted as D; then the remaining particles were weighed and averaged, and their mass was denoted as M. Finally, the apparent density was calculated according to Eq. 1:
Volume density: take 20 particles of each sample randomly as the measurement unit, weighed the mass of each group of particles, denoted as m. Tetrahydrofuran was used as the volumetric effluent, take the initial value V0 as 1, then put the particles into the measuring cylinder, the reading is recorded as Va, Va-V0, that is, the volume V of each group of particles, and finally, calculated its volume density according to Eq. 2:
3.4 Determination of water uptake (%)
The measured diameters of temporary plugging agent gel with different concentrations are denoted as (D0). Then, the temporary plugging agent gel was put into a beaker containing a certain amount of distilled water (NaCl solution, CaCl2 solution and Mg(NO3)2 solution), and at regular intervals the gel was taken out and its diameter after absorption was measured as (D). Until it reaches equilibrium. And the water absorption was measured at different temperatures (18). And finally, calculated its water uptake according to Eq. 3:
3.5 Determination of strength
There are many kinds of testing methods for the relative strength of temporary plugging agent particles. In this experiment, a self-made temporary plugging agent particle strength tester was used to test the strength of the prepared temporary plugging agent particles. The design of the particle strength tester for temporary plugging agent is shown in Figure 2. The pellets with different concentrations were placed in distilled water at different temperatures for 4 h. Then the temporary plugging agent particles were immediately placed in the tester to make the end face of the pressure rod contact the gel. Then add weights to the pallet until the gel breaks. The strength of the temporary plugging agent particles was calculated according to Eq. 4:
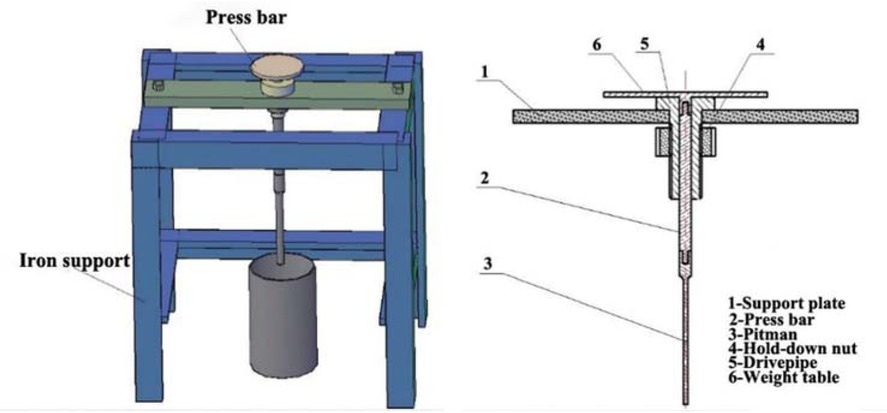
Processing diagram of gel strength tester.
where: S – particle strength of temporary plugging agent (KPa); m0 – total initial mass of counterweight, connecting bar and pressure bar (g); G1 – farmar weight; A – cross-sectional area of pressure rod head; f – friction is negligible when the surface of the pressure rod and connecting rod and the casing wall are sufficiently smooth; g – gravitational acceleration, 9.8 m/s2.
3.6 Determination of aging resistance
The temporary plugging agent gel was prepared into an aqueous solution with a mass concentration of 5%, and then put in 90°C oven, every time out, weighing, until completely dissolved, recording time.
4 Results and discussion
4.1 Structure and morphology of PAM gel particle
By using microfluidics technology, the large size gel particles temporary plugging agent with regular morphology, uniform size was prepared by using droplet as the reaction vessel and single mode single cavity structure. The infrared spectrum and morphology of PAM gel particles is shown in Figure 3. It can be seen from Figure 3a the peak at 3428 cm-1 is attributed to the stretching vibration of overlab of OH group of acrylic acid with NH2 of acrylamide. the peak at 2935 cm-1 are attributed to C–H stretch vibrations on methylene (–CH2–), the peak at 1664 cm-1 correspond to carbonyl group (C=O) of amide (polyacrylamide). Moreover, the characteristic peaks of –OH in the carboxyl group (–COOH) at 1409 cm-1 overlapped with those of the ester group (COO–) at 1409 cm-1, so that the characteristic absorption peak was strengthened. This indicates that the temporary plugging agent of PAM gel particles has been successfully prepared.
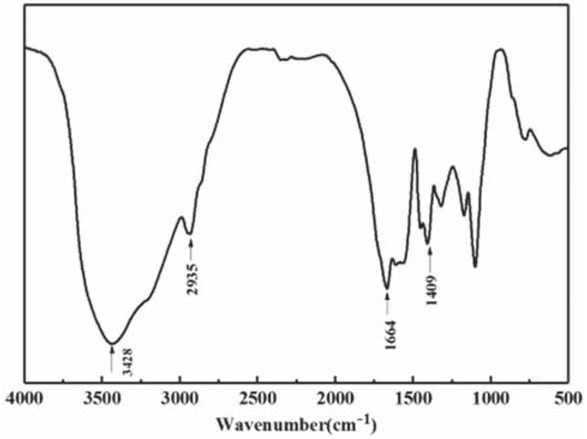
Infrared spectrum of PAM gel particles.
Figure 4 shows the Morphology of PAM gel particles and SEM images of cross section of PAM gel particles. As shown in Figure 4a the prepared PAM gel particles are regular spherical with uniform particle size, and the particle size distribution is between 2 and 2.3 mm. It can be seen from Figure 4b the cross section morphology of PAM gel particles is rough, porous and compact. This type of morphology provides more water absorbing sites and certain strength.
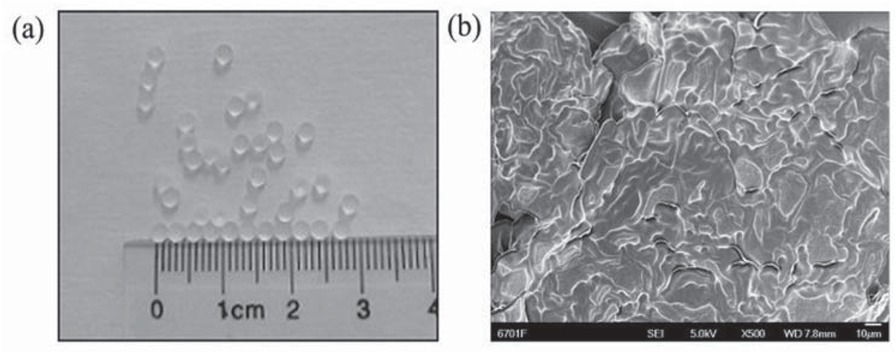
(a) Morphology of PAM gel particles, (b) SEM images of cross section of PAM gel particles.
