Abstract
C46H60N6O2Se2, triclinic, P
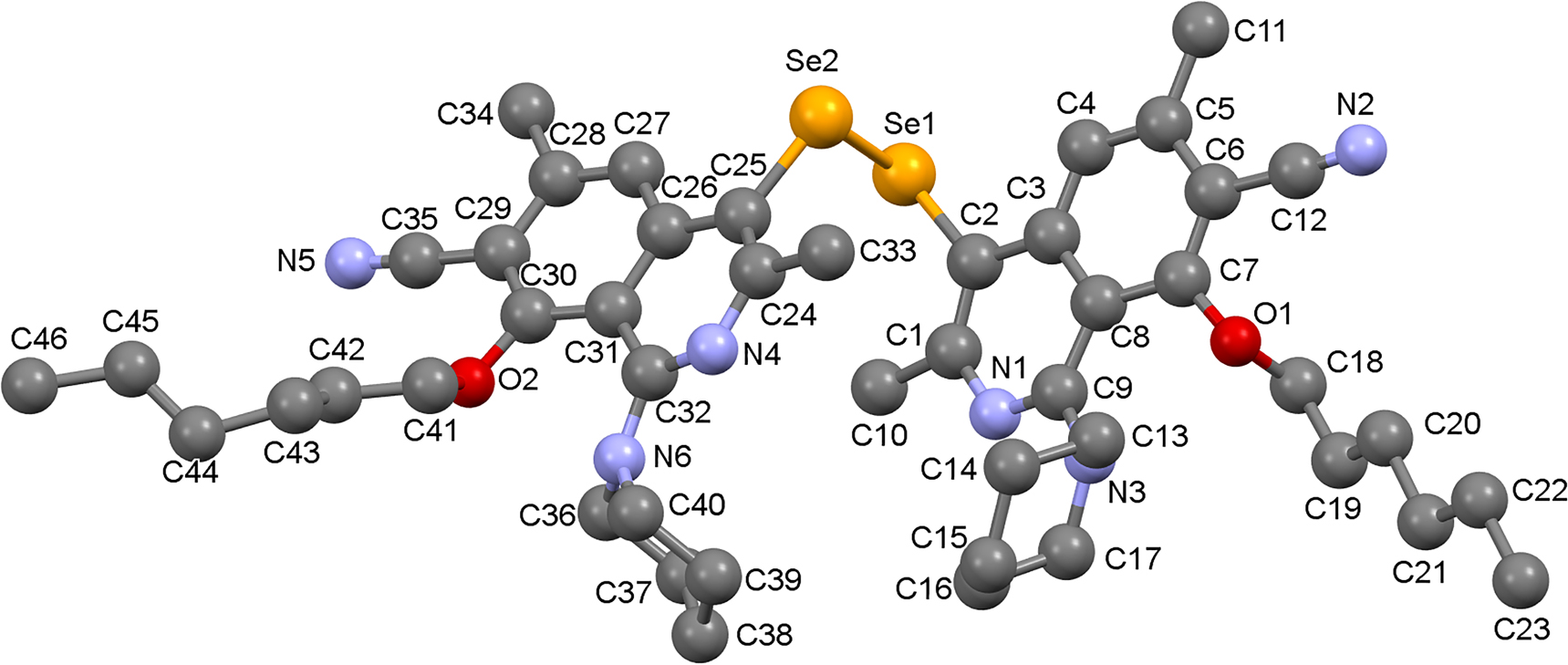
The molecular structure is shown in the Figure (Hydrogen atoms are omitted for clarity). Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red prismatic |
| Size: | 0.16 × 0.13 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.69 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 22,203, 8296, 0.046 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5396 |
| N(param)refined: | 566 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Se1 | 0.05978 (4) | 0.62847 (4) | 0.22989 (2) | 0.05821 (15) |
| Se2 | 0.14856 (4) | 0.70435 (4) | 0.33767 (2) | 0.05218 (15) |
| O1 | 0.5998 (3) | 0.7304 (3) | 0.09844 (13) | 0.0533 (7) |
| O2 | 0.0805 (3) | 0.1629 (3) | 0.48349 (15) | 0.0573 (8) |
| N1 | 0.3575 (3) | 0.4411 (3) | 0.17419 (16) | 0.0492 (8) |
| N2 | 0.5986 (5) | 1.0485 (4) | 0.0715 (2) | 0.0953 (15) |
| N3 | 0.5664 (3) | 0.4950 (3) | 0.15481 (16) | 0.0480 (8) |
| N4 | 0.3563 (3) | 0.4107 (3) | 0.40291 (17) | 0.0506 (9) |
| N5 | −0.2423 (4) | 0.1623 (4) | 0.5104 (2) | 0.0720 (12) |
| N6 | 0.3027 (3) | 0.1998 (3) | 0.41680 (17) | 0.0489 (9) |
| C1 | 0.2430 (4) | 0.4697 (4) | 0.1904 (2) | 0.0504 (10) |
| C2 | 0.2146 (4) | 0.5927 (4) | 0.19630 (19) | 0.0462 (10) |
| C3 | 0.3005 (4) | 0.6916 (4) | 0.17560 (18) | 0.0439 (10) |
| C4 | 0.2682 (4) | 0.8173 (4) | 0.1709 (2) | 0.0523 (11) |
| H4 | 0.1929 | 0.8377 | 0.1862 | 0.063* |
| C5 | 0.3428 (4) | 0.9096 (4) | 0.1449 (2) | 0.0551 (11) |
| C6 | 0.4563 (4) | 0.8772 (4) | 0.1215 (2) | 0.0510 (11) |
| C7 | 0.4913 (4) | 0.7559 (4) | 0.12499 (19) | 0.0455 (10) |
| C8 | 0.4181 (4) | 0.6606 (4) | 0.15508 (18) | 0.0410 (9) |
| C9 | 0.4469 (4) | 0.5304 (4) | 0.16242 (18) | 0.0446 (10) |
| C10 | 0.1545 (5) | 0.3564 (4) | 0.2048 (3) | 0.0794 (16) |
| H10A | 0.1802 | 0.2813 | 0.1852 | 0.119* |
| H10B | 0.0668 | 0.3665 | 0.1865 | 0.119* |
| H10C | 0.1601 | 0.3494 | 0.2517 | 0.119* |
| C11 | 0.3042 (5) | 1.0423 (4) | 0.1383 (3) | 0.0821 (16) |
| H11A | 0.3672 | 1.1007 | 0.1656 | 0.123* |
| H11B | 0.2207 | 1.0460 | 0.1522 | 0.123* |
| H11C | 0.3002 | 1.0644 | 0.0931 | 0.123* |
| C12 | 0.5374 (5) | 0.9705 (5) | 0.0926 (2) | 0.0641 (13) |
| C13 | 0.6837 (4) | 0.5644 (4) | 0.1927 (2) | 0.0578 (12) |
| H13A | 0.6683 | 0.6515 | 0.2014 | 0.069* |
| H13B | 0.7548 | 0.5649 | 0.1669 | 0.069* |
