Abstract
Background and aims
Chronic pain is affecting a growing number of individuals including adolescents. Different endogenous pain inhibitory systems could confer protection against development of chronic pain. Decreased pain perception can be observed following intense pain (i.e. conditioned pain modulation – CPM) or after physical exercise (i.e. exercise-induced analgesia – EIA). Reduced effectiveness of pain inhibitory mechanisms have been reported in several chronic pain conditions. However, the extent of these dysfunctions has not been thoroughly investigated in adolescents suffering from chronic pain. Our hypothesis was that adolescents suffering from chronic pain have less effective CPM and EIA than pain-free teenagers.
Methods
Twenty-five healthy adolescent girls and 16 teenage girls with chronic pain participated in this study. Only girls were included in this investigation, since chronic pain is more prevalent in females. The effectiveness of CPM was assessed by comparing heat pain stimulations (individually adapted to induce mild pain intensity) performed with a thermode before and after a cold pressor test (CPT; 2 min, 10 °C). EIA was evaluated by comparing pain intensity produced by an ice cube placed on the forearm before and after a graded exercise test on a cycle ergometer.
Results
Pain intensity produced by heat pain stimulations decreased following CPT in healthy (p<0.05), but not in chronic pain adolescent girls (p=0.4). Pain intensity induced by the ice cube was reduced after exercise in healthy (p<0.05), but not in chronic pain adolescents (p=0.9). The effectiveness of CPM and EIA was inferior in teenage girls suffering from chronic pain compared to healthy participants (p<0.05).
Conclusions
Endogenous pain inhibitory mechanisms triggered by intense pain or by physical exercise are effective in healthy adolescent girls. Teenage girls living with chronic pain do not show diminished pain perception after a CPT or a graded exercise test. These results suggest that pain inhibitory mechanisms such as CPM and EIA are ineffective in adolescent girls suffering from chronic pain.
Implications
In a wider context, the findings of the present research could help understand better the mechanisms involved in the development of chronic pain. Improved comprehension of this subject might help prevent chronic pain conditions and thus, reduce the negative impacts of this burden.
1 Introduction
Chronic pain affects many children and adolescents [1], [2], [3]. The negative consequences of chronic pain on quality of life, psychological status and physical function are well established in the pediatric population, occasioning missed school days and reduced social activities [4]. Furthermore, the presence of chronic pain during childhood and adolescence can persist to adult age [5], [6]. Chronic pain is a challenging condition to treat requiring multidisciplinary approaches and long-term follow-up thus, generating an important socio-economic burden [7], [8]. Therefore, we consider that prevention of chronic pain is of great importance and a better comprehension of the mechanisms leading to pain chronicity is required.
The development of chronic pain is thought to depend on many variables (physiological, psychological, genetic, etc.) [9], [10]. Notably, different endogenous mechanisms can augment or inhibit pain sensations [11], [12]. For instance, conditioned pain modulation (CPM) relates to the reduced pain sensations experienced in the entire body following an intense pain sensation (i.e. counter-irritation or pain inhibits pain effect) [13]. Reduced CPM effectiveness has been reported in adults suffering from several chronic pain conditions such as fibromyalgia, irritable bowel syndrome and atypical trigeminal neuralgia [14]. Interestingly, healthy individuals with lower pre-operative CPM activity have increased risk to develop chronic pain following surgery [15]. However, few studies investigated CPM effectiveness in children or adolescents. Weaker CPM effects have been observed in children (aged between 7 and 11 years old) that were born preterm and that experienced numerous painful interventions during the neonatal period compared to preterm children that did not receive many painful medical procedures or full-term children [16]. These results suggest that painful stimuli, particularly at a very young age, could alter the development and function of pain inhibitory mechanisms such as CPM. A study conducted on children and adolescents (aged between 10 and 17 years old) showed that poorer CPM and female sex were good predictors of pain persistence 4 months following appearance of musculoskeletal pain complaints [17]. Thus, assessment of CPM effectiveness, and possibly other pain inhibitory systems, could help identify adolescent girls at risk of developing long-lasting/chronic pain.