4.2 Effects of different monomer proportions on the particle properties of PAM temporary plugging agent
Figure 5 shows the swelling ratio curve of different monomer proportions and PAM gel particles at different temperatures in the reservoir formation environment. It can be seen from Figure 5 that at different temperatures, PAM gel particle temporary plugging agent rapidly absorbed water within the first 500 min, and the water absorption rate first increased and then decreased with the extension of time. After 500 min, the water absorption rate of the gel particle began to decrease after reaching the maximum value, until the swelling equilibrium. When the mass ratio of AA to AM was 5:5, the water absorption rate of PAM gel particles was the highest. Figure 5f shows the relationship between temperature and swelling ratio of PAM gel particles reached the maximum water absorption rate with different monomer ratios. It can be seen from Figure 5f PAM gel particle swelling ratio increases with the rise of temperature first and then decreases, and reached the maximum at 40°C. This is because the materials used to prepare the gel particles are water-soluble monomers, and the prepared polymers are hydrolyze. Therefore, when the temperature rises to a certain degree, the hydrolysis rate of the polymers increases, leading to decreases in the water-holding capacity. It can be seen from Figure 5 that the swelling ratio of PAM gel particles first increases and then decreases with the increases of the mass ratio of AA and AM, at different temperatures, and when the ratio is 5:5, the swelling ratio of the gel particles is the highest. This is because when the AM content is high, the synergistic effect between the CONH2– group in the AM molecule and the COO– group in the AA molecule increases the water absorption ability of the gel microspheres. When the mass ratio of AA and AM is greater than 1, the intermolecular reaction tends to be the self-polymerization reaction between AA, and the molecular chain of the polymer gradually becomes longer and the crosslinking degree gradually increases, thus the water absorption and the water retention capacity of the polymer reduced. In addition, it can be seen that the PAM gel particle temporary plugging agent, after water absorption and swelling, has a wide range of particle size change between 2 and 10.8 mm·mm-1, which can be fully applied in the non-uniform distribution of pore throat in the formation environment and effectively block the pore throat in the formation.
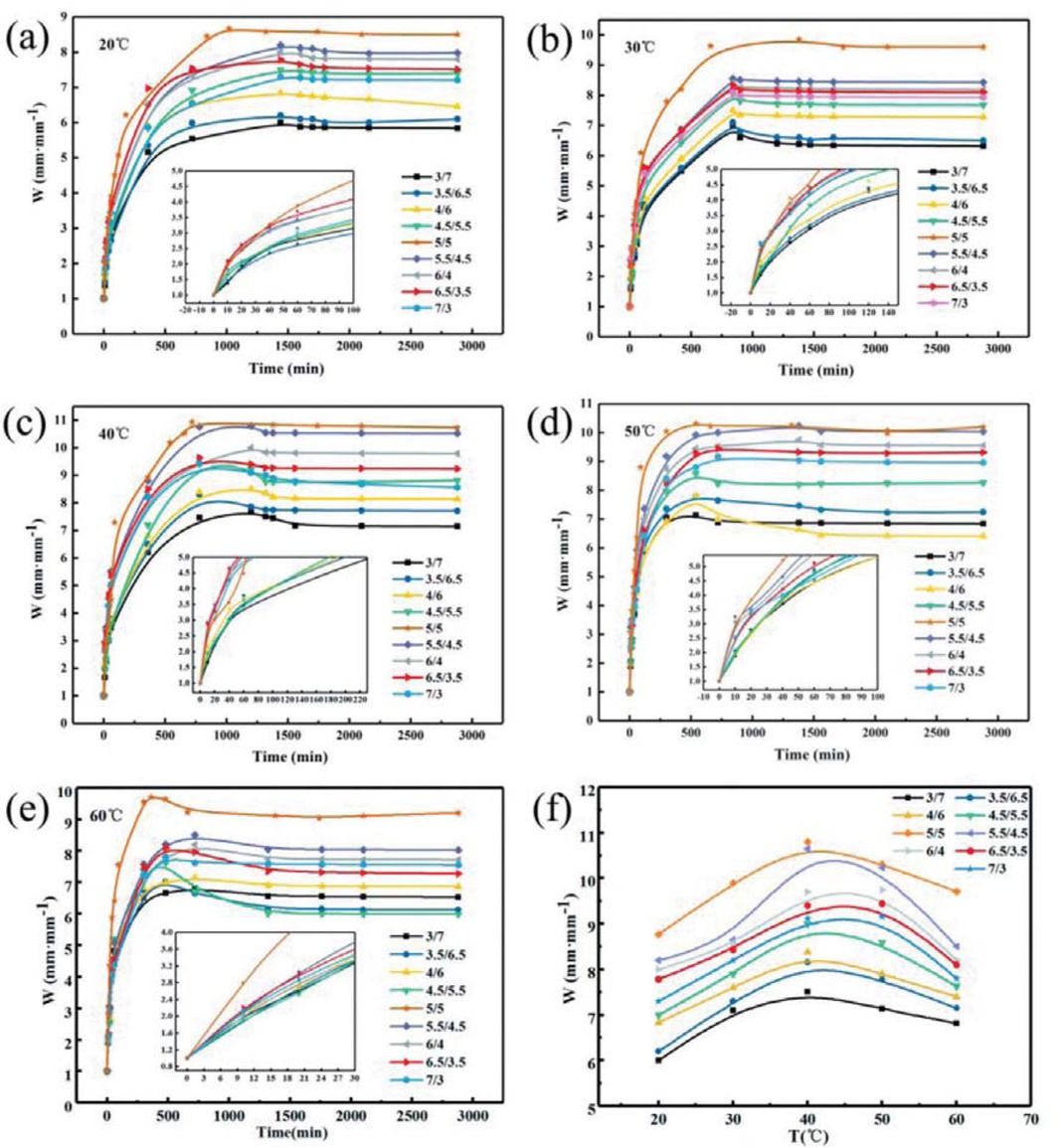
The relationship between monomer ratio and swelling ratio of PAM gel particles at different temperatures.
