Abstract
C17H14FNO2, monoclinic, P21/c (no. 14), a = 14.0279(13) Å, b = 7.0527(5) Å, c = 14.4150(16) Å, β = 113.165(12)°, V = 1311.2(2) Å3, Z = 4, Rgt(F) = 0.0524, wRref(F2) = 0.1358, T = 100 K.
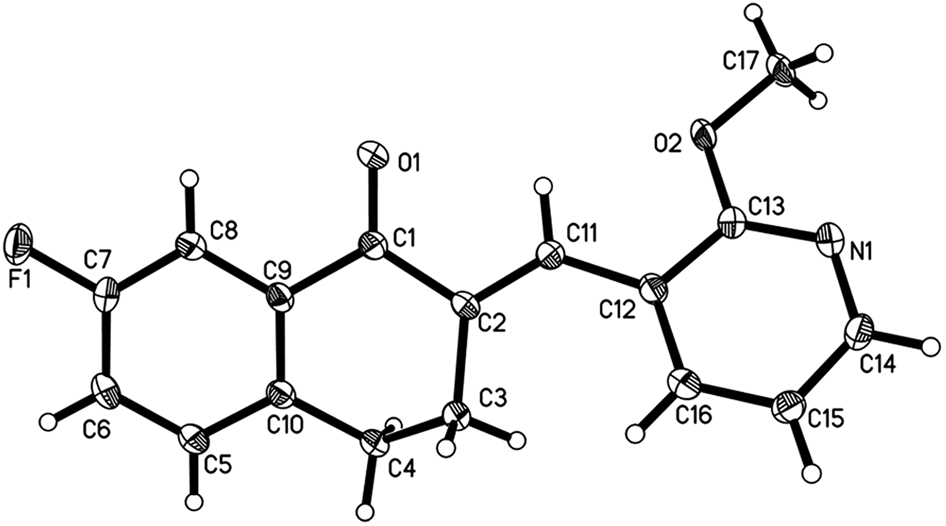
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.15 × 0.13 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | SuperNova |
| θmax, completeness: | 25.5°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 4957, 2438, 0.031 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1910 |
| N(param)refined: | 191 |
| Programs: | CrysAlisPRO [1], SHELX [2], [,3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| C1 | 0.47372 (16) | 0.5363 (3) | 0.60726 (15) | 0.0216 (5) |
| C2 | 0.52721 (16) | 0.7232 (3) | 0.64029 (15) | 0.0206 (5) |
| C3 | 0.46085 (16) | 0.8992 (3) | 0.62279 (16) | 0.0229 (5) |
| H3A | 0.447439 | 0.948526 | 0.555991 | 0.027* |
| H3B | 0.498772 | 0.995243 | 0.671525 | 0.027* |
| C4 | 0.35743 (16) | 0.8621 (3) | 0.63186 (17) | 0.0244 (5) |
| H4A | 0.369926 | 0.842488 | 0.702299 | 0.029* |
| H4B | 0.313485 | 0.972875 | 0.608510 | 0.029* |
| C5 | 0.19429 (16) | 0.6850 (3) | 0.52630 (16) | 0.0252 (5) |
| H5 | 0.155941 | 0.791073 | 0.528701 | 0.030* |
| C6 | 0.14291 (17) | 0.5233 (3) | 0.47724 (17) | 0.0277 (5) |
| H6 | 0.070919 | 0.520037 | 0.446411 | 0.033* |
| C7 | 0.20129 (17) | 0.3674 (3) | 0.47532 (16) | 0.0250 (5) |
| C8 | 0.30745 (16) | 0.3677 (3) | 0.51677 (16) | 0.0228 (5) |
| H8 | 0.344735 | 0.260862 | 0.513213 | 0.027* |
| C9 | 0.35843 (16) | 0.5333 (3) | 0.56472 (15) | 0.0201 (5) |
| C10 | 0.30175 (16) | 0.6922 (3) | 0.57194 (15) | 0.0213 (5) |
| C11 | 0.63138 (16) | 0.7192 (3) | 0.68050 (15) | 0.0220 (5) |
| H11 | 0.659874 | 0.598055 | 0.691636 | 0.026* |
| C12 | 0.70752 (16) | 0.8725 (3) | 0.70972 (16) | 0.0216 (5) |
| C13 | 0.81155 (16) | 0.8328 (3) | 0.77370 (16) | 0.0225 (5) |
| C14 | 0.86833 (17) | 1.1292 (3) | 0.75901 (17) | 0.0270 (5) |
| H14 | 0.922433 | 1.215867 | 0.774860 | 0.032* |
| C15 | 0.77038 (17) | 1.1868 (3) | 0.69688 (16) | 0.0254 (5) |
| H15 | 0.758412 | 1.310006 | 0.672016 | 0.031* |
| C16 | 0.69004 (17) | 1.0579 (3) | 0.67206 (16) | 0.0243 (5) |
| H16 | 0.623403 | 1.094834 | 0.629762 | 0.029* |
| C17 | 0.93629 (16) | 0.6116 (4) | 0.87542 (18) | 0.0304 (6) |
| H17A | 0.980048 | 0.637150 | 0.839771 | 0.046* |
| H17B | 0.942323 | 0.480371 | 0.894641 | 0.046* |
| H17C | 0.957201 | 0.689483 | 0.934725 | 0.046* |
| F1 | 0.15087 (10) | 0.2050 (2) | 0.42968 (10) | 0.0362 (4) |