| C14 | 0.7201 (4) | 0.5046 (4) | 0.2567 (2) | 0.0640 (13) |
| H14A | 0.6524 | 0.5118 | 0.2841 | 0.077* |
| H14B | 0.7996 | 0.5499 | 0.2797 | 0.077* |
| C15 | 0.7394 (5) | 0.3659 (5) | 0.2466 (2) | 0.0744 (14) |
| H15A | 0.8124 | 0.3584 | 0.2228 | 0.089* |
| H15B | 0.7579 | 0.3291 | 0.2890 | 0.089* |
| C16 | 0.6175 (5) | 0.2959 (4) | 0.2076 (2) | 0.0678 (13) |
| H16A | 0.6323 | 0.2092 | 0.1976 | 0.081* |
| H16B | 0.5473 | 0.2942 | 0.2341 | 0.081* |
| C17 | 0.5796 (4) | 0.3597 (4) | 0.1442 (2) | 0.0571 (12) |
| H17A | 0.6447 | 0.3506 | 0.1154 | 0.069* |
| H17B | 0.4979 | 0.3174 | 0.1219 | 0.069* |
| C18 | 0.5761 (5) | 0.6874 (6) | 0.0303 (2) | 0.0862 (15) |
| H18A | 0.5501 | 0.7556 | 0.0030 | 0.103* |
| H18B | 0.5070 | 0.6182 | 0.0233 | 0.103* |
| C19 | 0.6973 (6) | 0.6443 (7) | 0.0122 (3) | 0.1076 (15) |
| H19A | 0.7167 | 0.5717 | 0.0376 | 0.129* |
| H19B | 0.6813 | 0.6155 | −0.0340 | 0.129* |
| C20 | 0.8094 (6) | 0.7342 (8) | 0.0217 (3) | 0.1279 (17) |
| H20A | 0.8271 | 0.7632 | 0.0679 | 0.153* |
| H20B | 0.7919 | 0.8068 | −0.0041 | 0.153* |
| C21 | 0.9324 (7) | 0.6796 (8) | 0.0015 (4) | 0.1472 (19) |
| H21A | 0.9561 | 0.6139 | 0.0311 | 0.177* |
| H21B | 0.9110 | 0.6407 | −0.0428 | 0.177* |
| C22 | 1.0402 (7) | 0.7711 (9) | 0.0031 (4) | 0.164 (2) |
| H22A | 1.0627 | 0.8087 | 0.0476 | 0.197* |
| H22B | 1.0159 | 0.8377 | −0.0258 | 0.197* |
| C23 | 1.1583 (7) | 0.7192 (9) | −0.0177 (4) | 0.174 (3) |
| H23A | 1.1808 | 0.6506 | 0.0093 | 0.261* |
| H23B | 1.2295 | 0.7849 | −0.0125 | 0.261* |
| H23C | 1.1395 | 0.6891 | −0.0632 | 0.261* |
| C24 | 0.3215 (4) | 0.5257 (4) | 0.3856 (2) | 0.0480 (10) |
| C25 | 0.1938 (4) | 0.5506 (3) | 0.37558 (19) | 0.0426 (9) |
| C26 | 0.0974 (4) | 0.4621 (3) | 0.39697 (18) | 0.0387 (9) |
| C27 | −0.0306 (3) | 0.4893 (4) | 0.39952 (18) | 0.0405 (9) |
| H27 | −0.0567 | 0.5633 | 0.3817 | 0.049* |
| C28 | −0.1181 (4) | 0.4118 (4) | 0.42706 (18) | 0.0409 (9) |
| C29 | −0.0766 (4) | 0.3005 (4) | 0.45485 (19) | 0.0429 (9) |
| C30 | 0.0484 (4) | 0.2695 (4) | 0.45285 (19) | 0.0439 (10) |
| C31 | 0.1357 (4) | 0.3449 (3) | 0.42020 (19) | 0.0406 (9) |
| C32 | 0.2663 (4) | 0.3192 (4) | 0.41250 (19) | 0.0434 (9) |
| C33 | 0.4337 (4) | 0.6208 (4) | 0.3765 (3) | 0.0770 (16) |
| H33A | 0.5131 | 0.5926 | 0.3970 | 0.116* |
| H33B | 0.4224 | 0.7012 | 0.3966 | 0.116* |
| H33C | 0.4370 | 0.6292 | 0.3302 | 0.116* |