Figure 6 shows the relationship between different monomer ratios and the temporary plugging agent performance of PAM gel particles under the simulated reservoir formation environment. Figures 6a-c correspond to the density, apparent density to volume density ratio, compressive strength, aging resistance and flowback performance of PAM gel particles with different monomer ratios, respectively. It can be seen from Figure 6a with the increases of the mass ratio of AA to AM, the volume density and apparent density of the temporary plugging agent of gel particles first increases and then decreases, and the apparent density is slightly less than the volume density. This is because although the temporary plugging agent particles prepared by the infusion method are relatively regular but they are still some deviation from the ideal sphere, as can be seen from the ratio of apparent density to volume density of 0.75, the temporary plugging agent particles prepared are relatively regular in shape and uniform in size. As shown in Figure 6b that at different temperatures, the compressive strength of PAM gel particles firstly decreases and then increases as the mass ratio of AA and AM increases. And when the mass ratio of AA to AM is 1, the compressive strength is the minimum, because the change of AM content will affect the crosslinking degree of the polymer. As shown in Figure 6c the dissolution time of PAM gel particles first decreases and then increases with the increases of the mass ratio of AA to AM. When the mass ratio of AA to AM is 1, the minimum dissolution time is 8 h. According to Figure 3, when the mass ratio of AA and AM is 1, PAM gel particle temporary plugging agent has the fastest water absorption rate and the shortest swelling equilibrium time, so it can accelerate the dissolution rate of particle temporary plugging agent, and the viscosity distribution after dissolution is around 65 mPa·s, the system viscosity is relatively low, so has a good backflow performance.
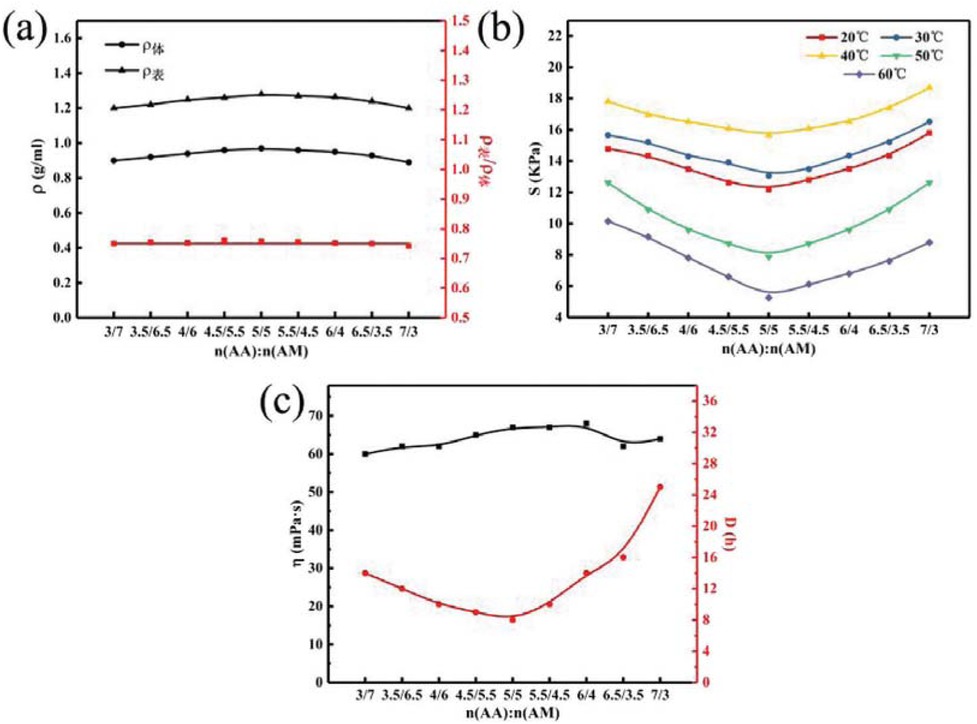
Relationship between monomer ratio and temporary plugging agent performance of PAM gel particles.
4.3 Effect of initiator concentration on the properties of temporary plugging agent particles
Figure 7 shows the relationship between the concentration of initiator and the swelling ratio of PAM gel particle temporary plugging agent at different temperatures in the simulated reservoir formation environment. As can be seen from Figure 7, at different temperatures and initiator concentrations, PAM gel particles rapidly absorb water in the first 500 min, after reaching the maximum value in 500 min, the water absorption rate decreases until the swelling equilibrium. And when the initiator concentration was 0.8-1.0%, the PAM gel particle temporary plugging agent had the highest water absorption rate. Figure 7f shows the relationship between temperature and swelling ratio of PAM temporary plugging agent gel particles reached the maximum water absorption rate with different initiator concentrations. It can be seen from Figure 7f that the PAM gel particle swelling ratio increased with the temperature increase and then reduce and reach the maximum at 40°C, and the swelling ratio of PAM gel particles first increases and then decreases with the increases of initiator concentration. When the initiator concentration is 0.8%, the swelling ratio of PAM gel particles temporary plugging agent reaches the maximum. This is because when the concentration of initiator is low, although the molecular chain length of the formed polymer is long, the content of small molecules is high. So it is good for water absorption but not good for water retention. When the initiator concentration is higher, the active free radical content of the polymer formed is higher and the molecular chain formed is shorter, which is beneficial to water absorption but not beneficial to water retention. Therefore, within a certain range, with the increase of initiator concentration, the water absorption rate of gel particles increases with the increases of initiator concentration, and the swelling ratio decreases with the increases of initiator concentration.