Exercise-induced analgesia (EIA) is another endogenous pain inhibitory mechanism implying reduced pain perception after an intense physical activity [18]. Physical exercise can be used to relieve pain in various chronic pain conditions such as chronic low back pain [19], chronic neck pain [20], osteoarthritis [21], migraine [22] and fibromyalgia [23]. Interestingly, endogenous pain inhibitory mechanisms such as CPM and EIA involve similar neuronal pathways [11]. Stolzman and Bement showed that more effective CPM is related to greater EIA in adolescents [24]. However, endogenous pain inhibitory mechanisms such as CPM and EIA have not been extensively investigated in adolescents suffering from chronic pain. Since chronic pain is more prevalent in women [25], [26], we decided to focus our study on female adolescents. Our hypothesis is that adolescent girls with chronic pain have reduced pain inhibition resulting from intense pain (i.e. CPM) and from physical exercise (i.e. EIA). The purpose of this investigation was to evaluate CPM and EIA effectiveness in adolescent girls suffering from chronic/recurrent pain and in healthy adolescent females.
2 Methods
2.1 Participants
Female adolescents were recruited in different high schools from Sherbrooke, Quebec, Canada. Inclusion criteria were female aged between 15 and 17 years old, suffering or not from chronic or recurrent pain. Only girls were included in this study based on the higher prevalence of chronic pain in females [25], [26]. Participants included in the chronic pain group had to be suffering from chronic or recurrent pain, defined as pain (headache/migraine, musculoskeletal, articular, gastrointestinal or gynecological) at an intensity of >3/10, at least once or twice a week for more than 3 months. Exclusion criteria were prematurity (<37 weeks of gestation), use of tobacco products, use of illicit drugs, cardiac or pulmonary disease (including asthma and upper respiratory tract infection at the moment of experiment), endocrine or metabolic disease. Participants were screened using a questionnaire, physical examination, electrocardiogram and spirometer to rule out any contraindications to cycle ergometer testing for aerobic fitness measurements.
All participants were asked to refrain from using analgesic medication at least 24 h prior to experimentation. Experimental procedures were held during the summertime not to interfere with school schedule. All procedures were approved by the Ethics Committee of the Centre Hospitalier Universitaire de Sherbrooke (CHUS). Participant and parent (or legal guardian) consent were obtained (complying with the requirements of the Declaration of Helsinky, 2013).
2.2 Pain evaluations
A numeric rating scale (0: no pain–10: most intense pain tolerable) was used to verbally rate pain intensity during the experimental pain stimulations. The numeric rating scale has been shown to have good validity and reliability for pain intensity evaluation in a pediatric population [27].
2.3 Heat pain stimulations (thermode)
A 30×30 mm thermode (TSA-II, Medoc advanced medical systems, Ramat Yishai, Israel) was used to induce thermal pain stimulations. Prior to actual testing, the thermode was placed in the palm of the left hand to familiarize participants to the heat pain stimuli, and to the experimental pain rating scale. During all subsequent heat pain tests the thermode was placed on the volar part of the left forearm. Thermode temperature was initially set to 37 °C and increased at a fixed rate of 0.3 °C/s. Participants were instructed to report when the sensation produced by the thermode changed from heat sensation to minimal pain (heat pain threshold) and when the pain could no longer be sustained (heat pain tolerance). This procedure was completed twice for every participant, and the mean of the two trials was retained. The thermode was placed on adjacent areas of the forearm for every trial to avoid primary skin hyperalgesia.
After heat pain threshold/tolerance assessments, the thermode was applied on the volar part of the left forearm for 5 s at constant temperature. During heat pain stimulations, the temperature was individually adapted to produce mild (30%) pain intensity (based on previously evaluated heat pain threshold and tolerance values. The following formula was used to determine the temperatures to use to induce mild pain: [0.3 (heat pain tolerance-heat pain threshold)]+heat pain threshold. Participants were asked to evaluate the pain intensity immediately after the heat pain stimulations (5 s). This procedure was done before and after the cold pressor test (CPT – see Section 2.4) using the same temperature. Participants were not informed the thermode temperature used before and after cold water immersion was the same to reduce expectations. The effectiveness of CPM was evaluated by comparing the pain intensity of the heat pain stimulation performed before and after the CPT.
2.4 Cold pressor test (CPT)
Participants had to immerse their right forearm in a bath of circulating cold water (10 °C) for 2 min. Participants were instructed not to move or contract their arm during the immersion. The CPT was used as a conditioning stimulus to activate CPM.
2.5 Cold pain stimulations (ice cube test)
A 2× 2 cm ice cube held with a plastic clamp was placed on four different sites of the left arm (volar portion) of the participants for 10 s. Pain intensity was evaluated with a verbal numeric rating scale at the end of the procedure. The ice cube tests were done before and after the graded exercise test (see Section 2.4). Mean pain intensity of the four trials (before and after physical exercise) are presented.
