Abstract
A novel chemical-consolidation method based foam amino resin system of sand control systems in the oilfield is reported. This sand control technique is more superior to the conventional method owing to its advantages such as the outstanding resistance and lower density as well as simple process preparation. The apparent density of the foam resin system ranges from 0.528 g/cm3 to 0.634 g/cm3 at room temperature. Moreover, the system has excellent foaming properties and excellent compatibility with the formation fluids. In addition, the foam amino resin sand consolidation system was optimized and investigated. Simultaneously, the sand-fixing performance of the foam resin system was comprehensively assessed. The optimized conditions are as follows: curing temperature, 60°C; curing time, 12 h; consolidated core compressive strength, 6.28 MPa. Furthermore, the consolidated core showed remarkable resistance to the formation fluids. In summary, the foam resin system effectively met the requirements of the sand control and the horizontal wells in the oilfield.
Graphical Abstract
1 Introduction
The movement of particles is problematic for producing wells in the oilfield. Typically, these particles could be formation sands. Recently, much attention has been paid to controlling the formation sands in theinjecting well, producing well, and even shale gas well (1, 2, 3). In recent years, mechanical method is the widely used and effective method of sand control in the oilfield. It includes the use of the slotted liner and gravel packing (4,5). This method successfully holds the sand on the consolidated reservoir, but is not useful for poorly and unconsolidated reservoirs (6). However, an alternative to the mechanical method is the chemical-consolidation method, where particles are bonded together to prevent movement. The chemical-consolidation method using resins including epoxies (7,8), phenolic aldehydes (9), and furans (10,11) has been widely utilized in a variety of applications in well production. Moreover, this method has been widely applied for the preparation of resin-coated proppants (12, 13, 14) and the strengthening of unconsolidated sands (15,16). Nevertheless, these three types of resins have higher density and poor rheological property, and their preparation process is complex, increasing the production cost.
Currently, the latest technology of consolidation chemicals is water-based resin systems (17). These systems have several benefits compared to conventional chemical resin technologies, including lower density, better rheological property, and easier cleanup equipment (18). The water-based system can also be foamed with nitrogen, helping to provide better coverage in longer intervals (19). Similarly, the melamine formaldehyde resin belonging to amino resin is a comprehensively utilized high-performance water-based resin. In addition, it is non-toxic and has high temperature resistance and excellent mechanical properties (20). At present, the melamine formaldehyde resin has found extensive application in all fields such as bonded plates and decorative materials as the main binder in the manufacture of wood-based panels. In particular, the melamine formaldehyde resin has not been investigated and applied in the chemical sand control in oilfield until now (21).
In this study, we proposed a one-step process to prepare a water-based foam resin system from melamine formaldehyde resin solutions using foaming agent and stabilizer for sand control in the oilfield. This study is divided into two parts. In the first part, the concentration and type of foaming agent and stabilizer were screened for the foam amino resin system; first, the compatibility between the foam system and the formation fluids was investigated by experimental methods. Second, the concentration of the curing agents, coupling agent, and the foam resin system was optimized for the foam resin sand consolidation system. Moreover, the adaptability and resistance properties of the consolidated core were analyzed in terms of sand particle size and the medium of formation fluids.
2 Experimental
2.1 Materials
All starting chemicals of analytical reagent grade were purchased from Aladdin and used unless otherwise stated. In addition, the amino resin of melamine formaldehyde resin (IND, 40%) was supplied by CSL resins. The curing agent, ammonium chloride (IND, >99%), and the foam stabilizer, sodium carboxymethyl cellulose (IND, >96%), were obtained from Guangdong New Material Co. Ltd. Furthermore, the silane coupling agent (IND, >98%) was purchased from Jining Chemical Co. Ltd. All the materials were utilized as received.
2.2 Evaluation methods of foam performance
At present, the concentration and type of foaming agent and stabilizer have been screened for a variety of foaming methods such as Din method, Ross-Miles, and Warning Blender method (22). Ultimately, we used Warning Blender method for the screening test of foaming agent and foam stabilizer with a speed of 600 r/min and a stirring time of 3 min. In contrast, in order to meet the requirements of oilfield operations, the foam system needs to carry enough of the resin solutions to achieve high compressive strength of consolidated core, which is intuitive for the performance for higher foaming volume V0. In contrast, to make the foam system entering the deep part of stratum, the foam system should have strong stability, i.e., longer foam decay half-life t1/2. However, the most significant evaluated parameters of the foam system are the foaming properties and stabilization performance, and both of them depend on each other. Accordingly, these properties were characterized as foam composite index Fc, applied to screen the type and concentration of the foam system.
where Fc is the foam composite index, mL · min; V0 is the foaming volume, mL; t1/2 is the foam decay half-life, min.