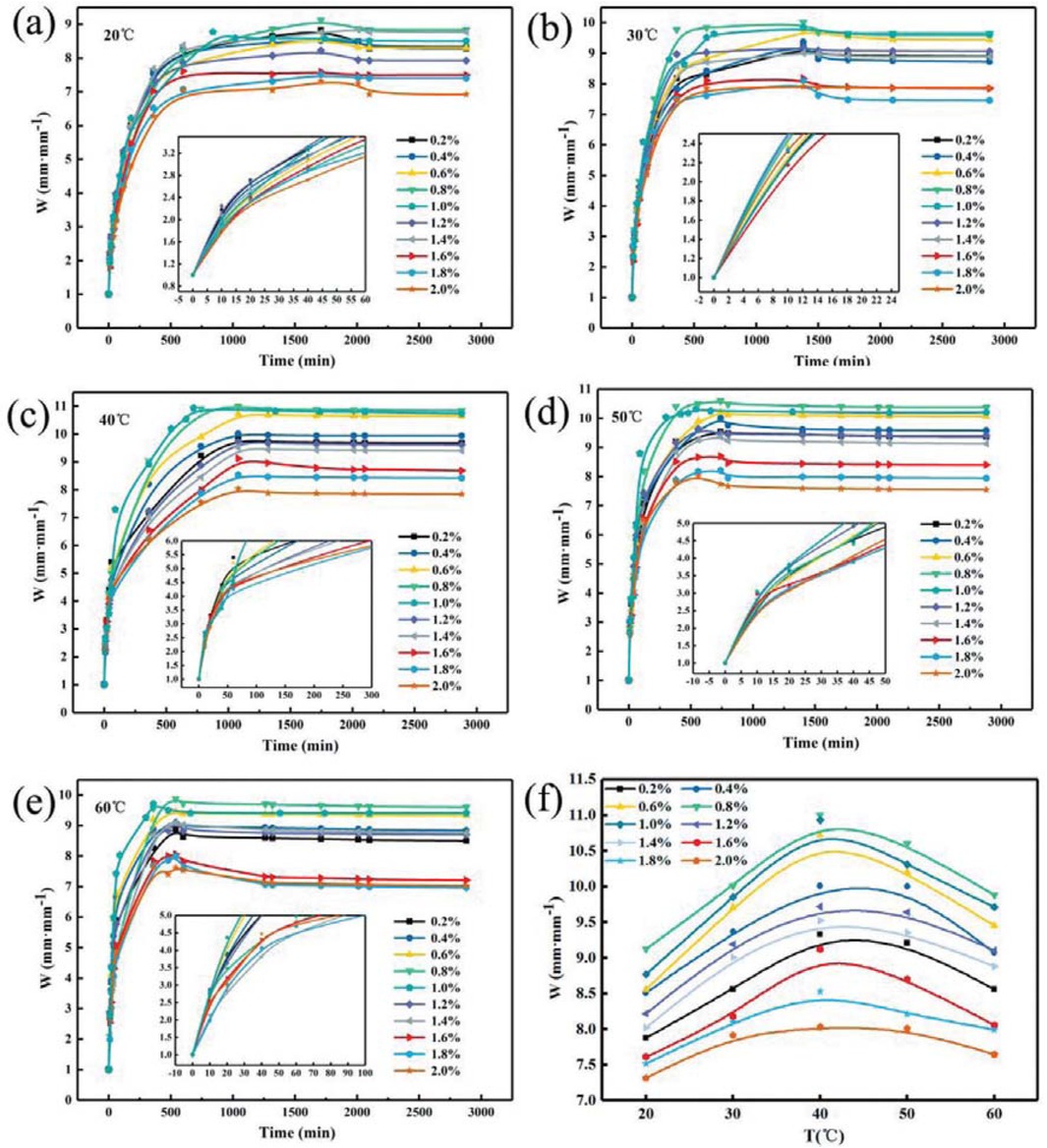
Relationship between initiator concentration and swelling ratio of PAM gel particle temporary plugging agent at different temperatures.
Figure 8 shows the relationship between the initiator concentration and the temporary plugging agent performance of PAM gel particles under the simulated reservoir formation environment. Figures 8a-c correspond to the curves between the density, apparent density to volume density ratio, compressive strength, aging resistance and backflow performance of PAM gel particle temporary plugging agent at different initiator concentrations, respectively. It can be seen from Figure 8a with the increases of initiator concentration, the volume density and apparent density of PAM gel particles first increases and then decreases, and the apparent density was slightly less than the volume density. This is mainly because the temporary plugging agent particles prepared by the infusion method are relatively regular, but still have a relative deviation from the ideal sphere. As can be seen from the ratio of apparent density to volume density of 0.77, the temporary plugging agent particles prepared have a relatively regular shape and uniform size. As can be seen from Figure 8b with the compressive strength of PAM gel particles temporary plugging agent increases first and then decreases with the increases of the initiator concentration at different temperatures. When the initiator concentration is 0.8%, the compressive strength reaches the maximum value. This is mainly because when the initiator concentration is low, the amount of active free radicals formed is small, and the polymerization reaction rate is slow, so a large number of oligomers are formed. However, with the increases of initiator concentration, the molecular weight of the polymer increases gradually, and its water-holding capacity and compressive strength increases. As the initiator concentration increased, the polymer molecular chain became shorter and the compressive strength decreased. From Figure 8c with the increases of initiator concentration, the aging resistance time of PAM gel particle temporary plugging agent first increases and then decreases, when the initiator concentration is 0.8%, the maximum complete dissolution time is 8 h, and the viscosity distribution after dissolution is about 60 mPa·s, the system viscosity is relatively low, so the gel microsphere has a good backflow performance.

Relation between initiator concentration and temporary plugging agent performance of PAM gel particles.