2.6 Graded exercise test
Participants performed a maximal oxygen capacity (VO2max) test on a cycle ergometer (Ergometrics 900, Ergoline GmbH, Bitz, Germany). The test began after a 3-min warm-up at 60 revolutions per minute (RPM) without resistance. Resistance was gradually added with a ramp protocol. Rate of workload increment was individualized to reach the maximal theoretical aerobic power between 8 and 12 min (increments 15–30 W/min) [28], [29]. Participants were encouraged to keep a constant rate of approximately 60 rpm throughout the procedure. Exercise continued until at least three of the VO2max criteria were obtained [30]: (1) a plateau of VO2 in spite of the increase of workload, (2) maximal heart rate (maximal predicted heart rate±5%), (3) respiratory exchange ratio>1.1, and (4) inability to maintain the pedaling frequency at 60 rpm (exhaustion).
2.7 Experimental procedure
Participants were comfortably seated in a calm room at the clinical research center of the CHUS. After determination of heat pain threshold and heat pain tolerance values, heat pain stimulations at mild intensity (30%) were completed. Heat pain stimulations were performed before and immediately after the CPT in order to evaluate CPM. After completing the CPM test, the participants filled-out different questionnaires to describe their socio-demographic and psychological characteristics. Socio-demographic information (age, time spent doing physical activities, etc.) was collected with a home-made questionnaire. Sleep quality was evaluated with the Pittsburgh Sleep Quality Inventory (PSQI). A standardized questionnaire on physical activity (including type, frequency, intensity and duration of physical activities) was completed by the participants [31]. The Pain Catastrophizing Scale, the State Trait Anxiety Inventory and the Children Depression Inventory were administered to assess psychological characteristics. Participants were allowed to eat a light meal or snack after completing the CPM evaluation.
Participants were then taken to the respiratory function lab at the CHUS. A 2-h interval separated CPM and EIA evaluations to avoid carry-over effects of CPM during EIA assessments. Physical characteristics (height, weight, sum of skinfold thickness) and spirometry were carried out. Pre-exercise ice cube tests were done and the participants then undertook the graded exercise test used to activate EIA. The post-exercise ice cube tests were realized after the end of the graded exercise test (<5 min after).
2.8 Statistical analyses
The effectiveness of CPM was evaluated by calculating the percentage of change in pain intensity for the heat pain stimulations (thermode) before and after the CPT using the following formula: 100[(thermode pain intensity before CPT-thermode pain intensity after CPT)/thermode pain intensity before CPT]. The effectiveness of EIA was assessed similarly by calculating the change in pain intensity of the ice cube test before and after the graded exercise test using the following formula: 100[(ice cube test pain intensity before exercise-ice cube test pain intensity after exercise)/ice cube test pain intensity before exercise]. Higher CPM or EIA values represent more important pain inhibition following the CPT or graded exercise test, respectively.
All analyses were conducted using SPSS (version 19.0; IBM, New York, NY, USA). Group differences (healthy and chronic pain participants) were evaluated with independent sample t-tests. Paired sample t-tests (repeated measures) were used to compare pain intensity assessments for the heat pain stimulations before and after CPT (or for the ice cube tests before and after graded exercise). All data are given as mean and standard error of the mean (SEM). Statistical significance was set at p<0.05.
3 Results
3.1 Participant characteristics
A total of 47 adolescent girls were recruited in this study. Six participants were excluded (two suffered from asthma, unknown prior to inclusion of the study; one was born prematurely and three did not complete the entire experimental procedures). The final analyses included 25 healthy (age=15.8±0.2 years); and 16 teenage girls with chronic/recurrent pain (age=15.7±0.2 years). Healthy girls had less sum of skinfold thickness compared to chronic pain teenagers (p=0.009) (Table 1). Healthy teens tended to be more physically active then girls suffering from chronic pain (p=0.06). Teenage girls with chronic/recurrent pain showed lower VO2max compared to healthy adolescents (p=0.004).
Physiological characteristics.
| Variable | Healthy (n=25) mean (SEM) | Pain (n=16) mean (SEM) |
|---|---|---|
| Age (years) | 15.8 (0.2) | 15.7 (0.2) |
| BMI (kg/m2) | 21.5 (0.5) | 21.5 (0.9) |
| Sum of skinfold thickness (mm) | 39.1 (0.9) | 43.0 (1.1)a |
| Physical activity (h/week) | 15.2 (1.7) | 10.4 (1.7) |
| VO2max (mL/min) | 1,978.9 (48.1) | 1,718.6 (73.5)a |
-
SEM=standard error of the mean; BMI=body mass index; VO2max=maximal oxygen uptake. ap<0.05 for difference between healthy and chronic pain groups.