2.3 Method of foam resin sand consolidation
The direct injection method used is as follows: 100 g quartz sand with size ranging from 40 mesh to 60 mesh was weighed and filled in the glass sand pack with φ25 × 200 mm. The surface of the sand was wet by injecting water with a plunger pump, and then the foam resin sand consolidation system was pumped into the sand pack, ensuring that both ends are compact. Finally, the sand pack was placed in a closed container at a pressure of 5 Mpa and 60°C. After a curing time of 12 h, the consolidated core was taken out.
2.4 Evaluation method of the system consolidated properties
The compressive strength and permeability of the consolidated core were measured, according to the determination (CNS, no. SY/T 2000-5276) of flexural strength and compressive strength, as well as the gas permeability of artificial core in the chemical sand control.
The core resistance to medium performance evaluation was investigated by the following tests. The consolidated core was immersed in a sealed container with different media solutions at room temperature for three days, and then the core was taken out from the solutions and its compressive strength was measured.
3 Preparation of foaming resin solution
3.1 Screening of foaming agent concentration
The foaming resin system is mainly made up of resin based solution and foaming agent as well as foam stabilizer. Simultaneously, sodium dodecyl sulfate (SDS) as a foaming agent with excellent compatibility with amino resin was selected through the preliminary experiments (23). In addition, the foaming properties of the foam resin system are directly affected by the concentration of foaming agent (SDS). Therefore, the foam composite index of the foaming resin system was calculated at different concentrations of SDS, aimed to evaluate the effect of the concentration of SDS on the foaming amino resin system.
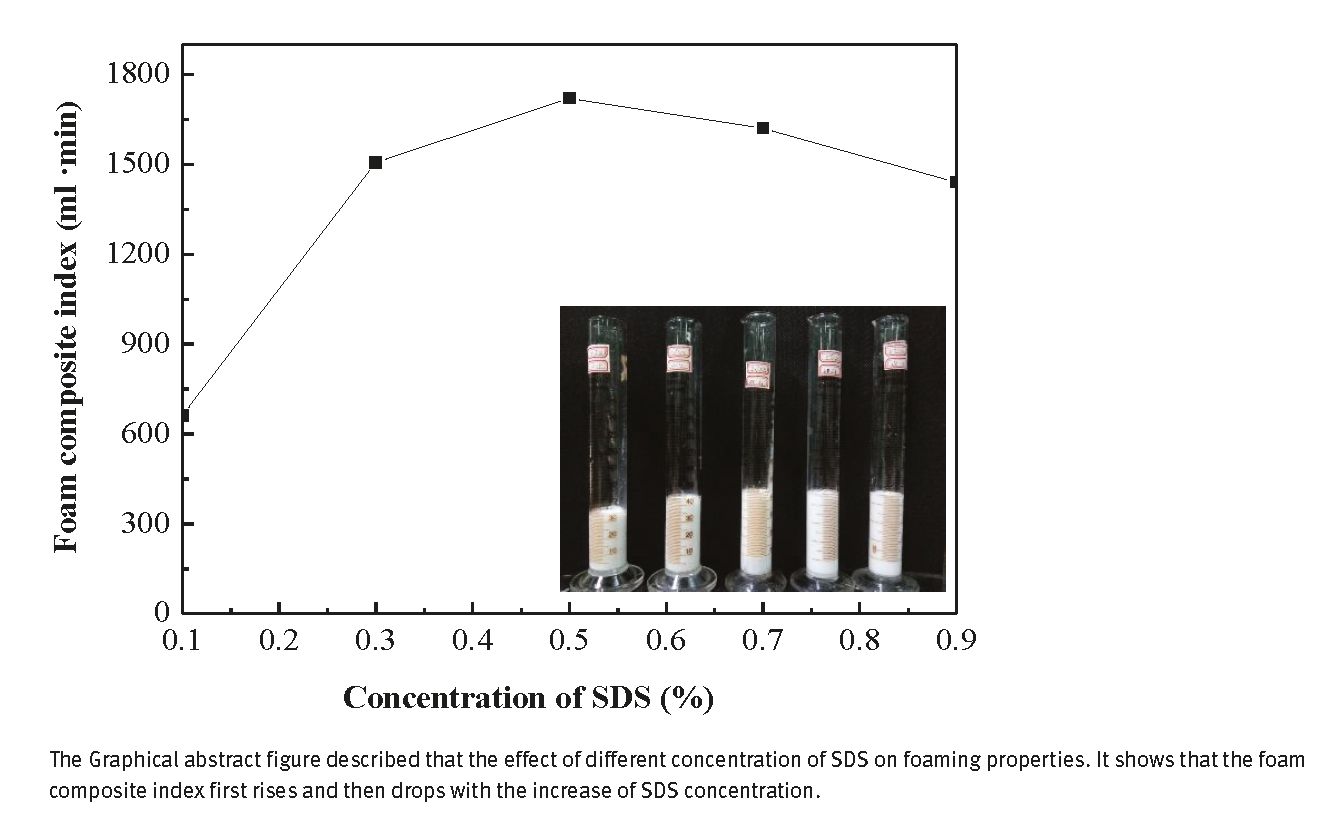
Figure 1 shows the effect of different concentrations of SDS on the foaming properties (the based fluids: 20 mL amino resin solutions and the SDS concentration ranges of 0.1–0.9 wt%). The graph shows that the foam composite index first rises and then decreases with increasing SDS concentration. Moreover, at 0.5 wt% SDS concentration, the foam composite index of the maximum reached 1720 mL · min. This can probably be explained by the fact that with increasing SDS concentration, the massive SDS molecules are adsorbed and closely arranged on the surface, increasing viscosity and enhancing the foam stability (24). Nevertheless, the SDS concentration exceeds the critical micelle concentration. Because of the presence of a significant amount of counter-ion in the diffusion layer, the electric double layer is compressed and the electrostatic repulsion is weakened, improving the deteriorated speed of destruction of foam stability. Finally, 0.5 wt% foaming agent SDS was selected and used in the subsequent experiments.