4.4 Effect of crosslinking agent concentration on the performance of temporary plugging agent particles
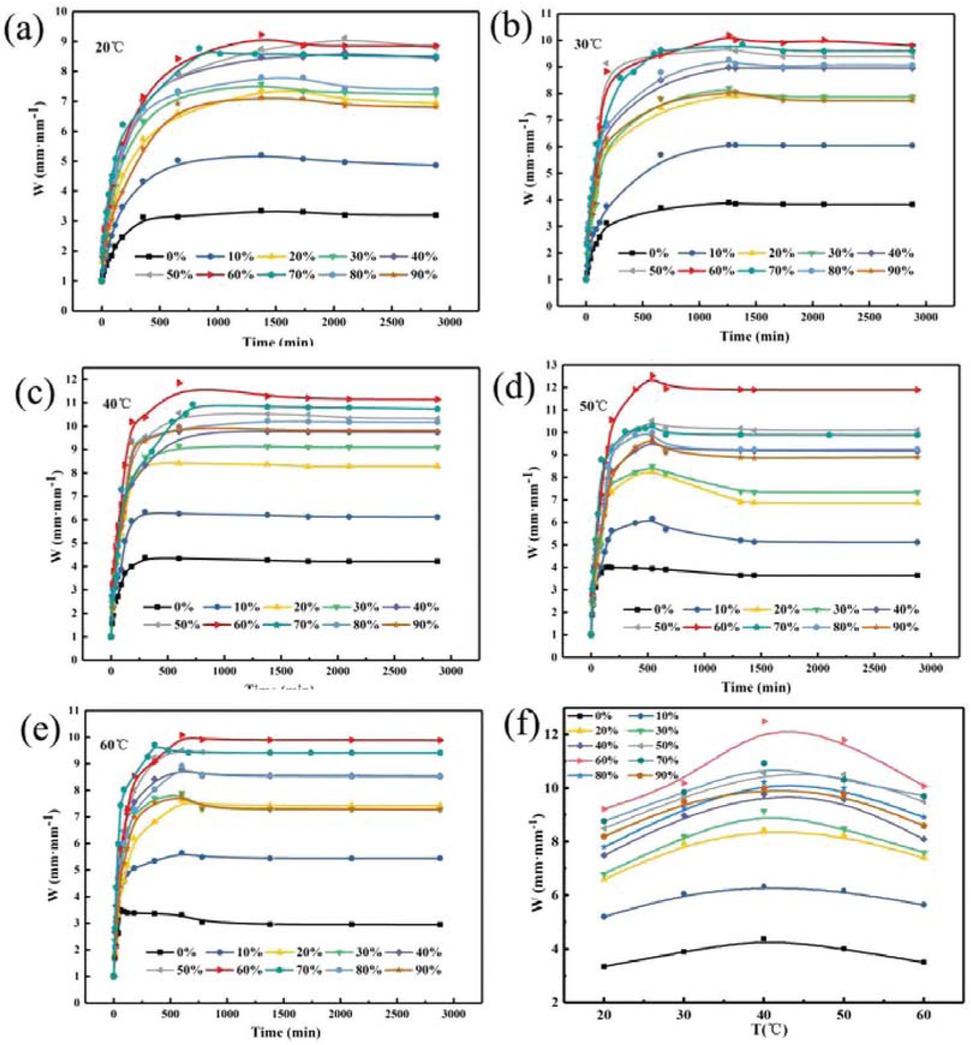
Figure 9 shows the relationship between the concentration of crosslinking agent and the swelling ratio of PAM gel particle temporary plugging agent at different temperatures in the simulated reservoir formation environment. It can be seen from Figure 9 that at different temperatures, PAM gel particle temporary plugging agent rapidly absorbs water within the first 500 min, and the water absorption rate first increases and then decreases with the extension of time. After reaching the maximum value in 500 min, the water absorption rate gradually slows down until the swelling equilibrium. And when the crosslinking agent concentration was 1.0%, the PAM gel particle temporary plugging agent had the highest water absorption rate. Figure 9f is the graph of the relationship between temperature and swelling ratio of PAM temporary plugging agent gel particles reached the maximum water absorption rate with different crosslinking agent concentrations. It can be seen that the PAM gel particle swelling ratio increases with the temperature increases and then reduces and reaches the maximum at 40°C, and it can be seen in Figure 9 that the swelling ratio of PAM gel particle temporary plugging agent first increases and then decreases with the increases of crosslinking agent concentration. And when the crosslinking agent concentration is 1.0%, the swelling ratio of gel particle temporary plugging agent reaches the maximum. This is mainly because the amount of crosslinking agent is small, the polymer does not form an ideal three-dimensional network structure, so the water solubility is large and the water absorption rate is low. When the dosage of crosslinking agent increases to a certain value, the water absorption rate reaches the maximum. When the amount of crosslinking agent is increases, the crosslinking point of the network structure increases, the structure is dense and the water molecules cannot enter the polymer, thus the water absorption rate decreases. To sum up, in the simulated formation temperature of 40°C, crosslinking agent concentration was 1.0%, the PAM gel particle swelling ratio of temporary plugging agent reached a maximum of 10.9 mm·mm-1, and it can be seen that PAM gel particle temporary plugging agent has a wide range of particle size change between 2 and 10.9 mm·mm-1 after water absorption and swelling, it can be fully applied to the non-uniform distribution of pore throats and effectively block the pore throat of stratum.
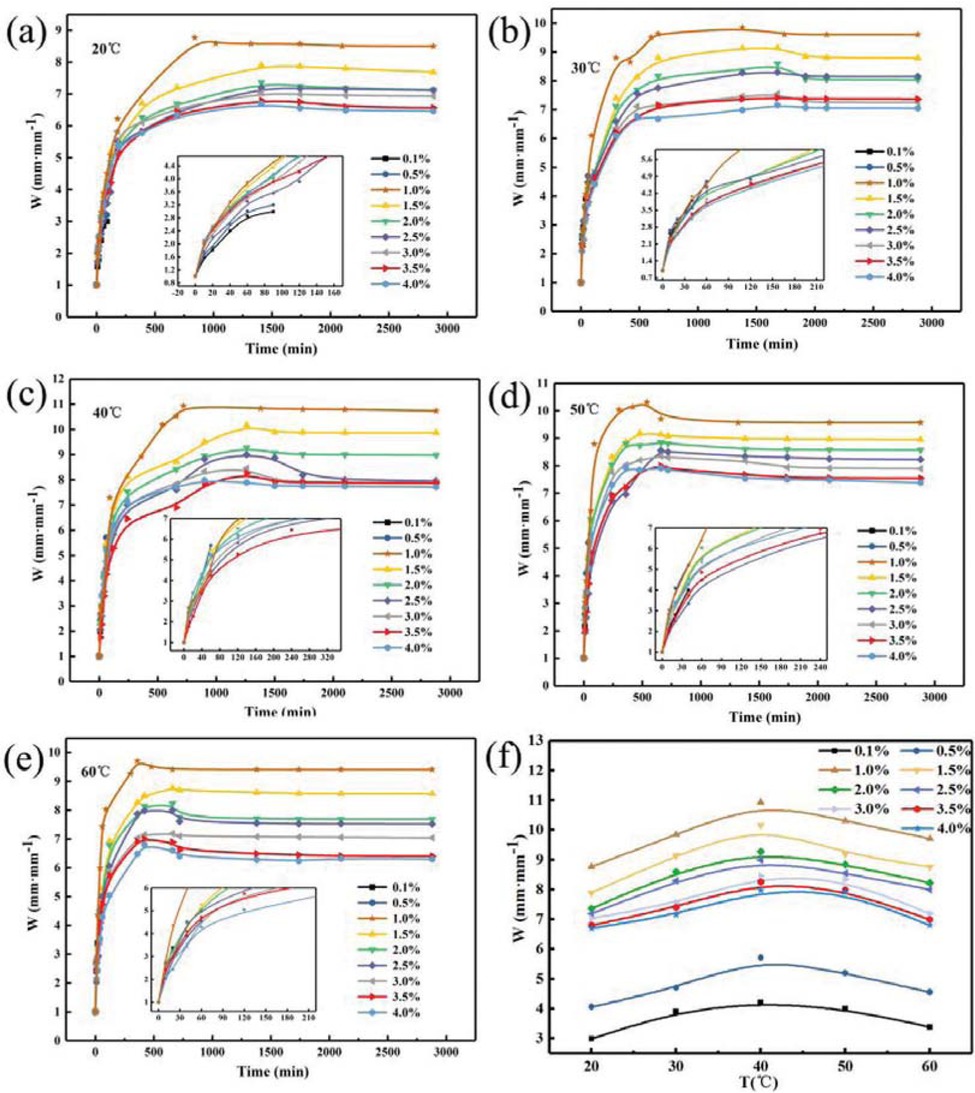
Relationship between concentration of crosslinking agent and swelling ratio of PAM gel particle temporary plugging agent at different temperature.