Psychological characteristics of the participants are presented in Table 2. State anxiety was comparable between groups, although trait anxiety tended to be higher in the chronic pain group compared to healthy girls (p=0.09). Teenage girls in the chronic pain group had more depressive symptoms and less healthy sleep quality compared to the pain-free group (p=0.002 and p=0.003, respectively).
Psychological characteristics.
| Variable | Healthy (n=25) mean (SEM) | Pain (n=16) mean (SEM) |
|---|---|---|
| State anxiety (STAI) | 32.2 (1.3) | 35.4 (1.9) |
| Trait anxiety (STAI) | 35.8 (1.9) | 39.8 (1.4) |
| Depression symptoms (CDI) | 8.8 (0.9) | 14.6 (1.7)a |
| Pain catastrophizing scale | 16.5 (1.8) | 19.9 (2.5) |
| Sleep quality (PSQI) | 4.3 (0.5) | 7.1 (0.8)a |
-
STAI=state and trait anxiety inventory; CDI=children’s depression inventory; PSQI=pittsburgh sleep quality inventory. ap<0.05 for difference between healthy and chronic pain groups.
3.2 Conditioned pain modulation (CPM)
A statistically significant reduction in pain intensity induced by heat pain stimulations performed after the CPT was observed in healthy adolescent girls (3.0±0.2 before CPT vs. 2.2±0.2 after CPT; t=4.0, p=0.0006). On the other hand, teenage girls suffering from chronic pain did not show pain intensity modifications during heat pain stimuli realized after the CPT (3.2±0.3 before CPT vs. 3.0±0.2 after CPT; t=0.8, p=0.4). Pain intensity evaluations for heat pain stimulations completed before the CPT were comparable between healthy and chronic pain adolescents (3.0±0.2 healthy vs. 3.2±0.3 pain; t=0.5, p=0.6). The effectiveness of CPM was greater in healthy participants compared to adolescent females suffering from chronic pain (26.7±8.1 vs. −1.9±9.6; t=2.2, p=0.03, Fig. 1).

Conditioned pain modulation (CPM) effectiveness (%) in healthy and in chronic pain adolescent girls. *p<0.05 for the difference in CPM effectiveness between groups.
3.3 Exercise-induced analgesia (EIA)
In the healthy adolescent girls group, pain intensity induced by the ice cube test significantly decreased after physical exercise (3.5±0.5 before vs. 2.7±0.4 after exercise; t=3.7, p=0.001). However, adolescents suffering from chronic pain did not show a significant reduction in pain intensity during the ice cube tests performed before and after the graded exercise test (3.1±0.7 before vs. 3.1±0.7 after exercise; t=0.2, p=0.9). Pain intensity evaluations for the ice cube test before the graded exercise test were comparable between healthy and chronic pain adolescents (3.5±0.5 healthy vs. 3.1±0.7 pain; t=0.6, p=0.6). The effectiveness of EIA was greater in healthy participants compared to adolescent females suffering from chronic pain (23.8±6.1 healthy vs. −12.0±11.2 pain t=3.1, p=0.004, Fig. 2).
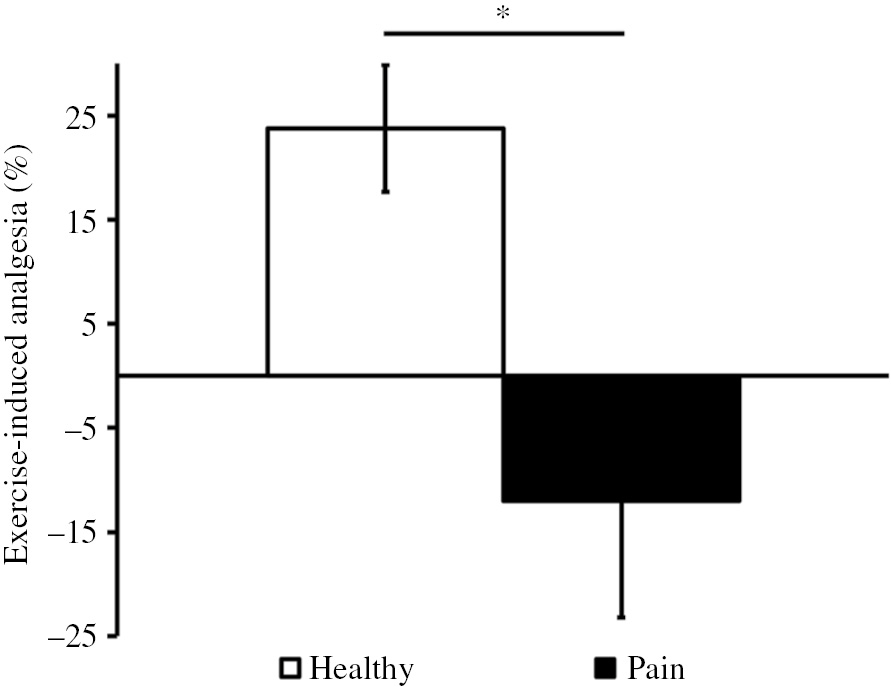
Exercise-induced analgesia (EIA) effectiveness (%) in healthy and in chronic pain adolescent girls. *p<0.05 for the difference in EIA effectiveness between groups.