Effect of SDS concentration on the foaming property (embedded image: volume change of foams with different SDS concentrations).
3.2 Screening of foam stabilizer concentration
Following foam stabilizer were selected: Sodium carboxymethyl cellulose (CMC), sodium fluoride polyvinyl alcohol, polyethylene glycol, solid particles (Na-montmorillonite), Nano SiO2, and sodium polyacrylate. Table 1 presents the effect of different types of stabilizer on the foam properties (the based fluids: 20 mL amino resin solutions, 0.5 wt% SDS, 1 wt% foam stabilizer). As listed in Table 1, the CMC stabilizer has excellent compatibility with the amino resin. Moreover, the foam composite index is up to 3388 mL·min, demonstrating the best foaming and stable performance. CMC may also have excellent compatibility with amino resin. The viscosity of the resin solution increases, and consequently the foam drainage speed decreases together with enhancing the rigidity of the liquid films and the gas permeability. Hence, the foam decay half-life and the foam stability improved. Therefore, CMC was screened as a foam stabilizer in this study.
The effect of different types of stabilizer on the foam properties.
| Stabilizing agents | Foaming volume (mL) | Half-time (min) | Foam composite index (mL·min) |
|---|---|---|---|
| Sodium soil | 48 | 47 | 2256 |
| Sodium carboxymethyl cellulose(CMC) | 44 | 77 | 3388 |
| sodium fluoride | 50 | 40 | 2000 |
| Polyvinyl alcohol(PVA) | 46 | 35 | 1610 |
| Polyethylene glycol(PEG) | 51 | 58 | 2958 |
| Nano SiO2 | 49 | 41 | 2009 |
| Sodium polyacrylate | 46 | 26 | 1196 |
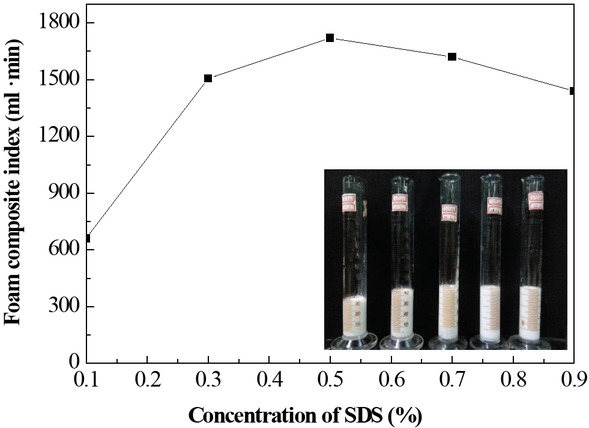
Not only the concentration of the stabilizer has critical effect on the foaming properties of the foam resin system, but also affects the economic benefit of the system for practical application. Figure 2 shows the effect of different CMC concentrations on the foam properties (the based fluids: 20 mL amino resin solutions, 0.5 wt% SDS, and the CMC concentration ranges 0.5–1.5 wt%), demonstrating that at CMC concentrations >1%, the foam composite index rises gradually with increasing CMC concentration. However, at CMC concentration >1%, the foam composite index decreases. The main reason is probably that when the concentration of CMC is >1%, the amount of CMC molecules increases in liquid films, improving the viscosity of the system. Consequently, the foam decay rate of the liquid films decreases, increasing the stability of the foam. Nevertheless, with further increase in the CMC concentration, the concentration of sodium ions rises and the liquid film thickness of the diffusion layer compressed together with increasing degree of molecular curling. Ultimately, the viscosity of the foam system is reduced, affecting the foam stability of the system. Therefore, the optimized concentration of CMC is identified as 1 wt%.
