Abstract
C26H19Br2ClN2, triclinic, P
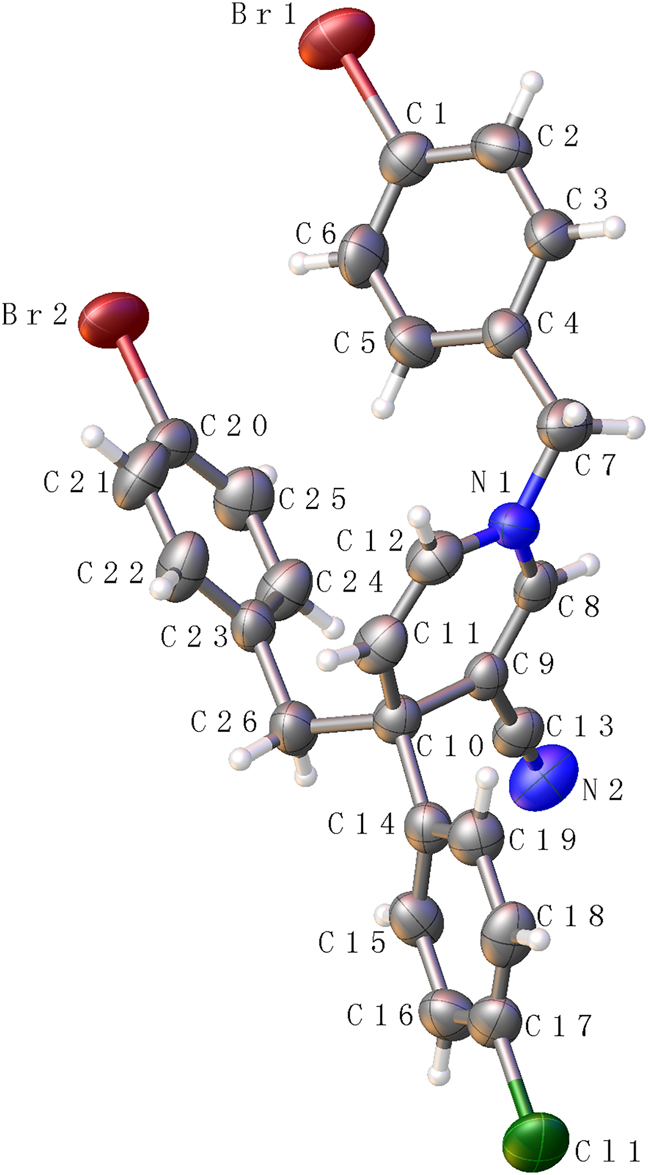
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.23 × 0.20 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 3.59 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 6017, 4093, 0.019 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2659 |
| N(param)refined: | 280 |
| Programs: | Bruker [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 1.17965 (6) | −0.35193 (5) | 0.11636 (5) | 0.0797 (2) |
| Br2 | 0.67877 (6) | −0.35025 (5) | −0.02730 (5) | 0.0887 (2) |
| C1 | 1.1809 (5) | −0.1564 (4) | 0.1957 (3) | 0.0504 (10) |
| C2 | 1.3033 (5) | −0.0638 (5) | 0.2763 (4) | 0.0602 (12) |
| H2 | 1.383229 | −0.097581 | 0.293601 | 0.072* |
| C3 | 1.3059 (5) | 0.0789 (5) | 0.3309 (3) | 0.0565 (11) |
| H3 | 1.388527 | 0.141614 | 0.385395 | 0.068* |
| C4 | 1.1883 (4) | 0.1318 (4) | 0.3067 (3) | 0.0445 (9) |
| C5 | 1.0664 (5) | 0.0355 (5) | 0.2270 (3) | 0.0585 (12) |
| H5 | 0.985221 | 0.068074 | 0.210377 | 0.070* |
| C6 | 1.0622 (5) | −0.1069 (4) | 0.1718 (4) | 0.0622 (12) |
| H6 | 0.978948 | −0.170232 | 0.118120 | 0.075* |
| C7 | 1.1986 (5) | 0.2892 (4) | 0.3704 (4) | 0.0611 (12) |
| H7A | 1.214411 | 0.307240 | 0.446825 | 0.073* |
| H7B | 1.284500 | 0.349228 | 0.361050 | 0.073* |
| C8 | 0.9575 (4) | 0.3312 (4) | 0.3923 (3) | 0.0423 (9) |
| H8 | 0.968981 | 0.305939 | 0.451529 | 0.051* |
| C9 | c | 0.3621 (3) | 0.3633 (3) | 0.0379 (9) |
| C10 | 0.7975 (4) | 0.4019 (4) | 0.2664 (3) | 0.0422 (9) |
| C11 | 0.9288 (5) | 0.4009 (4) | 0.2158 (3) | 0.0530 (11) |
| H11 | 0.924613 | 0.423173 | 0.155157 | 0.064* |
| C12 | 1.0499 (5) | 0.3704 (4) | 0.2517 (3) | 0.0522 (10) |
| H12 | 1.125874 | 0.373203 | 0.215401 | 0.063* |
| C13 | 0.7248 (5) | 0.3554 (4) | 0.4258 (3) | 0.0467 (10) |
| C14 | 0.7736 (4) | 0.5557 (4) | 0.3063 (3) | 0.0438 (9) |
| C15 | 0.6381 (5) | 0.5809 (4) | 0.3239 (3) | 0.0528 (11) |
| H15 | 0.557719 | 0.501221 | 0.307738 | 0.063* |
| C16 | 0.6187 (5) | 0.7177 (4) | 0.3640 (3) | 0.0578 (11) |
| H16 | 0.526779 | 0.730531 | 0.374585 | 0.069* |
| C17 | 0.7380 (5) | 0.8369 (4) | 0.3887 (3) | 0.0536 (11) |
| C18 | 0.8722 (5) | 0.8180 (4) | 0.3733 (3) | 0.0593 (12) |
| H18 | 0.951673 | 0.898466 | 0.389428 | 0.071* |
| C19 | 0.8907 (5) | 0.6778 (4) | 0.3332 (3) | 0.0531 (10) |
| H19 | 0.983463 | 0.666126 | 0.324449 | 0.064* |
