Abstract
The methylammonium iodide lead chloride-reduced graphene oxide (MAI:PbCl2-rGO) was prepared via the dipping deposition. In this work, the rGO composited in MAI:PbCl2 was introduced with different weight percents (wt%) between 7 and 10 wt%. All samples have been investigated via Fourier transform infrared, ultraviolet−visible spectrophotometer spectroscopy, scanning electron microscopy, X-ray diffraction (XRD), and the Hall effect to illustrate optical properties, morphological, and electrical properties. The XRD analysis result revealed that the mean grain size of MAI:PbCl2 is 27.46 nm, whereas a suitable crystallite size of 38.07 nm (and has a uniformity crystallite) is the case of the MAI:PbCl2-8wt%rGO obtained, leading to the lowest micro-strain of 0.15. Besides, the activation energy determined from the nature logarithm of conductivity and 1,000/T by using the temperature-dependent electrical transport data was displayed in a temperature range of 298–323 K. It was found that MAI:PbCl2-8 wt% rGO decreases the activation energy to 0.26 eV as well as yields the maximum conductivity of 72.55 S/cm. In this way, adding suitable rGO to MAI:PbCl2 is key to obtaining higher conductivity, which is used to ascertain and describe the nature of the semiconductor material.
1 Introduction
A photovoltaic cell (called a solar cell) can convert sunlight into electricity. The common solar device can produce an electrical current when an electric field is put into a circuit to sweep carriers out, and a semiconductor is used as an element in a solar device. A semiconductor material can absorb phonon energy from light. In this way, halide perovskite is widely studied and used as one of the semiconductor materials because it includes low production costs and has a high potential in perovskite solar cells (PSCs) whose electrical characteristics – such as a higher voltage. Also, PSCs have shown a rapid increase in higher power conversion efficiency (PCE), which has jumped from 3.8% in 2009 to 26.7% in the present [1,2,3,4]. Hence, the PSC is a promising candidate for the next generation of solar cells.
Halide perovskite materials are important absorption layers in a PSC, which has the general formula ABX3, where A is a monovalent cation (like methylammonium [CH3NH3 or MA], formamidinium [FA], or cesium [Cs]), B is a divalent metal (like lead [Pb], tin [Sn], and other rare metal), and X is a halide anion (like chloride [Cl], bromide [Br], and iodide [I]) [5,6,7]. The general ABX3 compounds as either methyl ammonium lead iodide (MAPbI3) or other mixed halides in the site of X like MAPbI3−x Cl x and MAPbI3−x Br x , where x subscript is between 1 and 3, are a primary perovskite structure [8,9,10]. Based on this, the cubic crystal structure of perovskite can adjust band gaps from approximately 1.3–2.9 eV [11,12]. In this work, lead chloride (PbCl2) is introduced because of Cl, which can improve the charge transport and increase the stability of perovskite when compared with I or Br, leading to boost PSC performance [13,14]. However, Kaiser et al. reported that residual Cl may have emerged from the difference ionic radii of Cl and I, leading to the defect in perovskite growth, which this solution has been mixed between PbCl2 with methylammonium iodide (CH3NH3I, MAI) in dimethylformamide (DMF) [15,16].
To address the above issue, reduced graphene oxide (rGO) is incorporated into a perovskite film. The addition of rGO aims to improve the performance of perovskite film growth and to improve charge transport behavior in perovskite compared to without rGO incorporation [17,18,19]. Kumaresan et al. explored the improvement of the performance of oxygen evolution reaction and supercapacitor with the combination of rGO and CeNiO3 (rGO/CeNiO3) perovskite [20]. Duan et al. improved the stability of photodetectors that were doped with SiW9Co3@, indicating that polyoxometalate-based composites have good application prospects for solar devices [21]. Kumar et al. demonstrated that the rGO nanomaterial can prove electronic properties after it is doped in CsSnBr3 perovskite, finding that the solar cell device boosts PCE from 3 to 5.27% [22]. Marchezi et al. exhibited that larger grains have affected the rate of degradation by adding the composition of rGO into CsxFA1−x Pb(Br y I1−y )3 [23]. Understanding the rGO performance via investigating the effect of rGO composited into the halide perovskite thin films was illustrated. Hereinafter, MAI:PbCl2 acts as a halide perovskite, also MAI:PbCl2-rGO composites were studied with four different weight percentages (wt%) of rGO are 7 wt% rGO (7-rGO), 8 wt% rGO (8-rGO), 9 wt% rGO (9-rGO), and 10 wt% rGO (10-rGO), which were deposited on the fluorine-doped tin oxide (FTO) substrate via the dipping process (Figure 1). In this way, this work elucidated the physical–chemical, optical properties, and structural properties of perovskite thin films, including the electrical properties due to the influence of rGO on the MAI:PbCl2 thin film.

The thin film deposition via the dipping method.
2 Experiment
2.1 Materials and methodst
rGO nanopowder was incorporated into the 1M MAI (purity 99.99%):1M PbCl2 (purity ≥98%), which dissolved in DMF to form the MAI:PbCl2 – rGO film on the fluorine-doped tin oxide (FTO, 15 Ω/cm2) glass substrate by the dip coating method in the air ambient for 90 s. Afterward, this film was annealed on the hotplate at 120°C for 10 min. In this work, the four different concentrations of rGO were considered (7-rGO, 8-rGO, 9-rGO, and 10-rGO).
2.2 Characterization
All samples were examined for the functional groups in the materials using a Fourier-transform infrared spectrum (FTIR), PerkinElmer Scientific Spectrometer). The optical properties of each thin film were presented using an ultraviolet−visible spectrophotometer (UV−vis spectrophotometer, Genesys 50/150/180). Observation of surface morphology and calculation of the chemical composition of materials are characterized by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS), respectively. Moreover, grazing incidence X-ray diffraction (GIXRD) and grain size of the MAI:PbCl2, rGO, and MAI:PbCl2–rGO film have been calculated using parameters from X-ray diffraction (XRD, the Rigaku Miniflex600), which the radiation source (Cu Kλ radiation, λ = 1.54 Å) was performed the range of 10° to 60°. A sample size of 1 × 1 cm2 was examined for electrical properties using a Hall effect (Linseis HCS 1).
3 Results and discussion
FTIR spectra are used to indicate molecular compounds and analyze characteristics of functional groups of rGO, MAI:PbCl2, and MAI:PbCl2–rGO. The FTIR spectrum displays the characteristics of the peak areas that have been recorded in the vibration range of 500–4,000 cm−1 (illustrated in Figure 2(a)). The observed wavenumber peak at 883 cm−1 is related to the bending of the CH3NH3 + rock in MAI:PbCl2 [24,25,26]. Besides, seven peaks on the FTIR pattern belong to MAI:PbCl2, observed (1) at 899 cm−1 where Pb–I–NH stretching is found, (2) at 1,136 cm−1 is identified as the C–H coordination bond, (3) at 1,446 and (4) 1,600 cm−1 were assigned to the formation of N–H stretch, around (5) 1,950 and (6) 2,200 cm−1 are related to C═O bonding and (7) approximately 3,800 cm−1 is O–H bonding [27,28,29].