4 Discussion
The main objective of this study was to evaluate the effectiveness of two endogenous pain inhibitory mechanisms (CPM and EIA) in healthy adolescent girls and in teenage girls suffering from chronic pain. Our results show that pain inhibition resulting from painful cold water immersion (i.e. CPM) or from intense physical activity (i.e. EIA) is lower in adolescents suffering from chronic pain compared to pain-free adolescents. More specifically, pain intensity significantly decreased after conditioning stimuli (CPT) and graded exercise test in healthy adolescent girls, but not in teenagers with chronic pain. This pattern of results suggests that central pain suppressing mechanisms are less effective in adolescent girls with chronic or recurrent pain.
Neuronal circuits that modulate pain are believed to confer protection from acute but, also chronic pain. Therefore, we believe that evaluation of endogenous pain inhibitory mechanisms such as CPM and EIA at a young age could help in the prevention of chronic pain. However, we do not know if endogenous pain inhibitory controls were ineffective prior to the development of chronic pain or if this is a consequence of persistent pain. A longitudinal study evaluating pain inhibitory mechanisms in healthy individuals could help determine the direction of this relationship. Yarnitsky and colleagues observed that adult pain-free patients with reduced CPM effectiveness before a surgical procedure (thoracotomy) had higher risk to develop postoperative chronic pain [15].
Our results confirm previous observations of altered psychological characteristics such as depressive symptoms and anxiety in children and adolescents suffering from chronic pain, even at this younger age [32], [33]. Adolescence is generally considered as a period of heightened stress and turmoil. Stress-induced analgesia, another endogenous pain inhibitory mechanism, should also be investigated in teenagers reporting chronic pain.
We also observed diminished sleep quality in adolescent girls with chronic/recurrent pain. Interestingly, sleep disturbances in adolescents living with chronic pain have been reported [34] and individuals suffering from chronic fatigue syndrome (CFS) have been shown to present hyperalgesia and abnormal pain processing during intense physical activity [35]. More research is needed to investigate the relationship between chronic pain, sleep disturbances and physical activity. The consequences of chronic pain on social activities or school performance were not evaluated in this study, but should be documented in future studies.
We acknowledge that some limits to the present investigations should be considered. The analyses presented were explorative and not of confirmatory nature. Our findings should be replicated in a larger sample. Post-exercise thermoregulatory processes such as peripheral vasodilatation and sweating could have affected cold and pain sensations during the ice cube tests done after the graded exercise test. Furthermore, the graded exercise test causes musculoskeletal pain in most participants. This pain could have triggered CPM effects in some individuals and may have influenced EIA evaluations. Persistent pain could also restrain the ability to perform intense physical activities and influence physical tests performance. The relative absence of pain-inhibitory effects (and potentially increased pain) following physical activities could dissuade teenagers to adopt the guidelines.
In conclusion, our results show that pain-free adolescent girls can effectively trigger endogenous pain inhibitory controls. More precisely, healthy adolescent girls have diminished pain after noxious immersion of the arm (CPM) and also following intense physical activity (EIA). On the other hand, adolescent girls suffering from chronic pain did not report a reduction in pain intensity to standardize experimental pain tests after CPT or intense physical exercise. The effectiveness of CPM and EIA was shown to be reduced in chronic pain compared to pain-free teenage girls. These results suggest that ineffective endogenous pain inhibitory mechanisms may be associated with chronic pain even at an early age.
Acknowledgments
We thank Pr. Tousigant-Laflamme from the École de Réadaptation for lending some experimental material. We thank the respiratory therapists from the Laboratoire de Fonction Respiratoire of CHUS for helping during the graded exercise tests.
-
Authors’ statements
-
Research funding: This study was supported by Dr. Lafrenaye’s research grants from the Foundation of Stars. Dr. Lafrenaye is a supported member of the Centre de Recherche Clinique Étienne-Le Bel du CHUS.