Effect of CMC concentration on the foam property (embedded image: volume change of foams with different CMC concentrations).
3.3 Compatibility of the foam resin system with formation fluids
3.3.1 Effect of pH
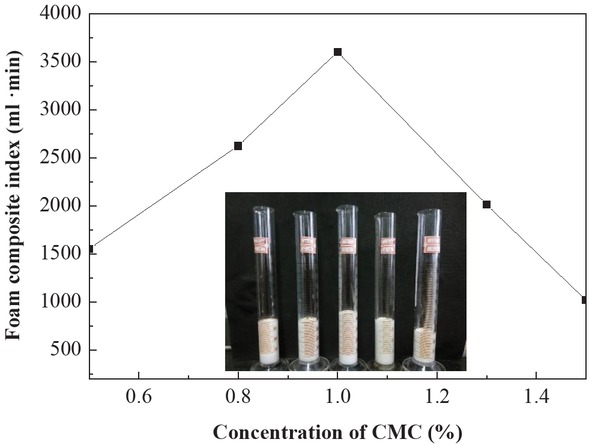
Through adjusting the pH of the base solution, the stability of the foam resin system was observed under different acid and alkali conditions. Figure 3 shows the effect of different pH solutions on the foam properties (formulation: 20 mL amino resin solutions, 0.5 wt% SDS and 1 wt% CMC). The graph shows a slight effect on the foam volume at different pH values of fluids; however, it significantly affects the foam decay half-life. This behavior can be attributed to the acidic nature of as the amino resin curing agent, in other words, resin long-chain structure easily forms a three-dimensional structure even when cured under the acidic conditions. Consequently, compared to the alkaline environment, the system has a better stability in acidic condition. Nevertheless, in acidic conditions (pH = 4) and alkaline conditions (pH = 10), the foam decay half-life was >40 min, hence the system has good stability in acidic and alkaline environment.
Effect of pH value on the foam property.
3.3.2 Effect of salinity
The salinity of the formation fluids also affects the foaming properties and stability of the foaming resin system. In the experiments, two different salts, NaCl and CaCl2, in the concentration range 2 × 104 – 10 × 104 mg/L to the foaming solutions (formulation: 20 mL amino resin solutions, 0.5 wt% SDS and 1 wt% CMC), indicating that the foam volume of the system is slightly different under different salinity conditions, but it has a significant effect on the decay half-life of the foam. Correspondingly, as shown in Figure 4, the foam decay half-life declines rapidly with larger salt concentration. The added salt ions in the foaming solution compress the thickness of the ionic atmosphere in the surfactant, resulting in a loose adsorbed layer (25), thus decreasing the foam decay half-life. The results further show that the decay half-life finally flattens after 45 min at a concentration of 2 × 104 mg/L salt. This behavior illustrates that foam resin system is seized of the strong salinity resistances.
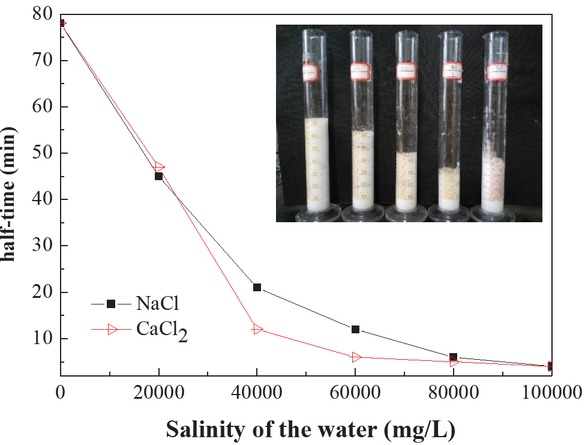
Effect of salinity on the half-time of foam (embedded image: volume change of foams with different salinity values).
3.3.3 Effect of oil saturation
Figure 5 shows the effect of oil saturation on the foam resin system (formulation: 20 mL amino resin solutions, 0.5 wt% SDS and 1 wt% CMC). The foam volume gradually decreases at the initial stage. In contrast, the foam decay half-life significantly increases with increasing oil saturation. The results indicated that the foam resin system has selective stability features of common foam. That is due to the expansion of oil drops in the gas–liquid interface, decreasing the thickness of the bubble film and forming a instable bridge across the foam film (26,27). Nevertheless, at a certain oil concentration, some of the crude oil is emulsified and has good dispersion properties in amino resin solutions. Simultaneously, the liquid film thickness is improved by small emulsified oil droplets, decreasing the liquid film decay rate. In other words, the foam resin system possesses excellent resistances to oil.