| C34 | −0.2535 (4) | 0.4443 (4) | 0.4304 (2) | 0.0537 (11) |
| H34A | −0.3147 | 0.3819 | 0.4048 | 0.081* |
| H34B | −0.2631 | 0.5260 | 0.4131 | 0.081* |
| H34C | −0.2692 | 0.4457 | 0.4756 | 0.081* |
| C35 | −0.1655 (4) | 0.2218 (4) | 0.4868 (2) | 0.0519 (11) |
| C36 | 0.2207 (4) | 0.0904 (4) | 0.3827 (2) | 0.0565 (12) |
| H36A | 0.1322 | 0.1110 | 0.3727 | 0.068* |
| H36B | 0.2208 | 0.0196 | 0.4113 | 0.068* |
| C37 | 0.2696 (5) | 0.0533 (4) | 0.3193 (2) | 0.0663 (13) |
| H37A | 0.2597 | 0.1204 | 0.2888 | 0.080* |
| H37B | 0.2177 | −0.0226 | 0.2991 | 0.080* |
| C38 | 0.4113 (5) | 0.0290 (5) | 0.3316 (3) | 0.0789 (15) |
| H38A | 0.4205 | −0.0439 | 0.3583 | 0.095* |
| H38B | 0.4413 | 0.0109 | 0.2898 | 0.095* |
| C39 | 0.4925 (4) | 0.1444 (4) | 0.3669 (2) | 0.0682 (13) |
| H39A | 0.5816 | 0.1261 | 0.3778 | 0.082* |
| H39B | 0.4914 | 0.2146 | 0.3380 | 0.082* |
| C40 | 0.4403 (4) | 0.1807 (4) | 0.4294 (2) | 0.0569 (11) |
| H40A | 0.4519 | 0.1147 | 0.4605 | 0.068* |
| H40B | 0.4895 | 0.2581 | 0.4494 | 0.068* |
| C41a | 0.1771 (9) | 0.1764 (8) | 0.5413 (4) | 0.0840 (19) |
| H41Aa | 0.1965 | 0.2656 | 0.5546 | 0.101* |
| H41Ba | 0.2561 | 0.1463 | 0.5300 | 0.101* |
| C42a | 0.1385 (9) | 0.1087 (8) | 0.5963 (4) | 0.0886 (18) |
| H42Aa | 0.1131 | 0.0204 | 0.5824 | 0.106* |
| H42Ba | 0.0638 | 0.1432 | 0.6102 | 0.106* |
| C43a | 0.2499 (9) | 0.1175 (13) | 0.6558 (5) | 0.100 (2) |
| H43Aa | 0.3297 | 0.0987 | 0.6402 | 0.120* |
| H43Ba | 0.2636 | 0.2029 | 0.6755 | 0.120* |
| C44b | 0.2169 (12) | 0.0248 (12) | 0.7074 (6) | 0.117 (2) |
| H44Ab | 0.1820 | −0.0553 | 0.6844 | 0.141* |
| H44Bb | 0.2975 | 0.0107 | 0.7344 | 0.141* |
| C45b | 0.1298 (13) | 0.0560 (14) | 0.7504 (7) | 0.125 (3) |
| H45Ab | 0.0472 | 0.0601 | 0.7229 | 0.150* |
| H45Bb | 0.1593 | 0.1417 | 0.7678 | 0.150* |
| C46b | 0.0999 (15) | −0.0211 (14) | 0.8100 (6) | 0.127 (4) |
| H46Ab | 0.1382 | −0.0993 | 0.8086 | 0.190* |
| H46Bb | 0.0077 | −0.0389 | 0.8083 | 0.190* |
| H46Cb | 0.1352 | 0.0268 | 0.8503 | 0.190* |
| C41′c | 0.0908 (17) | 0.157 (3) | 0.5515 (7) | 0.097 (4) |
| H41Cc | 0.0276 | 0.0912 | 0.5624 | 0.117* |
| H41Dc | 0.0713 | 0.2372 | 0.5702 | 0.117* |
| C42′c | 0.2265 (17) | 0.131 (3) | 0.5819 (9) | 0.104 (4) |
| H42Cc | 0.2919 | 0.1973 | 0.5733 | 0.124* |
| H42Dc | 0.2477 | 0.0501 | 0.5656 | 0.124* |