Figure 10 shows the relationship between different crosslinking agent concentrations and the temporary plugging agent properties of PAM gel particles under the simulated reservoir formation environment. Figures 10a-c correspond to the relationship between the density, apparent density to volume density ratio, compressive strength, aging resistance and backflow performance of PAM gel particle temporary plugging agent at different crosslinking agent concentrations, respectively. It can be seen from Figure 10a with the increases of crosslinking agent concentration, the volume density and apparent density of PAM gel particles first increases and then decreases, the apparent density is slightly less than the volume density. As can be seen from Figure 10b the compressive strength of PAM gel particles temporary plugging agent first decreases and then increases with the increases of crosslinking agent concentration at different temperatures. When the crosslinking agent concentration is 1.0%, the compressive strength reaches the minimum value; this is mainly because when the crosslinker concentration is low, the coordination mode between polymer molecular chains is surface chelation, forming a three-dimensional network structure, and the compressive strength is large; with the increases of crosslinking agent concentration, the coordination mode was gradually transformed from off-plane to in-plane. However, due to chelation competition, when the crosslinking agent concentration reached a certain value, the compressive strength of the polymer reached the minimum value, with the further increase of the crosslinking agent concentration, the concentration of Al3+ increases, so the crosslinking degree gradually increases and the compressive strength increases. It can be seen from Figure 10c that the aging resistance time of PAM gel particles first decreases and then increases with the increases of crosslinking concentration. When the crosslinking agent concentration is 1.0%, the minimum value appears and the time is 8.3 h, moreover, the viscosity distribution after dissolution is around 63 mPa·s, and the viscosity of the system is relatively low, so it has a better backflow performance.
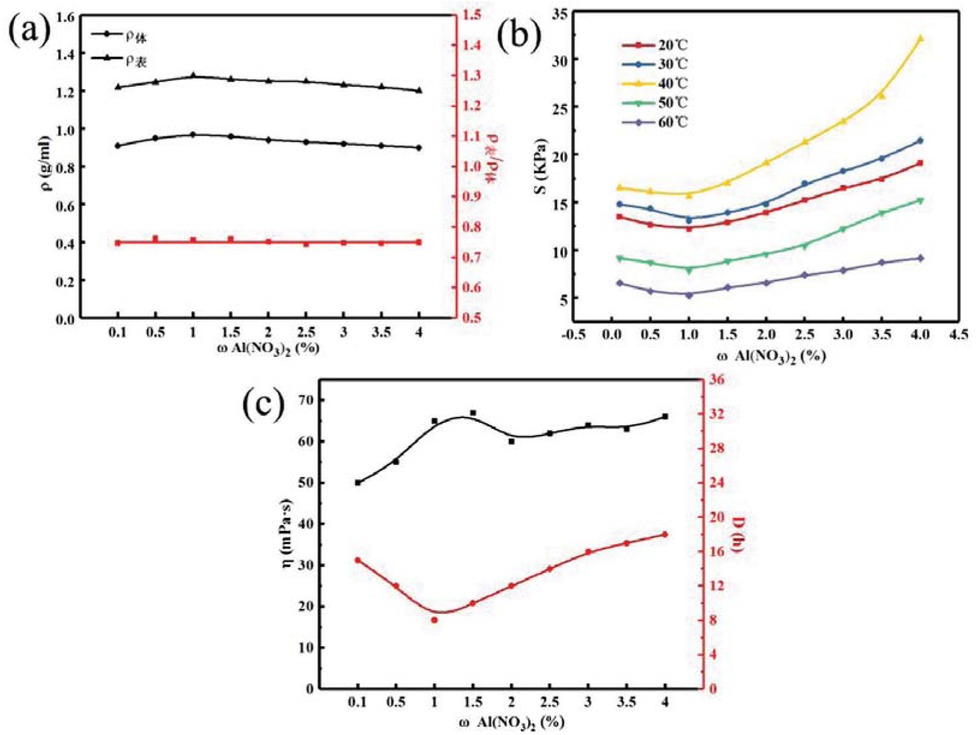
Relationship between crosslinking agent concentration and temporary plugging agent performance of PAM gel particles.
4.5 Effect of neutralization degree on the performance of temporary plugging agent particles
Figure 11 shows the relationship between different neutralization degrees and the swelling ratio of PAM gel particles temporary plugging agent at different reservoir temperatures. It can be seen from Figure 11 that at different temperatures, PAM gel particle temporary plugging agent absorbs water rapidly in the first 500 min, and after reaching the maximum value in 500 min, the water absorption rate gradually slows down and tends to balance. When the neutralization degree was 60%, the water absorption rate of PAM gel particle temporary plugging agent was the fastest. Figure 11f is the graph of the relationship between temperature and swelling ratio of PAM gel particles reached the maximum water absorption rate with different neutralization degrees. It can be seen in Figure 11f the PAM gel particle swelling ratio first increases and then decreases with the rise of temperature, and reach the maximum at 40°C. Moreover, the swelling ratio of PAM gel particles first increases and then decreases with the increases of neutralization degrees. When the neutralization degree was 60%, the swelling ratio of PAM gel particles reached the maximum. With the increases of neutralization degrees, through the synergistic effect of –CONH2 group in AM and –COO– group in AA, the crosslinking degrees of the polymer decreases and the network gap becomes larger, thus its water absorption and water retention capacity increases. When the neutralization degrees exceeds a certain value, the chelation mode between –COO– and Al3+ changes from low coordination to high coordination, the gap of the three-dimensional network structure becomes smaller gradually, so its ability of water absorption and water retention gradually weakens. To sum up, in the simulated formation temperature of 40°C, neutralization degrees was 60%, PAM swelling ratio of gel particles reached a maximum of 11.9 mm·mm-1. And it can be seen that PAM gel particle temporary plugging agent has a wide range of particle size change between 2 and 11.9 mm·mm-1 after water absorption and swelling. Therefore, it can be fully applied to the non-uniform distribution of pore throats in the formation environment and effectively block the pore throats in the formation.