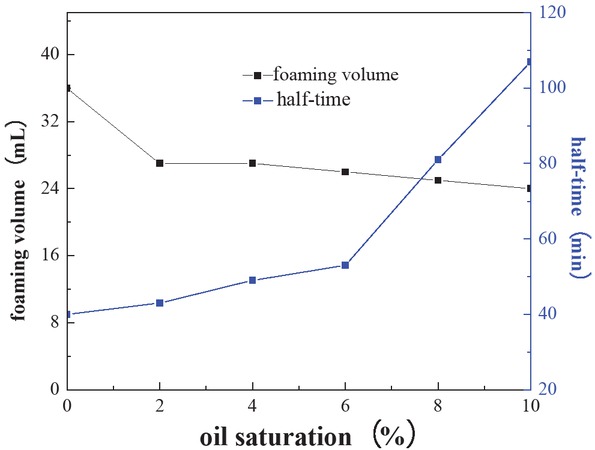
Effect of oil saturation on the foam resin system.
The abovementioned analysis proves the prominent compatibility between the foam resin system and simulated formation fluids. Therefore, when the foam resin system is injected into the formation, it maintains excellent foaming properties.
The microscopic image of the foam amino resin system was obtained using an Oslo Bahrain microscope. Figure 6 shows that the foam is dense and uniform in size. The remarkable foaming properties of the foam resin system are fully illustrated. In addition, the apparent density of the foam resin system was measured using a foam density meter and ranges from 0.528 g/cm3 to 0.634 g/cm3 and is lower than those of the conventional sand consolidation system (the density of unsaturated resin system ranges from 0.788 g/cm3 to 0.821g/cm3; the density of the emulsified epoxy resin system and urea formaldehyde resin system are 0.957 and 1.12 g/cm3, respectively). Therefore, the foam amino resin system was screened by the comparison tests (formation: 98.5 wt% MF resin solutions, 0.5 wt% SDS and 1 wt% CMC).
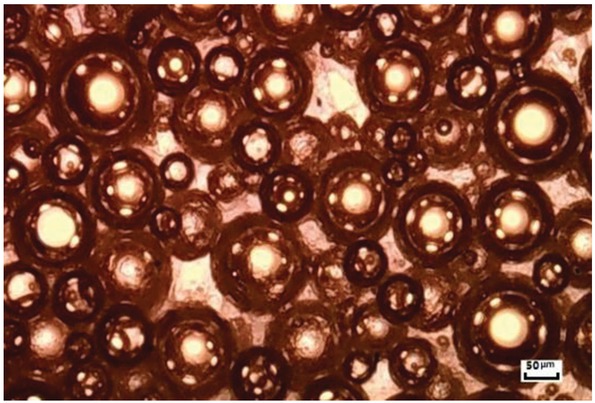
Microscopic image of the foam amino resin system.
4 Preparation of foaming resin sand consolidation system
4.1 Screening of curing agent concentration
For meeting the curing time requirements of more than three hours in the oilfield application, NH4Cl curing agent was screened through the preliminary tests. Figure 7 shows the effect of curing agent NH4Cl concentration on the sand consolidation properties of the foam resin system (formulation: 25 wt% solutions of foam resin system, the coupling agent KH-550 initial concentration is 0.2 wt%, and the curing agent NH4Cl concentration ranges of 0.5–2.5 wt%), indicating that with the larger concentration of curing agent, the compressive strength initially increases and then decreases. This behavior could be attributed to substantial phenolic hydroxyl and hydroxymethyl in the foam resin system. Correspondingly, with increasing curing agent concentration, the crosslinking density and the compressive strength of consolidated sand increase by degrees. Nevertheless, in the presence of excess concentration of the curing agent, the condensation reaction of the resin is suppressed. Accordingly, the pre-polymer formed by the resin polymerization is brittle, ultimately decreasing the compressive strength of the consolidated core. To sum up, 1% concentration of curing agent NH4Cl was used in the subsequent experiments.
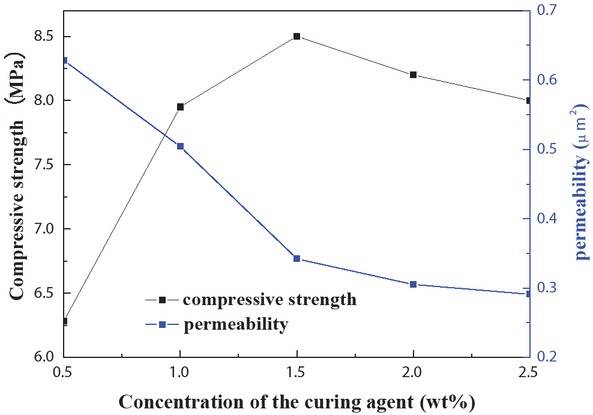
Effect of curing agent concentration on sand consolidation properties.