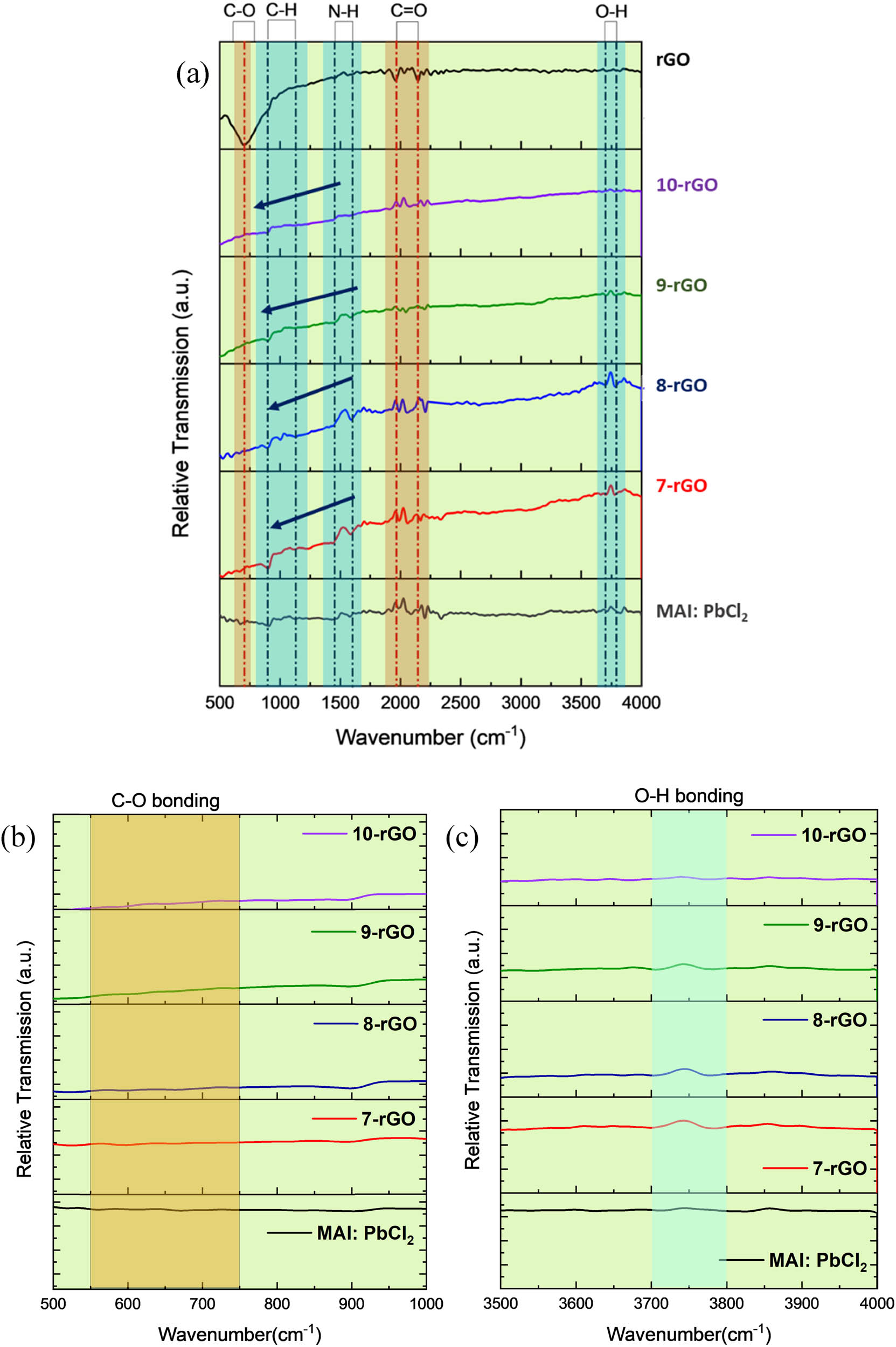
Typical FTIR spectra in the range of (a) 500–4,000 cm−1, (b) 500–1,000 cm−1, and (c) 3,500–4,000 cm−1 for rGO, MAI:PbCl2, and MAI:PbCl2–rGO composites, respectively.
Moreover, the intensity of the FTIR patterns demonstrated a downward trend in the range of around 500–1,500 cm−1, which fluctuated with the influence of the rGO composition on the MAI:PbCl2. Incidentally, a broad peak in the range of 600 cm−1 exhibited the FTIR spectrum of rGO, which can be indicated as C–O stretching vibration [30,31]. Interestingly, the oxygen-containing group of MAI:PbCl2–rGO almost disappears in MAI:PbCl2, confirming the existence of rGO, which is the spectrum approximately ranging from 600 and 3,800 cm−1. The influence of oxygen in moisture during perovskite fabrication under the ambient air has depicted a defect in driving the mass transportation of charge and leads to poor mechanical stability. Due to the oxygen in the air at the moisture of 60% ± 8% is from water vapor accelerating degradation processes, significantly impacting performance and lifetime. [32,33]. Thus, the peaks of O–H bonding and C–O bonding have appeared on the FTIR pattern, determining that this bonding is related to oxygen which is a part of water vapor. For this reason, the peak intensity of C–O and O–H bonding on the FTIR result shows the highest intensity for a pristine-MAI:PbCl2 and the lowest intensity for 8-rGO in Figure 2(b) and (c), respectively. Also, the incorporation of rGO into MAI:PbCl2 might be useful for the reduction of oxygen, meaning that the film has longer stability, leading to prevent carriers’ recombination [34].
Furthermore, the rGO incorporation in MAI:PbCl2 has affected reducing the electronic bandgap
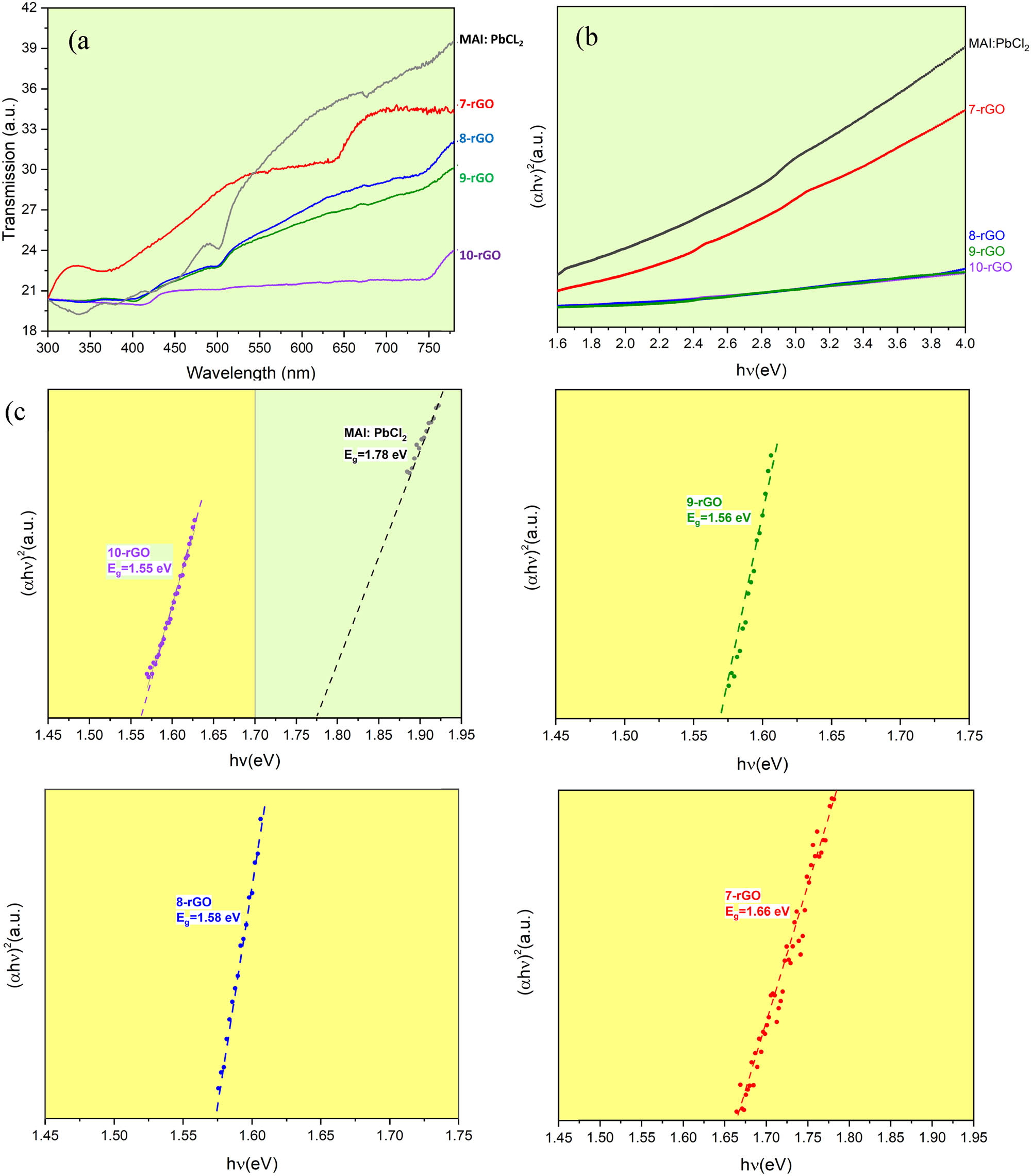
(a) The UV−vis transmission spectra of the pure MAI:PbCl2 and the composited MAI:PbCl2-rGO films deposited by a dip-coating process. (b) Tauc plots of the MAI:PbCl2 perovskite with different rGO content and (c) the electronic bandgap
The knee wavelength is related to the
where α corresponds to the absorption coefficient, and A′ is the constant [40]. MAI:PbCl2, 7-rGO, 8-rGO, 9-rGO, and 10-rGO have the
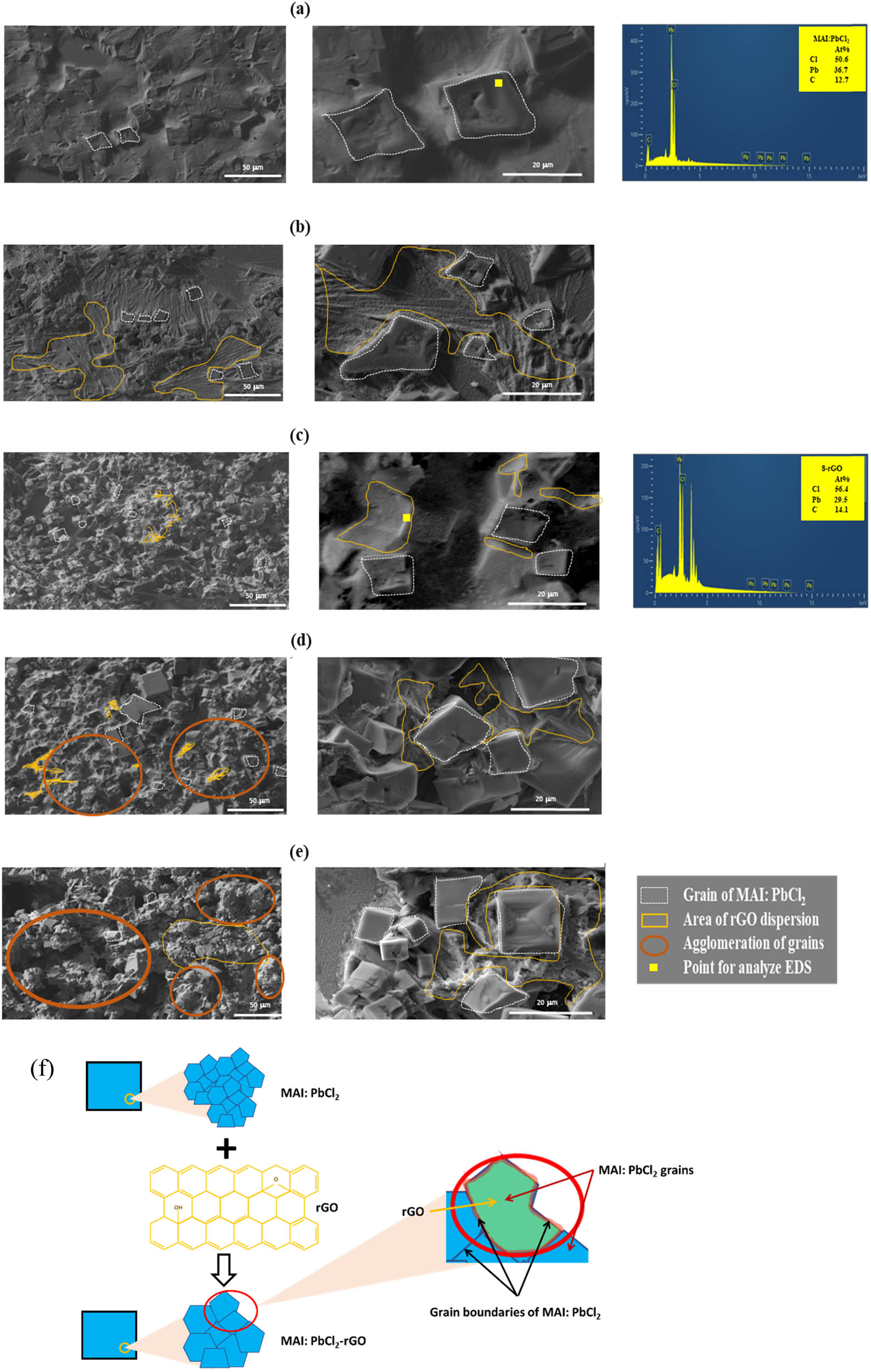
SEM profile of (a) a pure MAI:PbCl2 and MAI:PbCl2-rGO prepared by different wt% rGO such as (b) 7-rGO, (c) 8-rGO, (d) 9-rGO, and (e) 10-rGO. (f) The larger grain growth mechanism of MAI:PbCl2-rGO from the small grain size of a pure MAI:PbCl2.