-
Conflict of interest: The authors have no conflicts of interest and no financial relationships relevant to this article to declare.
-
Informed consent: The authors declare that informed content was obtained from all participants of the present study.
-
Ethical approval: This study was approved by the Ethics Committee of the Centre Hospitalier Universitaire de Sherbrooke (CHUS).
References
[1] King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain 2011;152:2729–38.10.1016/j.pain.2011.07.016Search in Google Scholar PubMed
[2] Ramage-Morin PL, Gilmour H. Chronic pain at ages 12 to 44. Health Rep 2010;21:53–61.Search in Google Scholar
[3] Van Dijk A, McGrath P, Pickett W, VanDenKerkhof EG. Pain prevalence in nine- to 13-year-old school children. Pain Res Manag 2006;11:234–40.10.1155/2006/835327Search in Google Scholar PubMed PubMed Central
[4] Huguet A, Miró J. The severity of chronic pediatric pain: an epidemiological study. J Pain 2008;9:226–36.10.1016/j.jpain.2007.10.015Search in Google Scholar PubMed
[5] El-Metwally A, Salminen JJ, Auvinen A, Kautiainen H, Mikkelsson M. Lower limb pain in a preadolescent population: prognosis and risk factors for chronicity – a prospective 1-and 4-year follow-up study. Pediatrics 2005;116:673–81.10.1542/peds.2004-1758Search in Google Scholar PubMed
[6] Ståhl M, Kautiainen H, El-Metwally A, Häkkinen A, Ylinen J, Salminen JJ, Mikkelsson M. Non-specific neck pain in schoolchildren: Prognosis and risk factors for occurrence and persistence. A 4-year follow-up study. Pain 2008;137: 316–22.10.1016/j.pain.2007.09.012Search in Google Scholar PubMed
[7] Pizzo PA, Clark NM. Alleviating suffering 101 – pain relief in the United States. N Engl J Med 2012;366:197–9.10.1056/NEJMp1109084Search in Google Scholar PubMed
[8] Groenewald CB, Palermo TM. The price of pain: the economics of chronic adolescent pain. Pain Manag 2015;5:61–4.10.2217/pmt.14.52Search in Google Scholar PubMed PubMed Central
[9] Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EAM. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur J Pain 2009;13:719–30.10.1016/j.ejpain.2008.07.015Search in Google Scholar PubMed
[10] Gerbershagen HJ, Özgür E, Dagtekin O, Straub K, Hahn M, Heidenreich A, Sabatowski R, Petzke F. Preoperative pain as a risk factor for chronic post-surgical pain – six month follow-up after radical prostatectomy. Eur J Pain 2009;13: 1054–61.10.1016/j.ejpain.2008.11.020Search in Google Scholar PubMed
[11] Gebhart G. Descending modulation of pain. Neurosci Biobehav Rev 2004;27:729–37.10.1016/j.neubiorev.2003.11.008Search in Google Scholar PubMed
[12] Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156: S24–31.10.1097/01.j.pain.0000460343.46847.58Search in Google Scholar PubMed
[13] Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7.10.1097/SPC.0000000000000126Search in Google Scholar PubMed
[14] Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44.10.1016/j.jpain.2012.07.005Search in Google Scholar PubMed
[15] Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–8.10.1016/j.pain.2007.10.033Search in Google Scholar PubMed
[16] Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, Marchand S. Preterm births: can neonatal pain alter the development of endogenous gating systems? Eur J Pain 2008;12:945–51.10.1016/j.ejpain.2008.01.003Search in Google Scholar PubMed
[17] Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents. Pain 2017;158:794–801.10.1097/j.pain.0000000000000817Search in Google Scholar PubMed PubMed Central
[18] Koltyn KF. Analgesia following exercise: a review. Sport Med 2000;29:85–98.10.2165/00007256-200029020-00002Search in Google Scholar PubMed
[19] Baena-Beato PA, Arroyo-Morales M, Delgado-Fernandez M, Gatto-Cardia MC, Artero EG. Effects of different frequencies (2–3 days/week) of aquatic therapy program in adults with chronic low back pain. A non-randomized comparison trial. Pain Med 2013;14:145–58.10.1111/pme.12002Search in Google Scholar PubMed
[20] Stewart MJ, Maher CG, Refshauge KM, Herbert RD, Bogduk N, Nicholas M. Randomized controlled trial of exercise for chronic whiplash-associated disorders. Pain 2007;128:59–68.10.1016/j.pain.2006.08.030Search in Google Scholar PubMed
[21] Jansen MJ, Viechtbauer W, Lenssen AF, Hendriks EJM, de Bie Rob AA. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J Physiother 2011;57:11–20.10.1016/S1836-9553(11)70002-9Search in Google Scholar PubMed
[22] Darling M. Exercise and migraine. A critical review. J Sports Med Phys Fitness 1991;31:294–302.Search in Google Scholar