4.2 Screening of coupling agent concentration
Figure 8 shows the effect of the coupling agent KH-550 on the sand consolidation properties of the system (formulation: 25 wt% solutions of foam resin system, 1 wt% curing agent as well as the coupling agent KH-550 concentration ranges 0.1–0.5 wt%). As shown in Figure 6, the effect of the compressive strength of the consolidated core rises to the maximum and then slightly drops. We believe that this is due to the possibility that the coupling agent plays a critical bridging role between the sand and resin molecules in the preliminary stage (27). Similarly, the concentration of the coupling agent is also better, attributed to the presence of HO–Si bond on the sand surface. With excess of KH-550, the Si–O–Si bonds are formed on the sand surface by the KH-550, decreasing the HO-Si bonds. Finally, its hydrogen bonding strength with the sand decreases, and the intensity decreases. In summary, the optimized concentration of coupling agent KH-550 was selected as 0.2 wt%.
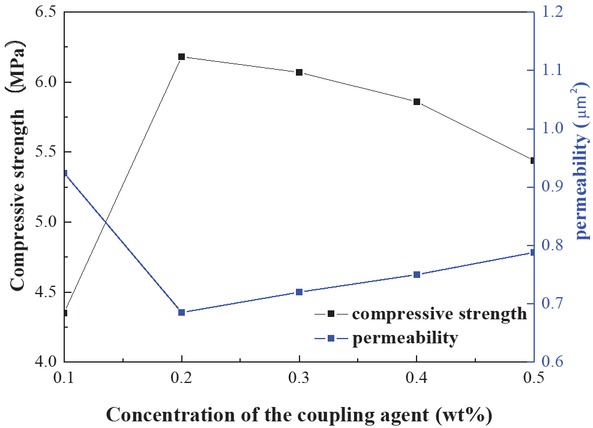
Effect of the coupling agent KH-550 in the sand consolidation properties.
4.3 Screening of foam resin system concentration
Figure 9 shows the effect of the concentration of foam resin system on the sand consolidation properties (formulation: 1 wt% curing agent and 0.2 wt%, the coupling agent KH-550 as well as the concentration of the foam resin system ranges 10–30 wt%). The graph represents that with increasing concentration of the foam resin system, the compressive strength of the core is gradually enhanced. Conversely, the permeability descends by degrees. This phenomenon can be explained by the fact that the substantial resin solutions is filled in sand pore, blocking the communication of pore communicated with each other. Ultimately, the permeability of the core decreases gradually. When the concentration of the foam resin system reached 20 wt%, the strength and permeability are relatively moderate. To sum up the above analyses, the optimum concentration of the foam resin system was identified as 20 wt%.
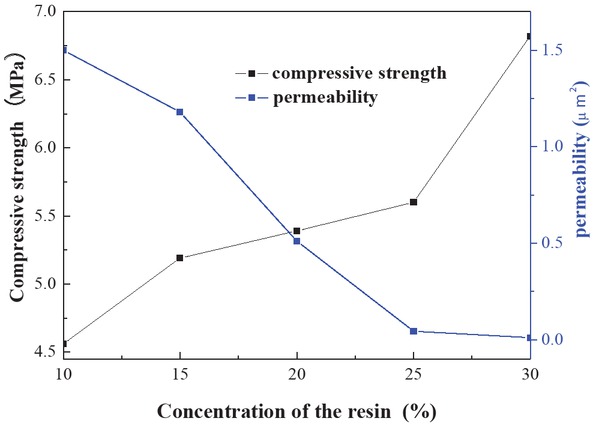
Effect of the concentration of foam resin system on sand consolidation properties.
4.4 Compatibility of system with sand particle size
The sand consolidated formulation of the foam amino resin system was screened (foam resin system: curing agent: coupling agent = 20 wt%:1 wt%:0.2 wt%). Simultaneously, 100 g sand with 20–40 mesh, 40–60 mesh, 60–80 mesh, 80–100 mesh as well as 100+ mesh was consolidated by the foam resin sand consolidation system, and the adaptability of the system with respect to sand particle sizes was investigated to prove the sand control ranges in the oil layer. The consolidated cores using different meshes of sands were shown in Figure 10. It shows that different meshes of sands can be consolidated to produce cores with the use of the system.

(A) 20 mesh ~ 40 mesh, (B) 40 mesh ~ 60 mesh, (C) 60 mesh ~ 80 mesh, (D) 80 mesh ~ 100 mesh: (E) 100+ mesh.
Table 2 shows that when the sand particle size is small, the compressive strength of the consolidated core is enhanced probably because the smaller sand particle size occupies a greater specific surface. In addition, the more sand surface is adsorbed by the resin molecules, increasing its compressive strength. Simultaneously, the pore volume is increased, and the effective permeability tends to descend. The system is better adapted to the formation sands with particle size >60–80 mesh. The analysis of the experiment result indicated excellent compatibility of the sand consolidation system with the formation sands.