The grain size was analyzed using an XRD pattern. Identify the MAI:PbCl2 peaks emerged at 15.76, 28.55, 31.62, and 40.75°, respectively, which can be associated with (110), (220), (310), and (400) diffractions, as shown in Figure 5(a) [43,44]. The calculation of the grain size is used to compare differences after increasing rGO content, using the Scherrer formula given in the following equation:
where G is defined as the grain size, FWHM is the full-width at half maximum in radians, λ is the X-ray wavelength (λ Cu Kα = 0.15405 nm), and θ is the diffraction angle of the XRD peak [45]. As mentioned above, the XRD pattern of each sample has been explored at 4 locations, results from the calculations obtained the average grain size of 28.36 nm for 7-rGO, 38.07 nm for 8-rGO, 49.78 nm for 9-rGO, and 35.12 nm for 10-rGO, whereas the average grain size of a pure MAI: PbCl2 was 27.46 nm. The present work finds the evolution of the grain size and shift of the electrical bandgap with rGO doping in MAI:PbCl2 thin films prepared by the dip-coating method. Hence, when rGO is composited in MAI:PbCl2 has led to a larger grain size, and the electric band gap decreases owing to the quantum confinement effect. Furthermore, the observed effect of MAI:PbCl2 anchored with rGO on the XRD pattern has revealed an inhomogeneous broadening of the peak at approximately 16° and 32° as depicted in Figure 5(b) and (c), respectively. The peak location of 9-rGO and 10-rGO shifted to higher 2θ, resulting from the disorder of the perovskite structure. This led to a changed band gap energy of MAI:PbCl2, which depends on the different incorporation with ratios of rGO content [46]. Moreover, the larger grain size and also uniformity of perovskite crystals are typically useful in semiconducting perovskite materials due to the expectation of decreasing the recombination defect, which results in easier charge transfer [47,48]. Nevertheless, a larger grain size is not enough to explain the effect of rGO on the MAI:PbCl2 film. Therefore, the incorporation of rGO nanomaterial into MAI:PbCl2 was further elucidated to gain insight into defects that about with the deformation of the lattice or disorder of the perovskite structure, which can be demonstrated with the presentation of high micro-strain (ε) value [49,50]. Changed perovskite structure causes the micro-strain in the lattice, leading to changes in the perovskite properties. Thus, the micro-strain value has been used to describe the physical property of perovskite semiconductors, illustrating and indicating that the micro-strain has changed after modulating the perovskite properties with the addition of rGO content [51,52].