[23] Häuser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schiltenwolf M, Busch A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79.10.1186/ar3002Search in Google Scholar PubMed PubMed Central
[24] Stolzman S, Bement MH. Does exercise decrease pain via conditioned pain modulation in adolescents? Pediatr Phys Ther 2016;28:470–3.10.1097/PEP.0000000000000312Search in Google Scholar PubMed PubMed Central
[25] Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85.10.1016/j.jpain.2008.12.001Search in Google Scholar PubMed PubMed Central
[26] Rustøen T, Wahl AK, Hanestad BR, Lerdal A, Paul S, Miaskowski C. Gender differences in chronic pain – findings from a population-based study of Norwegian adults. Pain Manag Nurs 2004;5:105–17.10.1016/j.pmn.2004.01.004Search in Google Scholar PubMed
[27] Bailey B, Daoust R, Doyon-Trottier E, Dauphin-Pierre S, Gravel J. Validation and properties of the verbal numeric scale in children with acute pain. Pain 2010;149:216–21.10.1016/j.pain.2009.12.008Search in Google Scholar PubMed
[28] Counil F-P, Varray A, Matecki S, Beurey A, Marchal P, Voisin M, Prefaut C. Training of aerobic and anaerobic fitness in children with asthma. J Pediatr 2003;142:179–84.10.1067/mpd.2003.83Search in Google Scholar PubMed
[29] Counil F-P, Voisin M. Physical fitness in children with asthma. Arch Pediatr 2006;13:1136–41.10.1016/j.arcped.2006.04.004Search in Google Scholar PubMed
[30] Spiro SG. Exercise testing in clinical medicine. Br J Dis Chest 1977;71:145–72.10.1016/0007-0971(77)90106-1Search in Google Scholar PubMed
[31] Schouten WJ, Verschuur R, Kemper HC. Habitual physical activity, strenuous exercise, and salivary immunoglobulin A levels in young adults: the Amsterdam Growth and Health Study. Int J Sports Med 1988;9:289–93.10.1055/s-2007-1025024Search in Google Scholar PubMed
[32] Youssef NN, Atienza K, Langseder AL, Strauss RS. Chronic abdominal pain and depressive symptoms: analysis of the national longitudinal study of adolescent health. Clin Gastroenterol Hepatol 2008;6:329–32.10.1016/j.cgh.2007.12.019Search in Google Scholar PubMed
[33] Varni JW, Rapoff MA, Waldron SA, Gragg RA, Bernstein BH, Lindsley CB. Chronic pain and emotional distress in children and adolescents. J Dev Behav Pediatr 1996;17:154–61.10.1097/00004703-199606000-00003Search in Google Scholar
[34] Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain 2007;23:812–20.10.1097/AJP.0b013e318156ca63Search in Google Scholar PubMed PubMed Central
[35] Meeus M, Hermans L, Ickmans K, Struyf F, Van Cauwenbergh D, Bronckaerts L, De Clerck LS, Moorken G, Grosemans S, Nijs J. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: a double-blind randomized controlled trial. Pain Pract 2015;15:98–106.10.1111/papr.12181Search in Google Scholar PubMed
©2018 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Articles in the same Issue
- Frontmatter
- Editorial comment
- Support for mirror therapy for phantom and stump pain in landmine-injured patients
- Lifting with straight legs and bent spine is not bad for your back
- Bipolar radiofrequency neurotomy for spinal pain – a promising technique but still some steps to go
- Topical review
- Prevalence, localization, perception and management of pain in dance: an overview
- Clinical pain research
- Pain assessment in native and non-native language: difficulties in reporting the affective dimensions of pain
- Colored body images reveal the perceived intensity and distribution of pain in women with breast cancer treated with adjuvant taxanes: a prospective multi-method study of pain experiences
- Physiotherapy pain curricula in Finland: a faculty survey
- Mirror therapy for phantom limb and stump pain: a randomized controlled clinical trial in landmine amputees in Cambodia
- Pain and alcohol: a comparison of two cohorts of 60 year old women and men: findings from the Good Aging in Skåne study
- Prolonged, widespread, disabling musculoskeletal pain of adolescents among referrals to the Pediatric Rheumatology Outpatient Clinic from the Päijät-Häme Hospital District in southern Finland
- Impact of the economic crisis on pain research: a bibliometric analysis of pain research publications from Ireland, Greece, and Portugal between 1997 and 2017
- Measurement of skin conductance responses to evaluate procedural pain in the perioperative setting
- Original experimental
- An observational study of pain self-management strategies and outcomes: does type of pain, age, or gender, matter?
