Abstract
C14H18N4O4S, triclinic,
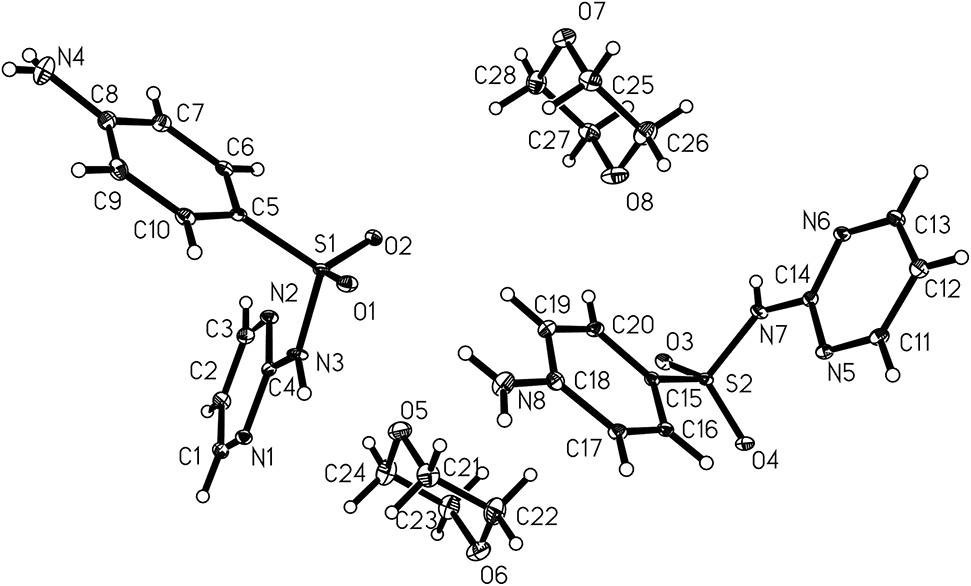
The asymmetric unit of the title crystal structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.25 × 0.25 × 0.24 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 2.13 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θmax, completeness: | 71.6°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 10,393, 10,393, n/a |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 8935 |
| N(param)refined: | 560 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.70055 (5) | 0.76559 (4) | −0.02130 (3) | 0.01230 (13) |
| O1 | 0.83651 (15) | 0.86711 (14) | −0.02257 (8) | 0.0171 (3) |
| O2 | 0.69737 (16) | 0.66355 (13) | 0.03622 (8) | 0.0173 (3) |
| N1 | 0.32800 (19) | 0.87896 (15) | 0.00767 (9) | 0.0140 (3) |
| N2 | 0.36910 (19) | 0.66512 (16) | 0.00348 (10) | 0.0152 (3) |
| N3 | 0.56958 (19) | 0.84546 (16) | −0.00126 (10) | 0.0150 (3) |
| N4 | 0.5857 (2) | 0.5395 (2) | −0.35366 (11) | 0.0281 (4) |
| C1 | 0.1800 (2) | 0.8302 (2) | 0.01505 (11) | 0.0161 (4) |
| C2 | 0.1187 (2) | 0.6987 (2) | 0.01865 (12) | 0.0181 (4) |
| C3 | 0.2200 (2) | 0.62005 (19) | 0.01153 (12) | 0.0171 (4) |
| C4 | 0.4160 (2) | 0.79264 (18) | 0.00352 (10) | 0.0130 (4) |
| C5 | 0.6583 (2) | 0.69942 (18) | −0.11967 (11) | 0.0129 (4) |
| C6 | 0.5817 (2) | 0.57095 (19) | −0.12994 (12) | 0.0143 (4) |
| C7 | 0.5559 (2) | 0.5185 (2) | −0.20808 (12) | 0.0175 (4) |
| C8 | 0.6052 (2) | 0.5938 (2) | −0.27684 (12) | 0.0181 (4) |
| C9 | 0.6784 (2) | 0.7241 (2) | −0.26533 (12) | 0.0189 (4) |
| C10 | 0.7052 (2) | 0.7767 (2) | −0.18750 (12) | 0.0161 (4) |
| S2 | 0.80284 (5) | 0.73578 (4) | 0.46990 (3) | 0.01199 (13) |
| O3 | 0.66526 (16) | 0.63589 (14) | 0.46025 (8) | 0.0171 (3) |
| O4 | 0.80708 (16) | 0.83199 (13) | 0.53211 (8) | 0.0169 (3) |
| N5 | 1.13583 (19) | 0.83277 (16) | 0.50015 (10) | 0.0145 (3) |
| N6 | 1.17464 (19) | 0.61817 (16) | 0.50330 (10) | 0.0145 (3) |
| N7 | 0.93310 (19) | 0.65333 (16) | 0.49030 (10) | 0.0144 (3) |