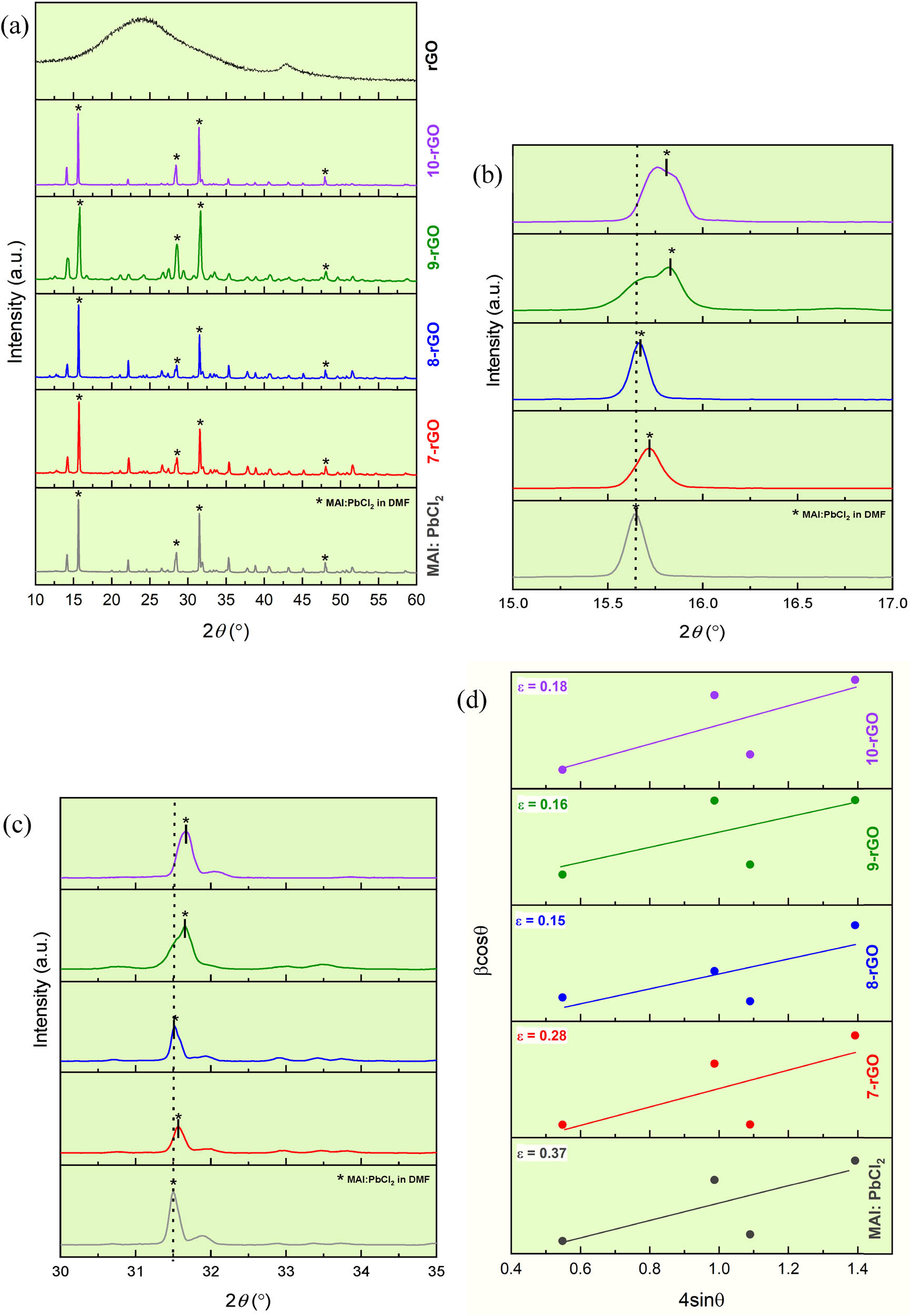
XRD patterns rGO, MAI:PbCl2, MAI:PbCl2-rGO films (a) in the range of (a) 10°–60°, at approximately (b) 16° and (c) 32° (* is for MAI:PbCl2 perovskite structure). (d) The micro-strain of MAI:PbCl2, and MAI:PbCl2-rGO.
To monitor the micro-strain, a theoretical formula of Williamson-Hall was used to assess the corresponding in-plane residual stress in MAI:PbCl2 perovskite via grazing incidence GIXRD measurements, according to the following equation:
where G represents the grain size, β is the full width at half maximum in terms of radian, K is the Scherrer constant, and C is a constant [53,54]. The calculated micro-strain is between 0.32 (MAI:PbCl2) and 0.15 (8-rGO), observing the micro-strain significantly decreases as grain size increases, especially that of 8-rGO. The different micro-strains are derived from the XRD data to plot as a linear equation, then calculated as slope, and the reason for this change occurred when rGO content was added, as depicted in Figure 5(d) and tabulated in Table 1.
Refinement data for composited MAI:PbCl2 with rGO and a pure MAI:PbCl2 based on the XRD pattern
| Sample | Grain size (nm) | Micro strain |
|---|---|---|
| 10-rGO | 35.96 ± 19.62 | 0.18 |
| 9-rGO | 49.78 ± 21.83 | 0.16 |
| 8-rGO | 38.98 ± 10.89 | 0.15 |
| 7-rGO | 29.04 ± 12.50 | 0.28 |
| MAI:PbCl2 | 28.12 ± 12.06 | 0.32 |
Found that the perovskite films have a significant residual micro-strain, revealing inhomogeneity or roughness in the rGO-composed perovskite thin film, which is expressed and confirmed with the SEM image and the largest micro-strain (ε = 0.28 for rGO content of 7 wt%). Due to changes in the morphological results, both the top-view and calculation of grain size depicted the disorder in the perovskite structure and found that the micro-strain was reduced after adding rGO into MAI:PbCl2. A decrease in the micro-strain indicated that the suitable rGO content not only maintained the structure of MAI:PbCl2, which is a cubic crystal perovskite structure, but also acted as intermediator to easier drive charge carriers.
Hence, the micro-strain value is worth noting because it might describe the effect of the carrier mobilities. However, these calculations of a micro-strain may not accurately guarantee how the micro-strain responds to improving charge mobility. Therefore, the electrical properties using Hall-effect measurement were used to clarify the effect of rGO incorporation into MAI:PbCl2. To investigate the MAI:PbCl2 and MAI:PbCl2-rGO electrical properties, all samples were analyzed by increasing temperature from room temperature to 323 K, as well-known temperature-dependence (in Figure 6). The conductivity (σ) of 8-rGO is higher (72.55 S/cm) than a pure MAI:PbCl2 (0.18 S/cm), while another rGO contents like 28.59 S/cm for 7-rGO, 55.30 S/cm for 9-rGO, and 47.12 S/cm for 10-rGO. A maximum σ value of each sample was compared at 303 K. Remarkably, the highest σ value of 8-rGO was attributable to electrical parameters, both the lowest sheet resistance (R sh) and the higher carrier concentration (µ), as the equation: R sh = pL/A = qnµA/L (where q is the electron charge in the unite of C/cm2, and n is the mobility in the unit of cm2/Vs), as depicted in Figures 6(a)–(c) and as listed in Table 2. In this way, conductivity (σ) is defined as the reciprocal of resistivity (ρ), that is σ = ρ = L/AR; R = ρ(L/A), where R is denoted as resistance, L is the length of the sample, and A is the area of the sample. Therefore, the defect on the MAI:PbCl2 has been solved, such as the small grain size and the disorder of crystal structure, which led to blocking charge drive and increasing the opportunity of recombination of electron–hole pairs.