The compatibility of the sand consolidation system sand particle size.
| Sand size, Mesh | 20-40 | 40-60 | 60-80 | 80-100 | 100+ |
|---|---|---|---|---|---|
| Compressive strength, MPa | 5.23 | 5.65 | 6.28 | 6.91 | 7.74 |
| Permeability, μm2 | 0.284 | 0.24 | 0.168 | 0.017 | 0.001 |
4.5 Medium resistance of the consolidated core
In the comparative experiments, urea formaldehyde resin (UF) sand consolidation system were used in the oilfield as a reference, and the effects of the consolidated core in water, 10 wt% HCl solution, 10 wt% NaOH solution, and NaCl solution (2 × 104 mg/L salinity), as well as diesel oil were investigated. Table 3 shows that when the consolidated core immerses in water, diesel fuel, and
The effects of the consolidated core on the simulated medium fluids of formation.
| Medium solutions | NaCl solutions | diesel oil | Water | 10% NaOH | 10% HCl |
|---|---|---|---|---|---|
| Compressive strength, MPa (MF) | 5.23 | 6.28 | 5.86 | 4.20 | 3.35 |
| Compressive strength, MPa (UF) | 3.72 | 3.14 | 3.64 | 3.46 | 1.67 |
NaCl solutions, the compressive strength (MF) is almost unaffected. Compared to the compressive strength (UF), the foam resin (MF) system demonstrated remarkable alkaline and oil resistance; moreover, the consolidated core met the requirements of formation salinity; however, in 10% hydrochloric acid solution, the strength is comparatively lower, and its resistance towards acid needs further investigation.
5 Conclusions
In this study, the foam amino resin sand consolidating system was optimized. Indeed, the optimum formula of the system is as follows: 97.3 wt% amino resin solutions, 0.5 wt% SDS, 1 wt% CMC and 1 wt% NH4Cl, and 0.2 wt% coupling agent (KH-550). Simultaneously, the apparent density ranges from 0.528 g/cm3 to 0.634 g/cm3 at room temperature. In addition, the system has excellent foam properties and remarkable compatibility with the formation fluid. Comprehensively, the sand consolidation performance of the foam resin system was assessed. At a curing temperature of 60°C, the compressive strength and permeability of the consolidated core is in the range 5.12–6.28 MPa and 0.92–2.7 μm2, respectively, after a curing time of 12 h. In contrast, the consolidated core has remarkable resistance to water, oil, and alkali fluids as well as salinity variation.
The performance of this novel sand control method based foam amino resin system was tested in the lab, and by April 2016, this technology was applied in two testing wells in the Shengli Oilfield in China.
Acknowledgements
This research is financially supported by National Natural Science Foundation of China (Grant 51704310, 51774307), Natural Science Foundation of Shandong Province, China (Grant ZR2017BEE034), PetroChina Innovation Foundation (Grant 2017D-5007-0203), and Fundamental Research Funds for the Central Universities (Grant 18CX02159A, 18CX02163A).
References
1 Hu K., Schmidt A., Barhaug J., Wong J., Tian J., Sand, resin-coated sand or ceramic proppant? The effect of different proppants on the long-term production of Bakken shale wells. Paper SPE 174816, 2015.10.2118/174816-MSSuche in Google Scholar
2 Jones P.J., London B.A., Tennison L.B., Karcher J.D., Unconventional remediation in the utica shale using advanced resin technologies. Paper SPE 165699, 2014.10.2118/165699-MSSuche in Google Scholar
3 Araujo O., Rodriguez L., Lopez E., Use of liquid resin to enhance and maintain conductivity in fractured wells better than use of curable resin proppants: a case from Burgos basin. Paper SPE 156131, 2012.10.2118/156131-MSSuche in Google Scholar
4 Li Y.L., Hu G.W., Liu C.L., Wu N.Y., Chen Q., Liu L., Gravel sizing method for sand control packing in hydrate production test wells. Petrol Explor Develop, 2017, 44(6), 1016-1021.10.1016/S1876-3804(17)30114-3Suche in Google Scholar
5 Khamehchi E., Ameri O., Alizadeh A., Choosing an optimum sand control method. Egypt J Pet, 2015, 24(2), 193-202.10.1016/j.ejpe.2015.05.009Suche in Google Scholar
6 Agunloye E., Utunedi E., Optimizing sand control design using sand screen retention testing. Paper SPE 172488, 2014.10.2118/172488-MSSuche in Google Scholar