| C20 | 0.6781 (4) | −0.1517 (4) | 0.0382 (3) | 0.0538 (11) |
| C21 | 0.7218 (6) | −0.0596 (5) | −0.0093 (4) | 0.0702 (13) |
| H21 | 0.754525 | −0.092189 | −0.072939 | 0.084* |
| C22 | 0.7171 (5) | 0.0838 (5) | 0.0381 (3) | 0.0652 (13) |
| H22 | 0.747291 | 0.146973 | 0.005407 | 0.078* |
| C23 | 0.6692 (4) | 0.1350 (4) | 0.1317 (3) | 0.0467 (10) |
| C24 | 0.6283 (5) | 0.0393 (4) | 0.1786 (3) | 0.0587 (11) |
| H24 | 0.597341 | 0.072132 | 0.242972 | 0.070* |
| C25 | 0.6317 (5) | −0.1047 (4) | 0.1330 (4) | 0.0631 (12) |
| H25 | 0.603221 | −0.168006 | 0.165849 | 0.076* |
| C26 | 0.6566 (5) | 0.2892 (4) | 0.1801 (3) | 0.0544 (11) |
| H26A | 0.572856 | 0.289083 | 0.213708 | 0.065* |
| H26B | 0.635981 | 0.321094 | 0.121861 | 0.065* |
| Cl1 | 0.71559 (16) | 1.01196 (11) | 0.43980 (10) | 0.0775 (4) |
| N1 | 1.0690 (4) | 0.3347 (3) | 0.3405 (3) | 0.0484 (8) |
| N2 | 0.6382 (5) | 0.3502 (4) | 0.4761 (3) | 0.0708 (11) |
Source of material
Dissolve 3-cyanopyridine 4.16 g (40 mmol) in acetonitrile (40 mL), add 4-bromobenzyl bromide (40 mmol), and reflux the mixed system for 7 h. TLC monitors the end of the reaction. After the reaction is over, use acetonitrile (30 mL) to wash the product to obtain a white powder pyridine salt. Add 1-(4-bromobenzyl)-3-cyanopyridin-1-ium (10 mmol) to the reaction flask, add 70 mL of anhydrous tetrahydrofuran to obtain a suspension. At this time, avoid light on an ice bath, add 0.5 mmol CuI while stirring, add 10 mL of 4-chlorophenyl magnesium bromide under nitrogen – after 2 h, TLC analysis indicated the reaction was complete. Brine was then added, and the mixture extracted with ethyl acetate. The combined ethyl acetate layers were dried over magnesium sulfate. Finally, the obtained solution was evaporated to dryness in vacuum, and recrystallized from methanol to obtain the material for the next step [4], [5]. The 1-(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile (5 mmol) was dissolved in dry tetrahydrofuran (30 mL) under stirring, placed in a quartz reactor and degassed. At room temperature, the light source (10 W, 410 nm, LED lamp) was 60 cm away from the sample. After 48 h, TLC analysis indicated the reaction was complete. The target derivative was formed, and the reaction solvent was removed by rotary evaporation. The silica gel column chromatography was used to obtain the title compound with high purity.