- Fibromyalgia patients and healthy volunteers express difficulties and variability in rating experimental pain: a qualitative study
- Effect of the market withdrawal of dextropropoxyphene on use of other prescribed analgesics
- Observational study
- Winning or not losing? The impact of non-pain goal focus on attentional bias to learned pain signals
- Gabapentin and NMDA receptor antagonists interacts synergistically to alleviate allodynia in two rat models of neuropathic pain
- Offset analgesia is not affected by cold pressor induced analgesia
- Central and peripheral pain sensitization during an ultra-marathon competition
- Reduced endogenous pain inhibition in adolescent girls with chronic pain
- Evaluation of implicit associations between back posture and safety of bending and lifting in people without pain
- Assessment of CPM reliability: quantification of the within-subject reliability of 10 different protocols
- Cerebrospinal fluid cutaneous fistula after neuraxial anesthesia: an effective treatment approach
- Pain in the hand caused by a previously undescribed mechanism with possible relevance for understanding regional pain
- The response to radiofrequency neurotomy of medial branches including a bipolar system for thoracic facet joints
- Letter to the Editor
- Diagnosis of carpal tunnel syndrome – implications for therapy
- Reply to the Letter to the Editor by Ly-Pen and Andréu
- Letter to the Editor regarding “CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer”
- Reply to comments from Ulf Kongsgaard to our study
Articles in the same Issue
- Frontmatter
- Editorial comment
- Support for mirror therapy for phantom and stump pain in landmine-injured patients
- Lifting with straight legs and bent spine is not bad for your back
- Bipolar radiofrequency neurotomy for spinal pain – a promising technique but still some steps to go
- Topical review
- Prevalence, localization, perception and management of pain in dance: an overview
- Clinical pain research
- Pain assessment in native and non-native language: difficulties in reporting the affective dimensions of pain
- Colored body images reveal the perceived intensity and distribution of pain in women with breast cancer treated with adjuvant taxanes: a prospective multi-method study of pain experiences
- Physiotherapy pain curricula in Finland: a faculty survey
- Mirror therapy for phantom limb and stump pain: a randomized controlled clinical trial in landmine amputees in Cambodia
- Pain and alcohol: a comparison of two cohorts of 60 year old women and men: findings from the Good Aging in Skåne study
- Prolonged, widespread, disabling musculoskeletal pain of adolescents among referrals to the Pediatric Rheumatology Outpatient Clinic from the Päijät-Häme Hospital District in southern Finland
- Impact of the economic crisis on pain research: a bibliometric analysis of pain research publications from Ireland, Greece, and Portugal between 1997 and 2017
- Measurement of skin conductance responses to evaluate procedural pain in the perioperative setting
- Original experimental
- An observational study of pain self-management strategies and outcomes: does type of pain, age, or gender, matter?
- Fibromyalgia patients and healthy volunteers express difficulties and variability in rating experimental pain: a qualitative study
- Effect of the market withdrawal of dextropropoxyphene on use of other prescribed analgesics
- Observational study
- Winning or not losing? The impact of non-pain goal focus on attentional bias to learned pain signals
- Gabapentin and NMDA receptor antagonists interacts synergistically to alleviate allodynia in two rat models of neuropathic pain
- Offset analgesia is not affected by cold pressor induced analgesia
- Central and peripheral pain sensitization during an ultra-marathon competition
- Reduced endogenous pain inhibition in adolescent girls with chronic pain
- Evaluation of implicit associations between back posture and safety of bending and lifting in people without pain
- Assessment of CPM reliability: quantification of the within-subject reliability of 10 different protocols
- Cerebrospinal fluid cutaneous fistula after neuraxial anesthesia: an effective treatment approach
- Pain in the hand caused by a previously undescribed mechanism with possible relevance for understanding regional pain
- The response to radiofrequency neurotomy of medial branches including a bipolar system for thoracic facet joints
- Letter to the Editor
- Diagnosis of carpal tunnel syndrome – implications for therapy
- Reply to the Letter to the Editor by Ly-Pen and Andréu
- Letter to the Editor regarding “CT guided neurolytic blockade of the coeliac plexus in patients with advanced and intractably painful pancreatic cancer”
- Reply to comments from Ulf Kongsgaard to our study