| N1 | 0.88976 (14) | 0.9528 (3) | 0.79815 (13) | 0.0254 (5) |
| O1 | 0.52231 (11) | 0.3891 (2) | 0.61177 (12) | 0.0296 (4) |
| O2 | 0.83063 (11) | 0.6529 (2) | 0.81134 (11) | 0.0279 (4) |
Source of material
7-Fluoro-3,4-dihydro-1(2H)-naphthalenone (0.7 g, 4.26 mmol) and 2-fluoro-3-formylpyridine (0.53 g, 4.26 mmol) were dissolved in 10 mL methanol. A sodium hydroxide aqueous solution (25%) was added to the mixture and stirred for 3 h at room temperature. The response endpoint was detected by thin layer chromatography (TLC, 254 nm). When 7-fluoro-3,4-dihydro-1(2H)-naphthalenone disappeared, the precipitate was filtered from the reaction mixture and dissolved with dichloromethane. The organic phase was washed respectively with deionized water and brine, dried over anhydrous sodium sulfate and condensed under vacuum. The crude product was purified by silica-gel column chromatography (petroleum ether: ethyl acetate = 10:1, v/v). Single crystal was obtained under ambient conditions via solvent evaporation in the mixed solvents of dichloromethane and methanol (1:1, v/v) and drying under vacuo at 333 K for 3 h.
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C—H) = 0.96 Å (methyl), Uiso(H) = 1.5Ueq(C), and d(C—H) = 0.97 Å (methylene), Uiso(H) = 1.2Ueq(C), and d(C—H) = 0.93 Å (aromatic), Uiso(H) = 1.2Ueq(C).
Comment
Microglia become activated under brain injury and immunological stimuli and undergo several alterations from a resting state to an active state. This activation and consequent neuroinflammation are substantially involved in the pathological development of inflammatory neurodegenerative diseases in the central nervous system (CNS) [4], [, 5]. It has been reported that pro-inflammatory cytokines [tumor necrosis factor (TNF-α), interleukin (IL)-6, IL-1β] secreted from M1 microglia increase blood-brain barrier (BBB) permeability by activating the nuclear factor kappa B (NF-κB) signaling pathway during inflammatory neurodegenerative diseases in CNS [6], [7], [8]. Concomitantly, the disruption of the blood-brain barrier can result in severe inflammatory response that aggravates the brain injury [9]. In addition, activated microglia can produce reactive oxygen species (ROS), which may indirectly induce neuroinflammation by activating NF-κB [10], [, 11]. Therefore, NF-κB inhibitor with anti-neuroinflammatory activity may represent a therapeutic option for the treatment of inflammatory neurodegenerative diseases [12], [13], [14].