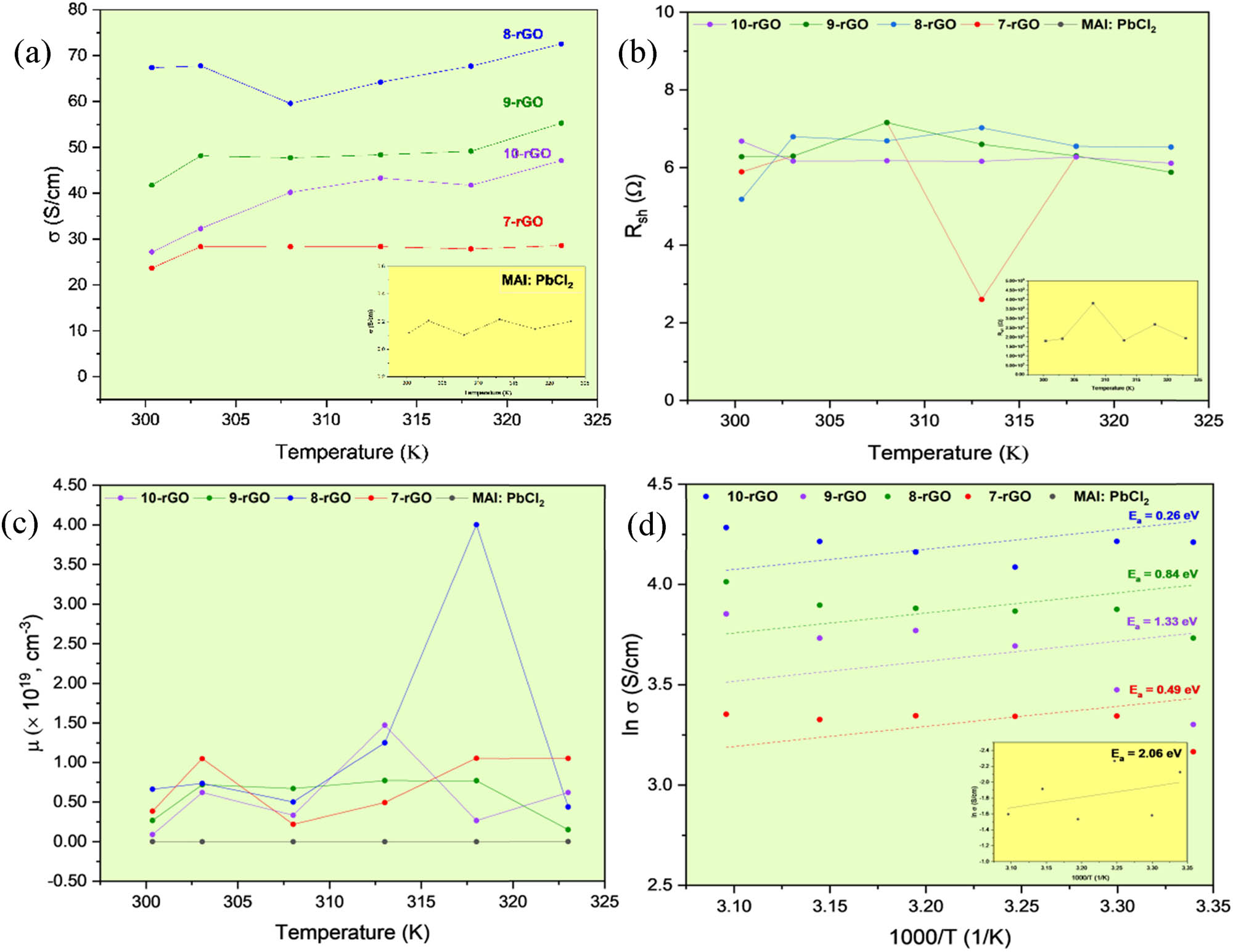
Temperature dependence of (a) the electrical conductivity (σ), (b) the sheet resistance (R
sh), (c) the concentration (μ), and (d) natural logarithm of electrical conductivity vs 1,000/T of rGO, MAI:PbCl2, and MAI:PbCl2-rGO samples before and after composite. The inset of 6(a), (b), and (d) illustrate the σ, R
sh, and
The electrical parameters for a pure MAI:PbCl2 and MAI:PbCl2-rGO
| Sample | Electrical characteristics | |||
|---|---|---|---|---|
| σ (S/cm) | R sh (Ω) | μ × 1019 (cm−3) | E a (eV) | |
| 10-rGO | 38.64 ± 7.46 (47.12) | 5.69 ± 1.58 (2.61) | 0.57 ± 0.05 (1.47) | 1.33 ± 0.16 |
| 9-rGO | 48.45 ± 4.30 (55.30) | 6.46 ± 0.65 (5.18) | 0.56 ± 0.28 (0.77) | 0.84 ± 0.76 |
| 8-rGO | 66.54 ± 4.32 (72.55) | 6.42 ± 0.43 (5.88) | 1.26 ± 0.14 (4.00) | 0.26 ± 0.13 |
| 7-rGO | 27.52 ± 1.88 (28.59) | 6.26 ± 0.21 (6.11) | 0.71 ± 0.04 (1.05) | 0.49 ± 0.39 |
| MAI:PbCl2 | 0.17 ± 0.05 (0.20) | 23.23 × 102 ± 7.90 × 102 (17.98 × 102) | 2.10 × 10−4 ± 0.02 × 10−4 (1.24 × 10−3) | 2.06 ± 0.85 |
Hence, the preparing rGO composite in MAI:PbCl2 has been used to improve the performance of MAI:PbCl2 thin film due to the above all results showing enhancing crystallite size, improving uniformity of the thin film, decreasing the micro-strain (ε), and declining electron–hole pairs phenomenon (as mean higher conductivity) [55,56].
To confirm the effect of rGO in MAI:PbCl2 with a second way of electrical properties, the increased temperature was introduced to elucidate the relation between conductivity and temperature [57]. This strategy could analyze reaction mechanisms using the Arrhenius equation defined by
where k is the dependence rate on the absolute temperature (T) in Kevin (K), R is the constant of molar gas, and the differences for each reaction are defined as
Hence, the kinetic value for parameters
The highest conductivity value (σ) is associated with the lowest active energy (
Typical, rGO stand-alone was a bulk material that could be easily dispersed and can readily composite with materials like perovskite semiconductors [61]. On the other hand, higher doping rGO levels not only increase agglomeration of rGO contents on MAI:PbCl2 or rGO might cover all MAI:PbCl2 surface, which might affect skew optical properties or reveal disorder MAI:PbCl2 structure, but also decrease driven charges in grains and at grain boundaries.
This mention was supported by the result of a top-view film surface using the SEM technique and degrading electrical performance in terms of 10-rGO via Hall-effect measurement, thus, high doping rGO was not selected for study in this work.
From the experimental, the highest σ value of 8-rGO was achieved when compared to the MAI:PbCl2 doped with other rGO contents, while a pure MAI:PbCl2 was obtained of σ = 0.18 S/cm and
4 Conclusion
In summary, the fabricated MAI:PbCl2 thin film was doped with different weight percentages (wt%) of rGO content (7, 8, 9, and 10 wt%). The results of the optical band gap changed from 1.77 eV for pure MAI:PbCl2 to 1.56 eV for 10-rGO, meaning a significant redshift in the transmission graph; it was found that the electronic bandgap declined linearly with the increase of rGO contents. The rGO addition can not only decrease the bandgaps but also enhance the grain size of MAI:PbCl2. Besides, grain size is one of the most important parameters in micro-strain calculation. These parameters are used to express the performance of each specimen. In this work, the optimum case for MAI:PbCl2 incorporation with rGO, that is, the 8-rGO, which has been demonstrated to have uniformity of perovskite crystal and a larger grain size, led to the lowest micro-strain of 0.16 eV. Furthermore, 8-rGO has the highest conductivity of 72.55 S/cm, while the pure material MAI:PbCl2 has a conductivity of 0.20 S/cm. Another reason for the higher conductivity of MAI:PbCl2 is the lower activation energy for rGO. Thus, improved conductivity via a suitable composition of rGO content into MAI:PbCl2 (8-rGO) can be a strategy to enhance the performance of organic halide perovskite MAI:PbCl2.