Relationship between neutralization degree and swelling ratio of PAM gel particles at different temperatures.
Figure 12 shows the relationship between different neutralization degrees and the temporary plugging agent performance of PAM gel particles under the simulated reservoir formation environment. Figures 12a-c correspond to the relationship between the density, apparent density to volume density ratio, compressive strength, aging resistance and backflow performance of the temporary plugging agent of gel particles at different neutralization degrees, respectively. It can be seen in Figure 12a with the increases of crosslinking agent concentration, the volume density and apparent density of PAM gel particles first increases and then decreases, the apparent density is slightly less than the volume density, but the shape and size of the temporary plugging agent particles are regular and uniform. It can be seen from Figure 12b that at different temperatures, with the increases of neutralization degrees, the compressive strength of PAM gel particles first decreases and then increases. When the neutralization degrees is 60%, the compressive strength of gel microspheres reaches the minimum value; this is mainly due to the high reactivity of AA when the neutralization degrees is low and forming the macromolecular chain polymers with high degree of crosslinking and the three-dimensional network structure is dense, so its compressive strength is high, with the increases of neutralization degrees, through the synergetic effect of the CONH2 group in AM and the –COO– group in AA, the cross-linking degree of the polymer decreases and the network gap becomes larger, thus the compressive strength gradually decreases. When the neutralization degrees is higher than a certain value, the chelation mode of –COO– and Al3+ changes from low coordination to high coordination, as a result, the gap of the three-dimensional network structure becomes smaller gradually, and the compressive strength of the gel microsphere increases gradually. As shown in Figure 12c with the increases of neutralization degrees, the aging resistance time of PAM gel particles temporary plugging agent first decreases and then increases. When the neutralization degrees is 60%, the minimum value is 7 h, and the viscosity distribution after dissolution is around 60 mPa·s. The system viscosity is relatively low and the backflow performance is good.
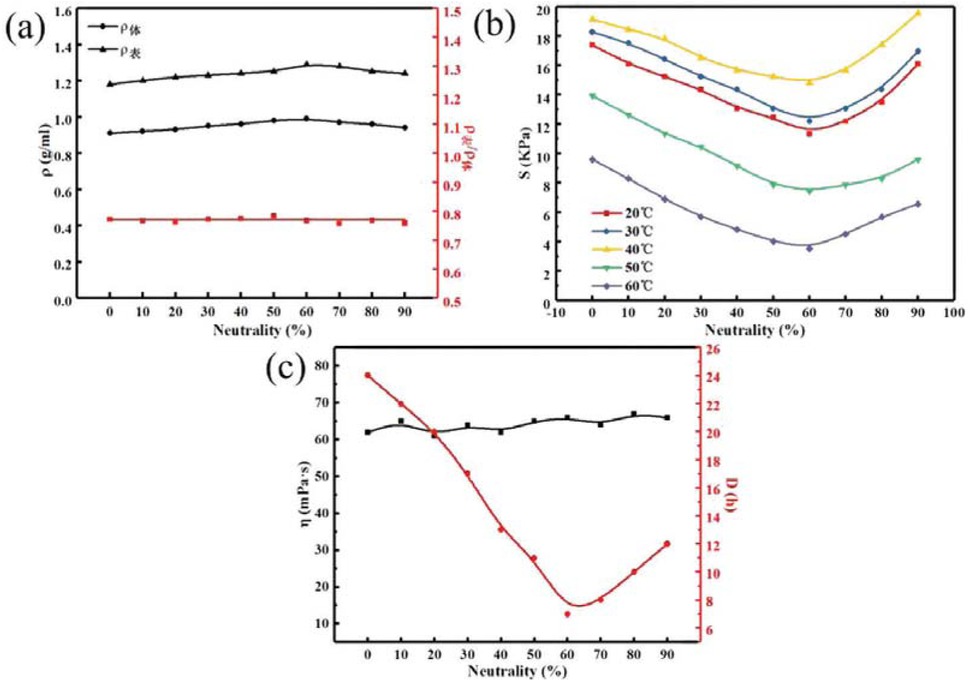
Relationship between neutralization degrees and temporary plugging agent performance of PAM gel particles.
4.6 Effects of different salinity on water absorption of gel microspheres
Figure 13 is the graph of water absorption ratio of PAM gel particles with different salinity. It can be seen in Figure 13, the prepared gel particles temporary plugging agent has a certain salt tolerance, and the water absorption ratio of gel particles decreases with the increases of NaCl, Mg(NO3)2 and CaCl2 content in aqueous solution. This is because with the amount of inorganic salt increases, the concentration of ions in the solution increases, the osmotic pressure inside and outside decreases of the copolymer, which makes it difficult for water to enter into the particle temporary plugging agent. In addition, the cationic entering the polymer molecules through osmosis will shield the anionic, the repulsion between different anions in the polymer is reduced, so the water absorption ratio is also reduced (19); as can be seen from Figure 13, the order of influence of the three inorganic salts on the water absorption rate of the temporary plugging agent particles is CaCl2 > Mg(NO3)2 > NaCl, this is because the ionization concentrations of the three inorganic salts in unit volume are successively CaCl2 > Mg(NO3)2 > NaCl.