3,4-Dihydronaphthalen-1(2H)-one (DHN) derivatives with antitumor and anti-inflammatory activities have been investigated as novel modulators of allergic and inflammatory responses [15], [, 16]. Our interests lie in developing these derivatives as anti-neuroinflammatory drugs. In this study, we designed and synthesized a new DHN derivative through Claisen–Schmidt condensation reaction.
Single crystals of the title compound were prepared under ambient conditions, with crystallization obtained via solvent evaporation in the mixed solvents of methanol and dichloromethane. Single-crystal structure analysis revealed that the title compound, here termed XF-1-4-2, crystallized in the monoclinic space group P21/c. The ORTEP diagram is presented in the Figure. There is only a drug molecule in the asymmetric unit. With respect to the C(12) = C(11) olefinic bonds, 2-methoxyphenyl and carbonyl groups adopt the E stereochemistry [17]. Because of the distorting effect of 3,4-dihydrobenzo[b]oxepin-5(2H)-one, the 7-fluorophenyl and 2-methoxyphenyl groups are not coplanar with each other, with a dihedral angle of approximately 37.8(3)°. This twisted configuration may increase the likelihood of interactions with bioactive molecules or the purposes of creating more potent biological activity [18], [, 19]. All geometric parameters are in the expected ranges [20]. Displacement ellipsoids are drawn at the 50% probability level.
Funding source: Science and Technology Innovation Development Plan of Yantai
Award Identifier / Grant number: 2020XDRH105
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: Science and Technology Innovation Development Plan of Yantai (No. 2020XDRH105) and the National Natural Science Foundation of China (No. 81473104).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Goldmann, T., Prinz, M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013, 2013, 208093; https://doi.org/10.1155/2013/208093.Search in Google Scholar PubMed PubMed Central
5. Li, N., Xin, W. Y., Yao, B. R., Wang, C. H., Cong, W., Zhao, F., Li, H. J., Hou, Y., Meng, Q. G., Hou, G. G. Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur. J. Med. Chem. 2018, 147, 21–33; https://doi.org/10.1016/j.ejmech.2018.01.088.Search in Google Scholar PubMed
6. Correale, J. The role of microglial activation in disease progression. Mult. Scler. J. 2014, 20, 1288–1295; https://doi.org/10.1177/1352458514533230.Search in Google Scholar PubMed
7. Zhang, J. Q., Zhang, Q., Xu, Y. R., Li, H. X., Zhao, F. L., Wang, C. M., Liu, Z., Liu, P., Liu, Y. N., Meng, Q. G., Zhao, F. Synthesis and in vivo anti-inflammatory activity of C20 epimeric ocotillol-type triterpenes and protopanaxadiol. Planta Med. 2019, 85, 292–301; https://doi.org/10.1055/a-0770-0994.Search in Google Scholar PubMed
8. Wang, C. M., Liu, J., Deng, J. Q., Wang, J. Z., Weng, W. Z., Chu, H. X., Meng, Q. G. Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J. Ginseng Res. 2020, 44, 14–23; https://doi.org/10.1016/j.jgr.2019.01.005.Search in Google Scholar PubMed PubMed Central