| N8 | 0.9288 (2) | 0.9920 (2) | 0.15291 (11) | 0.0247 (4) |
| C11 | 1.2865 (2) | 0.87710 (19) | 0.50864 (12) | 0.0165 (4) |
| C12 | 1.3878 (2) | 0.7971 (2) | 0.51571 (12) | 0.0178 (4) |
| C13 | 1.3243 (2) | 0.6661 (2) | 0.51213 (12) | 0.0167 (4) |
| C14 | 1.0878 (2) | 0.70482 (18) | 0.49824 (11) | 0.0133 (4) |
| C15 | 0.8453 (2) | 0.81120 (18) | 0.37639 (11) | 0.0126 (4) |
| C16 | 0.9121 (2) | 0.94203 (19) | 0.37439 (11) | 0.0139 (4) |
| C17 | 0.9409 (2) | 1.00187 (19) | 0.29988 (12) | 0.0163 (4) |
| C18 | 0.9031 (2) | 0.9312 (2) | 0.22590 (12) | 0.0168 (4) |
| C19 | 0.8399 (2) | 0.7980 (2) | 0.22960 (12) | 0.0185 (4) |
| C20 | 0.8108 (2) | 0.73825 (19) | 0.30374 (12) | 0.0156 (4) |
| O5 | 0.3965 (2) | 0.80037 (16) | 0.18729 (10) | 0.0296 (4) |
| O6 | 0.3588 (2) | 0.94760 (17) | 0.32547 (10) | 0.0309 (4) |
| C21 | 0.4937 (3) | 0.9234 (3) | 0.20434 (15) | 0.0287 (5) |
| C22 | 0.5051 (3) | 0.9507 (3) | 0.29524 (15) | 0.0336 (6) |
| C23 | 0.2598 (3) | 0.8258 (3) | 0.30770 (14) | 0.0298 (5) |
| C24 | 0.2504 (3) | 0.7973 (3) | 0.21728 (14) | 0.0304 (5) |
| O7 | 0.92768 (18) | 0.28278 (16) | 0.18253 (9) | 0.0248 (3) |
| O8 | 0.8340 (2) | 0.43373 (16) | 0.30382 (10) | 0.0313 (4) |
| C25 | 1.0058 (3) | 0.4131 (2) | 0.19764 (14) | 0.0248 (5) |
| C26 | 0.9901 (3) | 0.4503 (2) | 0.28561 (15) | 0.0300 (5) |
| C27 | 0.7564 (3) | 0.3039 (2) | 0.28844 (14) | 0.0244 (5) |
| C28 | 0.7718 (3) | 0.2667 (3) | 0.20069 (14) | 0.0283 (5) |
| H16 | 0.937 (3) | 0.983 (2) | 0.4224 (14) | 0.009 (5)* |
| H2 | 0.015 (3) | 0.667 (3) | 0.0246 (15) | 0.023 (6)* |
| H13 | 1.383 (3) | 0.607 (3) | 0.5155 (15) | 0.019 (6)* |
| H6 | 0.551 (3) | 0.524 (3) | −0.0862 (15) | 0.018 (6)* |
| H11 | 1.317 (3) | 0.966 (3) | 0.5105 (16) | 0.024 (7)* |
| H3 | 0.595 (3) | 0.926 (3) | −0.0056 (17) | 0.030 (7)* |
| H1 | 0.124 (3) | 0.888 (3) | 0.0191 (15) | 0.023 (7)* |
| H20 | 0.772 (3) | 0.655 (3) | 0.3058 (14) | 0.018 (6)* |
| H19 | 0.815 (3) | 0.752 (3) | 0.1847 (16) | 0.018 (6)* |
| H27A | 0.800 (3) | 0.252 (3) | 0.3276 (16) | 0.024 (6)* |