Experimental details
All hydrogen atoms were placed in the calculated positions.
Comment
Photochemical reactions have attracted much attention in the development of novel tandem reactions due to mild reaction conditions, efficient conversion, non-toxic, abundant and economy [6], [7]. In particular, six-membered rings with symmetrical double bond structure are often used as starting materials for photoreaction. For example, 1,4-dihydropyridine [8], 1,4-dihydropyrazine [9] and 1,4-oxazine. The group characteristics and structure-activity relationship of the photoreactive raw materials have a great influence on the yield of the title compound. When a strong electron withdrawing group is attached to the fourth group, the yield of the photoproduct is significantly improved. In the crystal structure, several important bond angle data are involved as follows C4—C7—N1 = 114.70(3) Å, C26—C10—C14 = 109.70(3) Å, C26—C10—C23 = 32.07 Å. The bond lengths and angles are in the expected ranges.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT. Version 8.23B; Bruker AXS Inc.: Madison, Wisconsin, USA, 2013.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
4. Hilgeroth, A., Baumeister, U. Formation of novel photodimers from 4-aryl-1, 4-dihydropyridines. Chem. Eur. J. 2001, 7, 4599–4603; https://doi.org/10.1002/1521-3765(20011105)7:21<4599::aid-chem4599>3.0.co;2-u.10.1002/1521-3765(20011105)7:21<4599::AID-CHEM4599>3.0.CO;2-USearch in Google Scholar
5. Paul, C. E., Gargiulo, S., Opperman, D. J., Lavandera, I., Gotor-Fernandez, V., Gotor, V., Taglieber, A., Arends, I. W. C. E., Hollmann, F. Mimicking nature: synthetic nicotinamide cofactors for C=C bioreduction using enoate reductases. Org. Lett. 2013, 15, 180–183; https://doi.org/10.1021/ol303240a.Search in Google Scholar
6. Iguchi, D., Erra-Balsells, R., Bonesi, S. M. Formation of 2,2-dimethylchroman-4-ones during the photoinduced rearrangement of some aryl 3-methyl-2-butenoate esters. A mechanistic insight. Tetrahedron 2016, 72, 1903–1910; https://doi.org/10.1016/j.tet.2016.02.039.Search in Google Scholar
7. Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008, 108, 1052–1103; https://doi.org/10.1021/cr0680336.Search in Google Scholar
8. Chen, S., Zhong, Q., Zhu, H., Liu, C., Zhuang, P., Sun, W. Visible-light-induced C–C coupling reaction to synthesize bipyridine from 3-cyano-1,4-dihydropyridines. Front. Chem. 2020, 7, 940; https://doi.org/10.3389/fchem.2019.00940.Search in Google Scholar
9. Xin, H., Sun, W., Yan, H. Photochemical ring contraction of 1-aryl-1,4-dihydropyrazine to 1-aryl-1H-imidazole. J. Photochem. Photobiol. 2013, 267, 49–54; https://doi.org/10.1016/j.jphotochem.2013.06.010.Search in Google Scholar
© 2021 Han-Qiao Shao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6