9. Gao, C. L., Hou, G. G., Liu, J., Ru, T., Xu, Y. Z., Zhao, S. Y., Ye, H., Zhang, L. Y., Chen, K. X., Guo, Y. W., Pang, T., Li, X. W. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke. Angew. Chem. Int. Ed. 2020, 59, 2429–2439; https://doi.org/10.1002/anie.201912489.Search in Google Scholar PubMed
10. Bi, Y., Yang, J., Ma, C., Liu, Z. Y., Zhang, T. T., Zhang, X. C., Lu, J., Meng, Q. G. Design, synthesis and in vitro NO-releasing activities of ocotillol-type furoxans. Pharmazie 2015, 70, 213–218.Search in Google Scholar
11. Liu, J., Xu, Y. R., Yang, J. J., Wang, W. Z., Zhang, J. Q., Zhang, R. M., Meng, Q. G. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378; https://doi.org/10.1016/j.jgr.2017.01.001.Search in Google Scholar PubMed PubMed Central
12. Sun, Y., Zhou, Y. Q., Liu, Y. K., Zhang, H. Q., Hou, G. G., Meng, Q. G., Hou, Y. Potential anti-neuroinflammatory NF-κB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivatives. J. Enzyme Inhib. Med. Chem. 2020, 35, 1631–1640; https://doi.org/10.1080/14756366.2020.1804899.Search in Google Scholar PubMed PubMed Central
13. Zeng, K. W., Wang, S., Dong, X., Jiang, Y., Tu, P. F. Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-κB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine 2014, 21, 298–306; https://doi.org/10.1016/j.phymed.2013.08.016.Search in Google Scholar PubMed
14. Sun, Y., Gao, Z. F., Yan, W. B., Yao, B. R., Xin, W. Y., Wang, C. H., Meng, Q. G., Hou, G. G. Discovery of novel NF-κB inhibitor based on scaffold hopping: 1,4,5,6,7,8-hexahydropyrido[4,3-d] pyrimidine. Eur. J. Med. Chem. 2020, 198, 112366; https://doi.org/10.1016/j.ejmech.2020.112366.Search in Google Scholar PubMed
15. Barlow, J. W., Zhang, T., Woods, O., Byrne, A. J., Walsh, J. J. Novel mast cell-stabilising amine derivatives of 3,4 dihydronaphthalen- 1(2H)-one and 6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-one. Med. Chem. 2011, 7, 213–223; https://doi.org/10.2174/157340611795564222.Search in Google Scholar PubMed
16. Kirby, A. J., Le Lain, R., Maharlouie, F., Mason, P., Nicholls, P. J., Smith, H. J., Simons, C. Inhibition of retinoic acid metabolising enzymes by 2-(4-aminophenylmethyl)-6-hydroxy-3,4-dihydronaphthalen-1(2H)-one and related compounds. J. Enzyme Inhib. Med. Chem. 2003, 18, 27–33; https://doi.org/10.1080/1475636021000049221.Search in Google Scholar PubMed
17. Li, N., Xin, W. Y., Yao, B. R., Wang, C. H., Cong, W., Zhao, F., Li, H. J., Hou, Y., Meng, Q. G., Hou, G. G. Novel dissymmetric 3,5-bis(arylidene)-4-piperidones as potential antitumor agents with biological evaluation in vitro and in vivo. Eur. J. Med. Chem. 2018, 147, 21–33; https://doi.org/10.1016/j.ejmech.2018.01.088.Search in Google Scholar PubMed
18. Yang, Q. W., Wang, N., Zhang, J., Chen, G., Xu, H., Meng, Q. G., Du, Y., Yang, X., Fan, H. Y. In vitro and in silico evaluation of stereoselective effect of ginsenosideisomers on platelet P2Y12 receptor. Phytomedicine 2019, 64, 152899; https://doi.org/10.1016/j.phymed.2019.152899.Search in Google Scholar PubMed