| C43′c | 0.213 (2) | 0.130 (3) | 0.6564 (11) | 0.111 (4) |
| H43Cc | 0.3013 | 0.1300 | 0.6781 | 0.133* |
| H43Dc | 0.1888 | 0.2134 | 0.6665 | 0.133* |
| C44′d | 0.1350 (16) | 0.0450 (19) | 0.6963 (8) | 0.116 (4) |
| H44Cd | 0.1215 | −0.0395 | 0.6753 | 0.140* |
| H44Dd | 0.0506 | 0.0750 | 0.6964 | 0.140* |
| C45′d | 0.2002 (16) | 0.0379 (19) | 0.7688 (8) | 0.122 (4) |
| H45Cd | 0.2839 | 0.0052 | 0.7727 | 0.146* |
| H45Dd | 0.2052 | 0.1166 | 0.7949 | 0.146* |
| C46′d | 0.088 (2) | −0.061 (2) | 0.7811 (10) | 0.131 (5) |
| H46Dd | 0.0153 | −0.0184 | 0.7904 | 0.196* |
| H46Ed | 0.1164 | −0.1094 | 0.8180 | 0.196* |
| H46Fd | 0.0622 | −0.1168 | 0.7425 | 0.196* |
-
aOccupancy: 0.7, bOccupancy: 0.6, cOccupancy: 0.3, dOccupancy: 0.4
Source of material
The title compound was prepared from the reaction of an intermediate 8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile, which was obtained according to the published method [5], and selenium dioxide. The mixture of the intermediate (1.0 mmol), selenium dioxide (3.0 mmol), and xylene (15.0 mL) was stirred for 12 h at 100 °C. After the reaction solution was cooled to room temperature, the mixture was extracted with dichloromethane. After removing the solvent under reduced pressure, the crude product was purified by column chromatography using petroleum ether and ethyl acetate as the eluent to obtain the target product in 62% yield as light yellow solid. Crystals were obtained by slow evaporation from a mixture of ethyl acetate and petroleum ether.
Experimental details
H atoms bonded to C were positioned geometrically and refined using a riding model, with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all other H atoms.
Comment
Isoquinoline is an important structural motif in the construction of various compounds with biological activities in pharmaceutical chemistry [6, 7]. In recent years, some isoquinoline derivatives have been also found to be used as solid-state fluorescence sensors for pressure, organic steam, and acid gas [8], [9], [10]. It should be pointed out that intermolecular interactions and stacking arrangements in crystalline state are found to play an important role in the solid-state stimulus-responsive fluorescent materials [11]. Thus, the synthesis and crystal structures of novel isoquinoline derivatives have received increasing attention [12, 13].