-
Funding information: The author would like to express sincere gratitude to Xi’an International University (XAIU), China, for providing financial support through the Initiation Funds for High-level Talents Program of Xi’an International University (grant no. XAIU202521), which enabled the successful completion of this research.
-
Author contribution: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: Author states no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc. 2009 Apr;131(17):6050–1. 10.1021/ja809598r.Search in Google Scholar PubMed
[2] Ubani CA, Ibrahim MA, Teridi MAM. Moving into the domain of perovskite sensitized solar cell. Renewable Sustainable Energy Rev. 2017;72:907–15. 10.1016/j.rser.2017.01.025.Search in Google Scholar
[3] Liu C, Yang Y, Chen H, Xu J, Liu A, Bati ASR, et al. Bimolecularly passivated interface enables efficient and stable inverted perovskite solar cells. Science. 2023;382(6672):810–5. adk1633.Search in Google Scholar
[4] Zheng Y, Li Y, Zhuang R, Wu X, Tian C, Sun A, et al. Towards 26% efficiency in inverted perovskite solar cells via interfacial flipped band bending and suppressed deep-level traps. Energy Environ Sci. 2024;17(3):1153–62. 10.1039/D3EE03435F.Search in Google Scholar
[5] Roy P, Sinha NK, Tiwari S, Khare A. A review on perovskite solar cells: Evolution of architecture, fabrication techniques, commercialization issues and status. Solar Energy. 2020;198:665–88. 10.1016/j.solener.2020.01.080.Search in Google Scholar
[6] Park NG. Halide perovskite photovoltaics: History, progress, and perspectives. MRS Bulletin. 2018;43:527–33. 10.1557/mrs.2018.152.Search in Google Scholar
[7] Correa-Baena JP, Saliba M, Buonassisi T, Graetzel M, Abate A, Tress W, et al. Promises and challenges of perovskite solar cells. Science. 2017;358(6364):739–44. 10.1126/science.aam6323.Search in Google Scholar PubMed
[8] Grätzel M. The light and shade of perovskite solar cells. Nature Mater. 2014;13:838–42. 10.1038/nmat4065.Search in Google Scholar PubMed
[9] Baranowski M, Plochocka P. Excitons in metal-halide perovskites. Adv Energy Mater. 2020;10(26):1903659. 10.1002/aenm.201903659.Search in Google Scholar
[10] Kenichiro T, Takayuki T, Takuma B, Takashi K, Kazuhito U, Noboru M. Comparative study on the excitons in lead-halide-based perovskite-type crystals CH3NH3PbBr3 CH3NH3PbI3. Solid State Commun. 2003;127(9–10):619–23. 10.1016/S0038-1098(03)00566-0.Search in Google Scholar
[11] Stoumpos CC, Kanatzidis MG. The renaissance of halide perovskites and their evolution as emerging semiconductors. Acc Chem Res. 2015;48(10):2791–802. 10.1021/acs.accounts.5b00229.Search in Google Scholar PubMed
[12] Green MA. Solar cell fill factors: general graph and empirical expressions. Solid-State Electron. 1981;24:788–9. 10.1016/0038-1101(81)90062-9.Search in Google Scholar
[13] Tanveer Karim AMM, Khan MKR, Hossain MS. Temperature dependency of excitonic efective mass and charge carrier conduction mechanism in CH3NH3PbI3−xClx thin flms. Sci Rep. 2021;11(1):10772. 10.1038/s41598-021-90247-x.Search in Google Scholar PubMed PubMed Central
[14] Xue L, Guo Y, Lu S, Li R, Chen J. Advances in chloride additives for high-efficiency perovskite solar cells: multiple points of view. 2023;59:13394–405. 10.1039/D3CC04177H.Search in Google Scholar
[15] Kaiser W, Radicchi E, Mosconi E, Kachmar A, De Angelis F. Iodide vs chloride: The impact of different lead halides on the solution chemistry of perovskite precursors. ACS Appl Energy Mater. 2021;4(9):9827–35. 10.1021/acsaem.1c01874.Search in Google Scholar
[16] Pool VL, Gold-Parker A, McGehee MD, Toney MF. Chlorine in PbCl2-derived hybrid-perovskite solar absorbers. Chem Mater. 2015;27:7240–3. 10.1021/ACS.CHEMMATER.5B03581.Search in Google Scholar
[17] Manikandan M, Prasankumar T, Manikandan E, Papanasam E, Ramesh K, Ramesh S. Hydrothermal synthesis of rGO and MnCoS composite for enhanced supercapacitor application. Sci Rep. 2024;14:25596. 10.1038/s41598-024-77245-5.Search in Google Scholar PubMed PubMed Central
[18] Han X, Xuan H, Gao J, Liang T, Yang J, Xu Y, et al. Construction of manganese-cobalt-sulfide anchored onto rGO/Ni foam with a high capacity for hybrid supercapacitors. 2018;288:31–41. 10.1016/j.electacta.2018.08.063.Search in Google Scholar
[19] Li Z, Ji X, Han J, Hu Y, Guo R. NiCo2S4 nanoparticles anchored on reduced graphene oxide sheets: In-situ synthesis and enhanced capacitive performance. J Colloid Interface Sci. 2016;477:46–53. 10.1016/j.jcis.2016.05.038.Search in Google Scholar PubMed
[20] Kumaresan L, Palanisamy G, Lee J. Cost-effective synthesis of rGO/CeNiO3 perovskite nanocomposites for enhanced and stable supercapacitors and oxygen evolution reaction catalysts. J Mater Chem C. 2024;12(41):16916–34. 10.1039/D4TC03159H.Search in Google Scholar
[21] Duan S, Peng Y, Guan H, Chen W. SiW9Co3@rGO composite–doping improved the crystallization and stability of a perovskite film for efficient photodetection. Dalton Trans. 2024;53(12):5407–15. 10.1039/D3DT04214F.Search in Google Scholar PubMed
[22] Kumar A, Pandey N, Punetha D, Saha R, Chakrabarti S. Enhancement in the structural and optical properties after incorporation of reduced graphene oxide (rGO) nanocomposite in pristine CsSnBr3 for solar cell application. ACS Appl Electron Mater. 2023;5(6):3144–53. 10.1021/acsaelm.3c00224.Search in Google Scholar
[23] Marchezi PE, Therézio EM, Szostak R, Loureiro HC, Bruening K, Gold-Parker A, et al. Degradation mechanisms in mixed-cation and mixed-halide CsxFA1−xPb(BryI1−y)3 perovskite films under ambient conditions. J Mater Chem. 2020;8:9302–12. 10.1039/d0ta01201g.Search in Google Scholar
[24] Szostak R, Silva JC, Turren-Cruz SH, Soares MM, Freitas RO, Hagfeldt A, et al. Nanoscale mapping of chemical composition in organic-inorganic hybrid perovskite films. Sci Adv. 2019;5(10):eaaw6619. 10.1126/sciadv.aaw6619.Search in Google Scholar PubMed PubMed Central
[25] PatelRebecca JB, MilotAdam L, WrightLaura D, HerzMichael M, Johnston B. Formation dynamics of CH3NH3PbI3 perovskite following two-step layer deposition. J Phys Chem Lett. 2016;7(1):96–102. 10.1021/acs.jpclett.5b02495.Search in Google Scholar PubMed
[26] Pérez-Osorio MA, Milot RL, Filip MR, Patel JB, Herz LM, Johnston MB, et al. Vibrational properties of the organic–inorganic halide perovskite CH3NH3PbI3 from theory and experiment: Factor group analysis, first-principles calculations, and low-temperature infrared spectra. J Phys Chem C. 2015;119(46):25703–18. 10.1021/acs.jpcc.5b07432.Search in Google Scholar
[27] Yerezhep D, Omarova Z, Aldiyarov A, Shinbayeva A, Tokmoldin NIR. Spectroscopic degradation study of thin organometal halide perovskite films. Molecules. 2023;28(3):1288. 10.3390/molecules28031288.Search in Google Scholar PubMed PubMed Central
[28] Laila IKR, Mufti N, Maryam S, Fuad A, Taufiq A. Synthesis and characterization of ZnO nanorods by hydrothermal methods and its application on perovskite solar cells. J Phys Conf Ser. 2018;1093:012012. 10.1088/1742-6596/1093/1/012012.Search in Google Scholar