19. Li, N., Yao, B. Y., Wang, C. H., Meng, Q. G., Hou, G. G. Synthesis, crystal structure and activity evaluation of novel 3,4-dihydro-1-benzoxepin-5(2H)-one derivatives as protein– tyrosine kinase (PTK) inhibitors. Acta Crystallogr. 2017, C73, 1003–1009; https://doi.org/10.1107/s2053229617015145.Search in Google Scholar
20. Sun, Y., Gao, Z., Wang, C., Hou, G. Synthesis, crystal structures and anti-inflammatory activity of fluorine-substituted 1,4,5,6-tetrahydrobenzo[h]quinazolin-2-amine derivatives. Acta Crystallogr. 2019, C75, 1157–1165; https://doi.org/10.1107/s2053229619010118.Search in Google Scholar PubMed
© 2020 Xiao-Fan Zhang and Qing-Guo Meng, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidoethylidene)benzohydrazonato-κ5N,O,O′:N′,O′′)hexkis(pyridine-κ1N)trinickel(II) - pyridine (1/1), C63H57Cl2N13Ni3O6
- Crystal structure of [(μ2-succinato κ3O,O′:O′′)-bis-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane)]dinickel(II)] diperchlorate, dihydrate C36H82Cl2N8Ni2O15
- Crystal structure of catena-poly[aquabis(3-nitrobenzoato-κ2O:O′)-(μ2-pyrazine-N: N′)cadmium(II)], C18H14N4O9Cd
- Crystal structure of 4-(2,2-difluoroethyl)-2,4,6-trimethylisoquinoline-1,3(2H,4H)-dione, C14H15F2NO2
- The crystal structure of thioxanthen-9-one-10,10-dioxide, C13H8O3S – a second polymorph
- Crystal structure of (E)-2-((2-methoxy-3-pyridyl)methylene)-7-fluoro-3,4-dihydronaphthalen-1(2H)-one, C17H14FNO2
- The crystal structure of diaquahydrogen 2,5-dimethylbenzenesulphonate, C8H14O5S
- The crystal structure of N-(4-(cyclohexylimino)pent-2-en-2-yl)cyclohexanamine, C17H30N2
- The twinned crystal structure of 1,3-phenylenedimethanaminium dibromide, C8H14Br2N2
- Crystal structure of 2,4,7,9-tetranitro-10H-benzofuro[3,2-b]indole – dimethyl sulfoxide (1/1), C16H11N5O10S
- Crystal structure of 2,6-bis(2-(pyridin-3-yl)ethyl)pyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)-tetraone, C24H18N4O4
- The crystal structure of 3,4-dichlorobenzoic acid chloride, C7H3Cl3O
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-k2S:S)zinc(II), C26H18N6ZnS4
- Crystal structure of tetrakis(μ-naphthalene-1-carboxylato-κ2O,O′)bis(methanol)copper(II), C46H36Cu2O10
- Crystal structure of 9-methyl-3-methylene-1,2,3,9-tetrahydro-4H-carbazol-4-one, C14H13NO
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 3-nitrophthalate monohydrate, C12H19N9O7S2
- Crystal structure of 3,3′-(1,2-phenylene-bis(methylene))bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluorophosphate), C18H24F12N4P2
- The crystal structure of 5-hydroxy-6,8-dimethoxy-2-methyl-4H-benzo[g]chromen-4-one– rubrofusarin B, C16H14O5
- The crystal structure of bis(ethanol-kO)- bis(6-aminopicolinato-k2N,O)manganese(II), C16H22O6N4Mn
- The crystal structure of 3,3′-((carbonylbis(azanediyl))bis(ethane-2,1-diyl)) bis(1-methyl-1H-benzo[d]imidazol-3-ium) tetrafluoroborate monohydrate, C21H28N6O3B2F8
- Crystal structure of dimethanol-dichlorido-bis( μ2-2-(((1,5-dimethyl-3-oxo-2- phenyl-2,3-dihydro-1H-pyrazol-4-yl)imino)methyl)phenolato- κ4O:O,O′,N)dinickel (II), C20H24ClNiN3O4
- The crystal structure of methyl 5-(trifluoromethyl)-1H-pyrrole-2-carboxylate, C7H6F3NO2