| H7A | 0.903 (3) | 0.576 (3) | 0.4890 (15) | 0.020 (6)* |
| H12 | 1.491 (3) | 0.825 (3) | 0.5220 (15) | 0.021 (6)* |
| H10 | 0.749 (3) | 0.860 (3) | −0.1793 (15) | 0.021 (6)* |
| H17 | 0.983 (3) | 1.092 (3) | 0.2954 (15) | 0.020 (6)* |
| H23A | 0.298 (3) | 0.762 (3) | 0.3333 (16) | 0.024 (7)* |
| H7 | 0.503 (3) | 0.428 (3) | −0.2150 (15) | 0.019 (6)* |
| H8A | 0.912 (4) | 0.952 (3) | 0.109 (2) | 0.035 (8)* |
| H3A | 0.185 (3) | 0.529 (3) | 0.0152 (16) | 0.025 (7)* |
| H9 | 0.710 (4) | 0.777 (3) | −0.3113 (18) | 0.035 (8)* |
| H25A | 1.111 (3) | 0.420 (3) | 0.1876 (15) | 0.023 (6)* |
| H23B | 0.158 (4) | 0.821 (3) | 0.3258 (18) | 0.035 (8)* |
| H4A | 0.616 (4) | 0.584 (3) | −0.3955 (19) | 0.034 (8)* |
| H8B | 0.959 (3) | 1.071 (3) | 0.1516 (17) | 0.029 (7)* |
| H22A | 0.543 (3) | 0.879 (3) | 0.3200 (17) | 0.031 (7)* |
| H26A | 1.043 (4) | 0.400 (4) | 0.321 (2) | 0.042 (9)* |
| H25B | 0.962 (3) | 0.470 (3) | 0.1613 (17) | 0.027 (7)* |
| H28A | 0.722 (3) | 0.316 (3) | 0.1645 (17) | 0.029 (7)* |
| H27B | 0.654 (3) | 0.294 (3) | 0.3020 (16) | 0.027 (7)* |
| H21A | 0.460 (4) | 0.989 (3) | 0.1786 (18) | 0.037 (8)* |
| H26B | 1.037 (4) | 0.537 (3) | 0.2959 (18) | 0.036 (8)* |
| H21B | 0.599 (4) | 0.918 (3) | 0.1862 (18) | 0.036 (8)* |
| H24A | 0.206 (4) | 0.871 (4) | 0.191 (2) | 0.048 (9)* |
| H28B | 0.724 (4) | 0.181 (3) | 0.1904 (18) | 0.037 (8)* |
| H4B | 0.516 (4) | 0.470 (3) | −0.3636 (17) | 0.032 (7)* |
| H24B | 0.185 (4) | 0.717 (4) | 0.206 (2) | 0.048 (9)* |
| H22B | 0.570 (4) | 1.034 (4) | 0.306 (2) | 0.046 (9)* |
Source of material
4-Amino-N-(2-pyrimidinyl)benzenesulfonamide (30 mg, 0.12 mmol) was dissolved in a mixed solvent system of acetone and 1,4-dioxane in a ratio of 6:10 (16 ml) at room temperature. The solution was filtered, sealed, and left to stand. After two weeks, colorless and block crystals were collected through solvent evaporation.
Experimental details
Hydrogen atoms were fixed in geometrically constrained positions and treated as riding on the parent atoms with Uiso(H) = 1.2 Ueq(C) [4], [, 5].