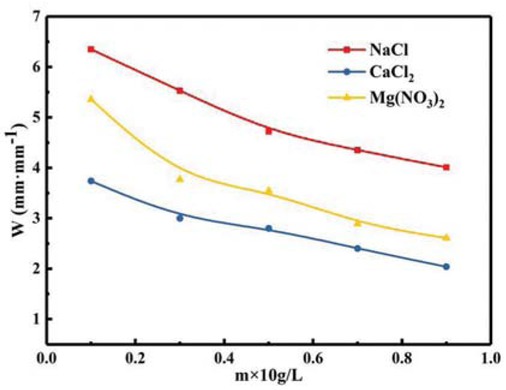
Relationship between different salinity and water absorption ratio of PAM gel particles temporary plugging agent.
5 Conclusions
By combining microfluidics technology with consolidation mechanism of suspension polymerization, used W/O emulsion templates, with droplet as micro-reaction vessel through free radical polymerization, prepared the polyacrylamide high strength temporary plugging agent particles with isotropy, regular shape and uniform size. And the effects of various variables on the properties of PAM gel particles temporary plugging agent (regularity, swelling rate, compressive strength, aging resistance and reflux) were also discussed, and it is concluded that when the n(AA) : n (AM) = 5:5, w(K2S2O8) = 0.8%, w(Al(NO3)3) = 1.0%, neutralization degrees was 60%, the PAM gel particles of temporary plugging agent has the maximum water absorption and the minimum compressive strength at 40°C can meet the requirements of its use in the fracturing process. And in the reservoir simulation environment conditions, the viscosity and salt tolerance of PAM gel particles after dissolution were determined, it can be seen that the viscosity of the series PAM gel particle temporary plugging agent prepared through the control variable after dissolution is between 50 and 65 mPa·s, the viscosity of the system is low, so it has a good backflow performance. In addition, the PAM gel particle temporary plugging agent has a certain salt tolerance. There are few samples analyzed in this experiment, thus these presented results are initial, however, its test results and rules are in line with the results of our laboratory analysis. Later, we will further explore and extend the results.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (Grant No. 51663013) and the Science Foundation of State Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals.
References
1 Wu Y.C., Gao B., Hou X., Yao K.D., The preparation and applications of founctional polyacrylamide microspheres. J. Polym. Mater. Sci., 2008, 24, 13-17.Suche in Google Scholar
2 Zhang Y.P., Ye Y.C., Guo Y.W., Zhang J.L., Research progress of temperature and salt resistant acrylamide copolymer. J. Appl. Chem., 2005, 34(10), 598-599.Suche in Google Scholar
3 Jiang C., Guan X.Q., Preparation, characterization, strength and swelling properties of new polyacrylamide gel. J. Shangdong Chem. Ind., 2018, 47(1), 13-14.Suche in Google Scholar
4 Huang Y.H., Research progress in reverse emulsion polymerization of polyacrylamide. J. Mod. Chem. Ind., 2005, 34(1), 56-59.Suche in Google Scholar
5 Wu M., Deng S.F., Wei F.L., Wang Q.B., Li Y.K., Synthesis technology of polyacrylamide and its application in oilfield development. J. Prog. Fine. Petro. Chem., 2011, 12(12), 1-4.Suche in Google Scholar
6 Yao C.J., Lei G.L., Gao X.M., Li L., Controllable preparation, Rheology and plugging property of micron-grade polyacrylamide microspheres as a novel profile control and flooding agent. J. Appl. Polym. Sci., 2013, 130, 1124-1130.10.1002/app.39283Suche in Google Scholar
7 Xia Y.M., Song X.F., Yu Z.S., Su Z.Q., Xu H., Cai H., Study on the preparation of polyacrylamide microspheres and its application in oil field profile control and plugging. J. Oilfield Chem., 2014, 43(6), 729-734.Suche in Google Scholar
8 Bardaiee G.R., Pourjavadi A., Soleyman R., Sheikh N., Irradiation mediated synthesis of a superabsorbent hydrogel network based on polyacrylamide grafted onto salep. J. Nucl. Instrum. Methods Phys. Res., Sect. B., 2008, 266(18), 3932-3938.10.1016/j.nimb.2008.06.023Suche in Google Scholar
9 Liu Y., Chang Q., Yu F.M., Li H.J., Application of polyacrylamide in oil field production. J. Petrochem. Appl., 2014, 33(4), 9-11.Suche in Google Scholar
10 Wang P.P., Liu F., Upconversion nanometer gel prepared by one-pot emulsion polymerization. J. China Adhesives, 2017, 5, 1-5.Suche in Google Scholar
11 Yang B., Cationic polyacrylamide aqueous emulsion was prepared by dispersion polymerization. J. Chem. Eng., 2018, 3, 8-11.10.1016/j.cej.2017.08.083Suche in Google Scholar
12 Li F.Y., Study on effective plugging radius of weak gel plugging system. PhD thesis, Chengdu University of Technology, Chengdu, 2016.Suche in Google Scholar
13 Liu D., Zhang H., Fontana F., Hirvonen J.T., Santors H.A., Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. J. Adv. Drug. Delivery Rev., 2018, 128, 54-83.10.1016/j.addr.2017.08.003Suche in Google Scholar PubMed
14 Kim J.Y., Fluri D.A., Marchan R., Boonen K., Mohanty S., Singh P., et al., 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J. Biotechnol., 2015, 205, 24-35.10.1016/j.jbiotec.2015.01.003Suche in Google Scholar PubMed
15 Prakash R., Pabbaraju K., Wong S., Wong A., Tellier R., Kaler K.V., Multiplex quantitative reverse transcription PCR detection of influenza viruses using droplet microfluidic technology. J. Micromachines, 2015, 6(1), 63-79.10.3390/mi6010063Suche in Google Scholar
16 Wei Z.L., Research progress on preparation of super absorbent resin by inverse suspension polymerization. J. New Chem. Mater., 2014, (10), 20-22.Suche in Google Scholar
17 Song Z.F., Wei J., Fu C.F., Li X., Chang Z.Q., Preparation of SDB-TPGDA porous microspheres with uniform particle size by microfluidics. J. Fine. Chem., 2014, 31(9), 41-44.Suche in Google Scholar
18 Zhao L., Study on the Synthesis and Properties of Salt-Resisting Superabsorbent based on Maleic anhydride. PhD thesis, Lanzhou University, Lanzhou, 2009.Suche in Google Scholar
19 Wu Y.F., Preparation and properties of water soluble temporary plugging agent with small particle size. PhD thesis, Lanzhou University of Technology, Lanzhou, 2019.Suche in Google Scholar
© 2019 Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