In the title compound, the pyridine ring and benzene ring in isoquinoline unit are not completely coplanar because of the existence of the piperidine and hexyloxy groups, a finding which is similar to previous reports in [8, 9]. Furthermore, two isoquinoline rings in molecule form in a “Z” shape and thus no π-π stacking is formed. The molecules are stabilized by a weak C36–H36B⃛N5 (2.564 Å) hydrogen bond, a C34–H34B⃛H41A (2.299 Å) interaction, an a weak C37–H37B⃛Se2 (3.078 Å) bond. The interaction between the N atom on the cyano unit and Se2 results in a distance of 3.346 Å. As a result, the molecules form a zigzag stacking mode with a reverse parallel arrangement between the upper and lower levels.
Funding source: Wenzhou University
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We acknowledge the financial support from Wenzhou University for the publication fee.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SMART and SAINT; AXS Inc.: Madison, WI, USA, 2007.Suche in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Chen, Y., Xie, Y., Shen, H., Lei, Y., Zhou, Y., Dai, W., Cai, Z., Liu, M., Huang, X., Wu, H. Tunable phosphorescence/fluorescence dual emissions of organic isoquinoline-benzophenone doped systems by alkoxy engineering. Chem. Eur. J. 2020, 26, 17376–17380.10.1002/chem.202004291Suche in Google Scholar PubMed
6. Kaneda, T., Takeuchi, Y., Matsui, H., Shimizu, K., Urakawa, N., Nakajyo, S. Inhibitory mechanism of papaverine on carbachol-induced contraction in bovine trachea. J. Pharmacol. Sci. 2005, 98, 275–282.10.1254/jphs.FPJ05013XSuche in Google Scholar
7. Baek, S. C., Ryu, H. W., Kang, M. G., Lee, H., Park, D., Cho, M. L., Oh, S. R., Kim, H. Selective inhibition of monoamine oxidase A by chelerythrine, an isoquinoline alkaloid. Bioorg. Med. Chem. Lett. 2018, 28, 2403–2407.10.1016/j.bmcl.2018.06.023Suche in Google Scholar PubMed
8. Chen, Y., Zhang, X., Wang, M., Peng, J., Zhou, Y., Huang, X., Gao, W., Liu, M., Wu, H. Mechanofluorochromism, polymorphism and thermochromism of novel D-pai-A piperidin-1-yl-substituted isoquinoline derivatives. J. Mater. Chem. C 2019, 7, 12580–12587.10.1039/C9TC03942BSuche in Google Scholar
9. Chen, Y., Dai, C., Xu, X., Zhou, Y., Lei, Y., Liu, M., Gao, W., Huang, X., Wu, H. Effect of connecting units on aggregation-induced emission and mechanofluorochromic properties of isoquinoline derivatives with malononitrile as the terminal Group. J. Phys. Chem. C 2021, 125, 24180–24188.10.1021/acs.jpcc.1c07410Suche in Google Scholar
10. Wang, D., Zhang, X., Han, X., Zhou, Y., Lei, Y., Gao, W., Liu, M., Huang, X., Wu, H. Ketone-enol tautomerism, polymorphism, mechanofluorochromism and solid- state acidochromism of isoquinolinone-arylidenehydrazine derivatives. J. Mater. Chem. C 2021, 9, 12868–12876.10.1039/D1TC03063ASuche in Google Scholar
11. Chi, Z., Zhang, X., Xu, B., Zhou, X., Ma, C., Zhang, Y., Liu, S., Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896.10.1039/c2cs35016eSuche in Google Scholar PubMed
12. Zhang, X., Wang, D., Shen, H., Wang, S., Zhou, Y., Lei, Y., Gao, W., Liu, M., Huang, X., Wu, H. 3,6-Diamino-7,8-dihydroisoquinoline-4-carbonitrile derivatives: unexpected facile synthesis, full-color-tunable solid-state emissions and mechanofluorochromic activities. Org. Chem. Front. 2021, 8, 856–867.10.1039/D0QO01527JSuche in Google Scholar
13. Zhang, X., Zhou, Y., Wang, M., Chen, Y., Zhou, Y., Gao, W., Liu, M., Huang, X., Wu, H. Metal-free facile synthesis of multisubstituted 1-aminoisoquinoline derivatives with dual-state emissions. Chem. Asian J. 2020, 15, 1692–1700.10.1002/asia.202000322Suche in Google Scholar PubMed
© 2021 Xin-Yue Xu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27