Comment
4-Amino-N-(2-pyrimidinyl)benzenesulfonamide, also known as sulfadiazine, is commonly used in clinical practice as a sulfonamide anti-infective drug. It has an inhibitory effect on sensitive bacteria and microorganisms, such as plasmodium, actinomycetes, and toxoplasma gondii, and is particularly effective in treating epidemic meningitis. However, sulfadiazine powder has poor properties, such as small particle size, poor fluidity, and low bulk density [6], which affect its solubility and preparation processes, such as tableting. Changing crystallization mode is a way to improve the properties of the powder but is ineffective in controlling crystal habit and particle size. Thus, sulfadiazine polymorphism screening has attracted interest [7]. Sulfadiazine solvate formation is convenient for controlling particle size and crystal habit. Different desolvent conditions may cause different crystalline phases, which are beneficial to the improvement of sulfadiazine production properties [8], [9], [10], [11]. Preparing sulfadiazine through phase conversion mediation is expected to improve the poor properties. Currently, reports on sulfadiazine crystal forms are rare. The crystal data include active pharmaceutical ingredients, metal complexes, solvates and cocrystals [12], [, 13]. Solvates have been reported, namely: 4-methylpyridine solvate, N,N-dimethylacetamide solvate, N-alkylpyrrolidone solvate, tetrahydrofuran solvate, and 1,4-dioxane solvate, which mostly have nitrogen/oxygen-containing ring structures, so do cocrystal formers [8], [14], [15], [16], [17], [18], [19]. However, 1,4-dioxane solvate lacks single-crystal data. In this study, we finally acquired 1,4-dioxane solvate of sulfadiazine through two weeks of slow evaporation in a mixed solvent of acetone and 1,4-dioxane.
The crystal was measured and analyzed. It presented a twinning phenomenon. The ratio of major and minor components is 0.5841(9):0.4159(9). The results showed the 1,4-dioxane solvate of sulfadiazine crystallized in the triclinic space group
Funding source: National Key R&D Program of China
Award Identifier / Grant number: 2016YFC1000900
Funding source: National Science and Technology Major Project of China
Award Identifier / Grant number: 2017ZX09101001003
Award Identifier / Grant number: 2018ZX09711001-001-013
Award Identifier / Grant number: 2018ZX09711001-010
Award Identifier / Grant number: 2018ZX09711001-003-022
Funding source: National Natural Science Foundation of China (NSFC)
Award Identifier / Grant number: 81703473
Funding source: CAMS Innovation Fund for Medical Sciences
Award Identifier / Grant number: 2017-I2M-3-010
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: National Key R&D Program of China (Grant No. 2016YFC1000900), National Science and Technology Major Project of China (Grant Nos. 2017ZX09101001003, 2018ZX09711001-001-013, 2018ZX09711001-010, 2018ZX09711001-003-022), National Natural Science Foundation of China (NSFC) (Grant No. 81703473) and CAMS Innovation Fund for Medical Sciences (Grant No. 2017-I2M-3-010).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO (Version 1.171.37.35). Agilent Technologies: Santa Clara, CA, USA, 2017.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Yang, D. Z., Wang, R. N., Jin, G. M., Zhang, B. X., Zhang, L., Lu, Y., Du, G. H. Structural and computational insights into cocrystal interactions: a case on cocrystals of antipyrine and aminophenazone. Cryst. Growth Des. 2019, 19, 347–361; https://doi.org/10.1021/acs.cgd.9b00591.Search in Google Scholar