[29] Arobi N, Zumahi SMAA, Ibrahim K, Rahman MM, Hossain MK, Bhuiyan MMR, et al. A holistic framework towards understanding the optical and dielectric behaviors of CH3NH3PbCl3 perovskites/graphene oxide hybrid films for light absorbing active layer. J Solid State Chem. 2021;298:122137. 10.1016/j.jssc.2021.122137.Search in Google Scholar
[30] Zamani A, Sadjadi MS, Mahjoub A, Youse M, Farhadyar N. Synthesis, characterization and investigation of photocatalytic activity of ZnFe2O4@MnO–GO and ZnFe2O4@MnO–rGO nanocomposites for degradation of dye Congo red from wastewater under visible light irradiation. Res Chem Intermed. 2020;46:33–61. 10.1007/s11164-019-03934-w.Search in Google Scholar
[31] Krishnamoorthy K, Kim GS, Kim SJ. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason Sonochem. 2013;20(2):644–9. 10.1016/j.ultsonch.2012.09.007.Search in Google Scholar PubMed
[32] Liu K, Luo Y, Jin Y, Liu T, Liang Y, Liu Y, et al. Moisture-triggered fast crystallization enables efficient and stable perovskite solar cells. Nat Commun. 2022;13:4891. 10.1038/s41467-022-32482-y.Search in Google Scholar PubMed PubMed Central
[33] Li Z, Jia C, Cao J, Shi J, Xue J, Liu X, et al. Boosting mechanical durability under high humidity by bioinspired multisite polymer for high-efficiency flexible perovskite solar cells. Nat Commun. 2025;16(1):1771. 10.1038/s41467-025-57102-3.Search in Google Scholar PubMed PubMed Central
[34] Kumar SRA, Mary DV, Josephine GAS, Ahamed MAR. Graphene/GO/rGO based nanocomposites: Emerging energy and environmental application– review. Hybrid Adv. 2024;5:100168. 10.1016/j.hybadv.2024.100168.Search in Google Scholar
[35] Gard FS. Desarrollo de hardware y software de un sistema de espectroscopia de reflectancia difusa (DRS) para Medir la temperatura del sustrato semiconductor in-situ. Revista Tecnología Y Ciencia. 2021;41:118–34. 10.33414/rtyc.41.118-134.Search in Google Scholar
[36] Lizarraga K, Enrique-Morán LA, Tejada A, Piñeiro M, Llontop P, Serquen E, et al. New optical dispersion models for the accurate description of the electrical permittivity in direct and indirect semiconductors. J Phys D: Appl Phys. 2023;56(36):365106. 10.1088/1361-6463/acd859.Search in Google Scholar
[37] Dangi S, Khichar K, Kumar U, Hashmi SZ, Choudhary B, Kumar S, et al. Investigation of optical properties of PVA-GO nanocomposites. AIP Conf Proc. 2020;2220:020082. 10.1063/5.0001129.Search in Google Scholar
[38] Shan F, Kim B, Liu G, Liu Z, Sohn J, Lee W, et al. Blueshift of near band edge emission in Mg doped ZnO thin films and aging. J Appl Phys. 2004;95(9):4772–6. 10.1063/1.1690091.Search in Google Scholar
[39] Chamberlin PM, Adu KW. Determining bandgap from simulated absorbance: The Tauc approach. MRS Adv. 2024;9:1031–6. 10.1557/s43580-024-00889-y.Search in Google Scholar
[40] Wang Y, Xiu J, Gan T, Zou H. Photocatalytic degradation of tetracycline hydrochloride by lanthanum doped TiO2@g-C3N4 activated persulfate under visible light irradiation. RSC Adv. 2023;13(12):8383–93. 10.1039/D3RA00729D.Search in Google Scholar
[41] Fang Z, Deng B, Jin Y, Yang L, Chen L, Zhong Y, et al. Surface reconstruction of wide-bandgap perovskites enables efficient perovskite/silicon tandem solar cells. Nat Commun. 2024;15(1):1–11. 10.1038/s41467-024-54925-4.Search in Google Scholar PubMed PubMed Central
[42] Ni Z, Bao C, Liu Y, Jiang Q, Wu WQ, Chen S, et al. Resolving spatial and energetic distributions of trap states in metal halide perovskite solar cells. Science. 2020;367:1352–8. 10.1126/science.aba0893.Search in Google Scholar PubMed
[43] Kyong S, Kim P, Ko S, So H, Ri J, Ryu K. Effect of polystyrene treatment on the efficiency and stability of fully printable mesoscopic perovskite solar cells with carbon electrode. J Mater Sci: Mater Electron. 2021;32:1–10. 10.1007/s10854-021-05922-6.Search in Google Scholar
[44] Butsriruk K, Sriwong C, Ruttanapan C. Effecting on electrical performance of Methylammonium Lead Chloride Iodide perovskite films composited by reduced graphene oxide for controlling double Schottky barrier. Materials Science and Engineering: B. 2024;14(304):117343. 10.1016/j.mseb.2024.117343.Search in Google Scholar
[45] Vinila VS, Isac J. Chapter 14 - Synthesis and structural studies of superconducting perovskite GdBa2Ca3Cu4O10.5+δ nanosystems. India: Elsevier; 2022. p. 319–41. 10.1016/B978-0-12-820558-7.00022-4.Search in Google Scholar
[46] Alaizeri ZM, Alhadlaq HA, Aldawood S, Akhtar MJ, Aziz AA, Ahamed M. Photocatalytic degradation of methylene blue and anticancer response of In2O3/RGO nanocomposites prepared by a microwave-assisted hydrothermal synthesis process. Molecules 28(13):5153. 10.3390/molecules28135153.Search in Google Scholar PubMed PubMed Central
[47] Li Z, Ren Z, Liang Q, Fong PWK, Liu H, Lu X, et al. Grain orientation management and recombination suppression for ultra-stable PeLEDs with record brightness. Joule. 2024;8(4):1176–90. 10.1016/j.joule.2024.03.004.Search in Google Scholar
[48] Jin B, Ming Y, Liang Z, Wang K, Wu C. Avoid pitfalls in identifying perovskite grain size. J Phys Chem Lett. 2022;13(31):7236–42. 10.1021/acs.jpclett.2c02060.Search in Google Scholar PubMed
[49] Peng SY, Tian YZ, Ni ZY, Lu S, Li S. Effect of grain size on the deformation mechanism and fracture behavior of a non-equiatomic CoCrNi alloy with low stacking fault energy. Int J Plast. 2024;182:104129. 10.1016/j.ijplas.2024.104129.Search in Google Scholar
[50] Zeng Q, Chen Y, Yang Z, Zhang L, Wang Z, Wang L, et al. Effect of grain size and grain boundary type on intergranular stress corrosion cracking of austenitic stainless steel: A phase-field study. Corros Sci. 2024;241:112557. 10.1016/j.corsci.2024.112557.Search in Google Scholar
[51] Nemla F, Cherrad D. Metallic amorphous electrodeposited molybdenum coating from aqueous electrolyte: Structural, electrical and morphological properties under current density. Appl Surf Sci. 2016;375:1–8. 10.1016/j.apsusc.2016.01.012.Search in Google Scholar
[52] Abdel-Rahman A, Sabry YA. An approach to the micro-strain distribution inside nanoparticle structure. Int J Non-Linear Mech. 2024;161:104670. 10.1016/j.ijnonlinmec.2024.104670.Search in Google Scholar
[53] van der Poll LM, van Silfhout N, Nespoli J, van der Meer M, Boekhoff RK, Bannenberg LJ, et al. Additive-free sequential thermal evaporation of near-intrinsic Pb-Sn perovskites. Small Methods. 2024;9(4):2401246. 10.1002/smtd.202401246.Search in Google Scholar PubMed PubMed Central
[54] Werzer O, Kowarik S, Gasser F, Jiang Z, Strzalka J, Nicklin C, et al. X-ray diffraction under grazing incidence conditions. Nat Rev Methods Primers. 2024;4(1):15. 10.1038/s43586-024-00293-8.Search in Google Scholar
[55] Shen Y, Tang Z, Chen J, Zhang T, Qian L, Xiang C. The improvement of tin-based perovskite thin film quality to enhance the performance of near-infrared PeLEDs through thiocyanate modification. Adv Opt Mater. 2024;12(20):2400398. 10.1002/adom.202400398.Search in Google Scholar
[56] Kim WY, Kim HE, Schuck A, Shin MK, Moon KC, Im H, et al. Improvement of the electrical properties of amorphous indium-gallium-zinc oxide thin-film transistors by cross-linked CYTOP passivation. Appl Surf Sci. 2024;653:159304. 10.1016/j.apsusc.2024.Search in Google Scholar