7 Nguyen P.D., Dusterhoft R.G., Stabilizing wellbores in unconsolidated, clay-laden formations. Paper SPE 86559, 2006.10.2118/86559-MSSuche in Google Scholar
8 Mallikarjuna S.R., Sumit S., Pratiksha M., Effective resin consolidation treatment methods and compositions for clay-laden formations. Paper SPE 178998, 2016.Suche in Google Scholar
9 Morris K.A., Deville J.P., Jones P., Resin-based cement alternatives for deep water well construction. Paper SPE 155613b, 2012.10.2118/155613-MSSuche in Google Scholar
10 Keith C., Azman A., Wijoseno D.A., Kasim M.H., Ishak M.F., Coil tubing furan resin sand consolidation treatment on multi layered formation in Peninsular Malaysia. Paper SPE 165911, 2013.10.2118/165911-MSSuche in Google Scholar
11 Pumama G.W., Laboratory studies: analysis of resin composition to handle sand problems on unconsolidated gas formation. Paper SPE 152362, 2011.10.2118/152362-STUSuche in Google Scholar
12 Geehan T., Deckert S.L., Johnson K., Arnold W.T., Use of resin-coated proppant to control shallow, low-temperature unconsolidated sands. Paper SPE 54631, 1999.10.2118/54631-MSSuche in Google Scholar
13 Pope C.D., Wiles T.J., Pierce B.R., Curable resin-coated sand controls proppant flowback. Paper SPE 16209, 1987.10.2118/16209-MSSuche in Google Scholar
14 Burke L.H., Roney D., Elgar T., Hersey A.N., Factors that impact the performance of resin coated proppant in low temperature reservoirs. Paper SPE 162792, 2012.10.2118/162792-MSSuche in Google Scholar
15 Nguyen P.D., Dusterhoft R.G., Ali S., Lockman R.R., Stabilizing wellbores in unconsolidated, clay-laden formations. Paper SPE 86559, 2004.10.2118/86559-MSSuche in Google Scholar
16 Wasnik A., Mete S., Ghosh B., Application of resin system for sand consolidation, mud-loss control, and channel repairing. Paper SPE 97771, 2005.10.2118/97771-MSSuche in Google Scholar
17 Villesca J., Loboguerrero S., Gracia J., Hansford A., Nguyen P., Rickman R., Development and field applications of an aqueous-based consolidation system for proppant remedial treatments. Paper SPE 128025, 2010.10.2118/128025-MSSuche in Google Scholar
18 Oubre B., Hasemann A., Particle consolidation as a means of sand control: Gulf of Mexico applications. Paper SPE 127829, 2010.10.2118/127829-MSSuche in Google Scholar
19 Pei X., Shi B., Chen L., Zheng L., Metal foam sand control screen. SPE 165829, 2013.10.2118/165829-MSSuche in Google Scholar
20 Costa N., Pereira J., Martins J., Ferra J., Cruz P., Alternative to latent catalysts for curing UF resins used in the production of low formaldehyde emission wood-based panels. Int J Adhes Adhes, 2012, 33(3), 56-60.10.1016/j.ijadhadh.2011.11.003Suche in Google Scholar
21 Yeh C.S., Grueschow E.R., Bhargava P., Burdette J.A., Barry M.D., Hecker M.T., et al., Technology innovation and integration enabling more flexible, adaptive, and reliable sand control in open-hole completions. Paper SPE159858, 2012.10.2118/159858-MSSuche in Google Scholar
22 Bai Y.R., Shang X.S., Wang Z.B., Zhao X.T., Dong C.Y., Experimental investigation of nanolaponite stabilized nitrogen foam for enhanced oil recovery. Energy Fuels, 2018, 32(3), 3163-3175.10.1021/acs.energyfuels.7b03798Suche in Google Scholar
23 Mishra S., Ojha K., Application of an improvised inorganic–organic chemical mixture to consolidate loose sand formations in oil fields. J Pet Sci Eng, 2016, 137, 1-9.10.1016/j.petrol.2015.11.008Suche in Google Scholar
24 Zhao X.T., Wang Q., Wang Z.B., Commonly used foaming agents in high temperature and high salinity reservoir. Mater Rev, 2016, 30(5), 75-80.Suche in Google Scholar
25 Zhang Y., Li C., Xiao J.X., Ma J.M., Effect of salt with high concentration on surface activities of equimolar mixtures of cationic-anionic surfactants. Acta Chim Sin, 2004, 62(16), 1491-1494.Suche in Google Scholar
26 Garrett P.R., Defoaming: theory and industrial applications. Marcel Dekker, New York, 1993, pp. 225.Suche in Google Scholar
27 Simjooa M., Andrianovd T.R., Zitha P.L., Foam stability in the presence of oil: effect of surfactant concentration and oil type. Colloids Surf A, 2013, 438, 148-158.10.1016/j.colsurfa.2013.05.062Suche in Google Scholar
© 2019 Shang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