5. Yuan, Y., Li, D. X., Wang, C. G., Chen, S. D., Kong, M. M., Deng, Z. W., Sun, C. Q. C., Zhang, H. L. Structural features of sulfamethizole and its cocrystals: beauty within. Cryst. Growth Des. 2019, 19, 7185–7192; https://doi.org/10.1021/acs.cgd.9b01060.Search in Google Scholar
6. Pan, F. F., Wang, R. M., Englert, U. Competing protonation sites in sulfadiazine: answers from chemistry and electron density. CrystEngComm 2013, 15, 1164–1172; https://doi.org/10.1039/c2ce26633d.Search in Google Scholar
7. Zhao, S. L., Wang, L. Y., Wu, S. G. Progress in the research of pharmaceutical polymorph. Chem. Ind. Lond. 2018, 35, 12–21.Search in Google Scholar
8. Wu, S. G. Analysis and Control of Polymorphism Transformation Process of Drug. PhD Degree Thesis; Tianjin University, 2017.Search in Google Scholar
9. Tianjin University. The Invention Relates to a Sulfadiazine N-Methyl Pyrrolidone Solvent Compound Crystal and a Preparation Method Thereof. Patent CN201410629017.3.2017.05.17.Search in Google Scholar
10. Macaringue, E. G. J. The Solvent Effect on the Solubility of D-Mannitol and on the Polymorphic Transformation of Sulfadiazine N-Methyl Pyrrolidone (NMP) Solvate. Master Degree Thesis; Tianjin University, 2017.Search in Google Scholar
11. Shin, H. S., Ihn, G. S., Kim, H. S., Koo, C. H. The crystal and molecular structure of sulfadiazine. J. Kor. Chem. Soc. 1974, 18, 329–340.Search in Google Scholar
12. Batista, L. C., de Souza, F. S., de Assis, V. M., Seabra, S. H., Bortoluzzi, A. J., Rennó, M. N., Horn, A., DaMatta, R. A., Fernandes, C. Antiproliferative activity and conversion of tachyzoite to bradyzoite of Toxoplasma gondii promoted by new zinc complexes containing sulfadiazine. RSC Adv. 2015, 5, 100606–100617; https://doi.org/10.1039/c5ra17690e.Search in Google Scholar
13. Sun, J., Li, X., Bao, Y. Desolvation kinetics of sulfadiazine N-methylpyrrolidone solvate. CIESC J. 2016, 67, 2349–2354.Search in Google Scholar
14. Zhang, X., Wang, C., Zhou, L., Yang, W. C., Zhou, L. N., Bao, Y., Zhang, M. J., Hou, B. H., Xu, Z., Yin, Q. X. Effects of hydrogen bond acceptor ability of solvents on molecular self-assembly of sulfadiazine solvates. J. Pharm. Sci. 2018, 107, 2823–2828; https://doi.org/10.1016/j.xphs.2018.06.026.Search in Google Scholar
15. Vemathi, G., Selvameena, R. Crystal structure of 4-amino-N-pyrimidin-2-ylbenzenesulfonamide. Bulg. Chem. Commun. 2018, 50, 33–36.Search in Google Scholar
16. Buist, A. R., Dennany, L., Kennedy, A. R., Manzie, C., McPhie, K., Walker, B. Eight salt forms of sulfadiazine. Acta Crystallogr. 2014, C70, 900–907; https://doi.org/10.1107/s2053229614018725.Search in Google Scholar
17. Coropceanu, E., Bologa, O., Arsene, I., Vitiu, A., Bulhac, I., Gorinchioy, N., Bourosh, P. Synthesis and characterization of inner-sphere substitution products in azide-containing cobalt (III) dioximates. Russ. J. Coord. Chem. 2016, 42, 516–538; https://doi.org/10.1134/s1070328416070046.Search in Google Scholar
18. Boughougal, A., Cherchali, F. Z., Messai, A., Attik, N., Decoret, D., Hologne, M., Sanglar, C., Pilet, G., Tommasino, J. B., Luneau, D. A new model of metalloantibiotic: synthesis, structure and biological activity of a zinc (II) mononuclear complex carrying two enrofloxacin and sulfadiazine antibiotics. New J. Chem. 2018, 42, 15346–15353; https://doi.org/10.1039/c8nj01774c.Search in Google Scholar
19. Elacqua, E., Bucar, D.-K., Henry, R. F., Zhang, G. G. Z., MacGillivray, L. R. Supramolecular complexes of sulfadiazine and pyridines: reconfigurable exteriors and chameleon-like behavior of tautomers at the co-crystal salt boundary. Cryst. Growth Des. 2013, 13, 393–403; https://doi.org/10.1021/cg301745x.Search in Google Scholar
© 2021 Ruonan Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10