[57] Zhang S, Liu Z, Pi H, Fang Z, Weng H, Wu Q. Complex field-, temperature-, and angle-dependent Hall effects from intrinsic Fermi surface revealed by first-principles calculations. Phys Rev B. 2024;110(20):205132. 10.1103/physrevb.110.205132.Search in Google Scholar
[58] Katz E. Electrochemical contributions: svante august arrhenius (1859–1927). Electrochem Sci Adv. 2024;4(4):e202400020. 10.1002/elsa.202400020.Search in Google Scholar
[59] Bai K, Chen X, Pan Y, Jin Z, Zhang B, Wang Y, et al. Evolution of activation energy for boron dissolution in the borosilicate glass during the initial leaching stage. J Non-Cryst Solids. 2024;646:123244. 10.1016/j.jnoncrysol.2024.123244.Search in Google Scholar
[60] Makarova TL, Sundqvist B, Scharff P, Gaevski M, Olsson E, Davydov VA, et al. Electrical properties of two-dimensional fullerene matrices. Carbon. 2001;39:2203–9. 10.1016/S0008-6223(01)00036-7.Search in Google Scholar
[61] Haque S, Nasar A, Rahman I, Rahman MM Applications of chitosan (CHI)-reduced graphene oxide (rGO) -polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci Rep. 2020;10:10428. 10.1038/s41598-020-67253-6.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Investigation on cutting of CFRP composite by nanosecond short-pulsed laser with rotary drilling method
- Antibody-functionalized nanoporous silicon particles as a selective doxorubicin vehicle to improve toxicity against HER2+ breast cancer cells
- Study on the effects of initial stress and imperfect interface on static and dynamic problems in thermoelastic laminated plates
- Analysis of the laser-assisted forming process of CF/PEEK composite corner structure: Effect of laser power and forming rate on the spring-back angle
- Phase transformation and property improvement of Al–0.6Mg–0.5Si alloys by addition of rare-earth Y
- A new-type intelligent monitoring anchor system for CFRP strand wires based on CFRP self-sensing
- Optical properties and artistic color characterization of nanocomposite polyurethane materials
- Effect of 200 days of cyclic weathering and salt spray on the performance of PU coating applied on a composite substrate
- Experimental analysis and numerical simulation of the effect of opening hole behavior after hygrothermal aging on the compression properties of laminates
- Engineering properties and thermal conductivity of lightweight concrete with polyester-coated pumice aggregates
- Optimization of rGO content in MAI:PbCl2 composites for enhanced conductivity
- Collagen fibers as biomass templates constructed multifunctional polyvinyl alcohol composite films for biocompatible wearable e-skins
- Early age temperature effect of cemented sand and gravel based on random aggregate model
- Properties and mechanism of ceramizable silicone rubber with enhanced flexural strength after high-temperature
- Buckling stability analysis of AFGM heavy columns with nonprismatic solid regular polygon cross-section and constant volume
- Reusable fibre composite crash boxes for sustainable and resource-efficient mobility
- Investigation into the nonlinear structural behavior of tapered axially functionally graded material beams utilizing absolute nodal coordinate formulations
- Mechanical experimental characteristics and constitutive model of cemented sand and gravel (CSG) material under cyclic loading with varying amplitudes
- Synthesis and properties of octahedral silsesquioxane with vinyl acetate side group
- The effect of radiation-induced vulcanization on the thermal and structural properties of Ethylene Propylene Diene Monomer (EPDM) rubber
- Review Articles
- State-of-the-art review on the influence of crumb rubber on the strength, durability, and morphological properties of concrete
- Recent advances in carbon and ceramic composites reinforced with nanomaterials: Manufacturing methods, and characteristics improvements
- Special Issue: Advanced modeling and design for composite materials and structures
- Validation of chromatographic method for impurity profiling of Baloxavir marboxil (Xofluza)
Articles in the same Issue
- Research Articles
- Investigation on cutting of CFRP composite by nanosecond short-pulsed laser with rotary drilling method
- Antibody-functionalized nanoporous silicon particles as a selective doxorubicin vehicle to improve toxicity against HER2+ breast cancer cells
- Study on the effects of initial stress and imperfect interface on static and dynamic problems in thermoelastic laminated plates
- Analysis of the laser-assisted forming process of CF/PEEK composite corner structure: Effect of laser power and forming rate on the spring-back angle
- Phase transformation and property improvement of Al–0.6Mg–0.5Si alloys by addition of rare-earth Y
- A new-type intelligent monitoring anchor system for CFRP strand wires based on CFRP self-sensing
- Optical properties and artistic color characterization of nanocomposite polyurethane materials
- Effect of 200 days of cyclic weathering and salt spray on the performance of PU coating applied on a composite substrate
- Experimental analysis and numerical simulation of the effect of opening hole behavior after hygrothermal aging on the compression properties of laminates
- Engineering properties and thermal conductivity of lightweight concrete with polyester-coated pumice aggregates
- Optimization of rGO content in MAI:PbCl2 composites for enhanced conductivity
- Collagen fibers as biomass templates constructed multifunctional polyvinyl alcohol composite films for biocompatible wearable e-skins
- Early age temperature effect of cemented sand and gravel based on random aggregate model
- Properties and mechanism of ceramizable silicone rubber with enhanced flexural strength after high-temperature
- Buckling stability analysis of AFGM heavy columns with nonprismatic solid regular polygon cross-section and constant volume
- Reusable fibre composite crash boxes for sustainable and resource-efficient mobility
- Investigation into the nonlinear structural behavior of tapered axially functionally graded material beams utilizing absolute nodal coordinate formulations
- Mechanical experimental characteristics and constitutive model of cemented sand and gravel (CSG) material under cyclic loading with varying amplitudes
- Synthesis and properties of octahedral silsesquioxane with vinyl acetate side group
- The effect of radiation-induced vulcanization on the thermal and structural properties of Ethylene Propylene Diene Monomer (EPDM) rubber
- Review Articles
- State-of-the-art review on the influence of crumb rubber on the strength, durability, and morphological properties of concrete
- Recent advances in carbon and ceramic composites reinforced with nanomaterials: Manufacturing methods, and characteristics improvements
- Special Issue: Advanced modeling and design for composite materials and structures
- Validation of chromatographic method for impurity profiling of Baloxavir marboxil (Xofluza)