- Crystal structure of (OC‐6‐13)‐aqua‐tris (3‐bromopyridine‐κ1N)‐bis(trifluoroacetato‐κ1O)cadmium(II) C19H14Br3CdF6N3O5
- Crystal structure of methyl (E)-3-(4-(2-ethoxy-2-oxoethoxy)phenyl) acrylate, C14H16O5
- Crystal structure of methyl 4-acetoxy-3,5-dimethoxybenzoate, C12H14O6
- The crystal structure of 2-(1H-benzimidazol-2-yl)-3-bromo-5-chlorophenol, C13H8BrClN2O
- The crystal structure of bis(μ2-5-chloro-N-(2-methyl-1-oxidopropylidene)-2-oxidobenzohydrazonate-κ5N,O,O′:N′,O′′)pentakis(pyridine-κ1N)tricopper(II), C47H45Cl2N9Cu3O6
- Synthesis and crystal structure of catena-poly[aqua-bis(nitrato-κ2O:O′)- (μ2-((1 H-imidazol-1-yl)methyl)benzene-κ2 N,N′)-H2O-κ2O]cadmium(II), C14H16N6O7Cd
- The crystal structure of pentakis(carbonyl)-{μ-[2,3-bis(sulfanyl)propan-1-olato]}-(triphenylphosphane)diiron (Fe–Fe)C26H21Fe2O6PS2
- Crystal structure of ethyl-2-(3-benzoylthioureido)propanoate, C13H16N2O3S
- Crystal structure of 2-methoxy-4b,5,14,15-tetrahydro-6H-isoquinolino[2′,1′:1,6] pyrazino[2,3-b]quinoxaline, C19H18N4O
- Crystal structure of 2,2′-[ethane-1,2-diylbis(azanylylidenemethylylidene)]bis(6-chlorophenol), C16H14Cl2N2O2
- The crystal structure of (Z)-3-((2-(2-(2-aminophenoxy)ethoxy)phenyl)amino)-1-phenylbut-2-en-1-one, C24H24N2O3
- The crystal structure of 10-(3,5-di(pyridin-4-yl)phenyl)-10H-phenoxazine dihydrate, C28H23N3O3
- Crystal structure of poly[dipoly[aqua-di(µ2-pyrazin-2-olato-κ2N:N′) zinc(II)], C8H8N4O3Zn
- Crystal structure of poly[tetra(μ2-cyanido-κ2N:O)-bis(N,N-dimethylformamide-κO)-manganese(II)-platinum(II)], C10H14MnN6O2Pt
- The crystal structure of aqua-chlorido-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(2,4-dichlorophenolato-κ4N,N′,O,O′)manganese(III), C16H12Cl5MnN2O3
- Crystal structure of [di(µ2-cyanido)-dicyanido-bis(dimethyl sulfoxide-κO)- bis(2,2′-(ethane-1,2-diylbis(azanylylidenemethanylylidene))diphenolato-κ4,N,N′,O,O′)- dimanganese(III)-platinum(II)], C40H40Mn2N8O6PtS2
- The crystal structure of (azido)-κ1N-6,6′-((cyclohexane-1,2-diylbis(azanylylidene)) bis(methanylylidene))bis(3-bromophenolato-κ4N,N,O,O)-(methanol)-manganese(III)–methanol(1/1), C22H26Br2MnN5O4
- Crystal structure of 7-chloro-N-(4-iodobenzyl)-1,2,3,4-tetrahydroacridin-9-amine, C20H18ClIN2
- Crystal structure of catena-poly[(1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N′′′)-bis(μ2-thiocyanato-κ2N:S)-bis(thiocyanato-κS)-nickel(II)palladium(II)], C14H24N8NiPdS4
- Crystal structure of 3-chloro-4-(4-ethylpiperazin-1-yl)aniline monohydrate, C12H20ClN3O
- Crystal structure of the 2D coordination polymer poly[diaqua-bis(μ2-3- methoxyisonicotinato-κ2N:O)cobalt(II)] — dimethylformamide (1/1), C20H30CoN4O10
- Crystal structure of 4-[(5-chloro-2-hydroxybenzylidene)amino]-3-propyl-1H-1,2,4-triazole-5(4H)-thione, C12H13ClN4OS
- Crystal structure of N-(5-(2-(benzyl(1-(4-methoxyphenyl)propan-2-yl)amino)-1-hydroxyethyl)-2-(benzyloxy)phenyl)formamide, C33H36N2O4
- Crystal structure of 3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]heptane-2-carboxylic acid, C9H12O5
- The crystal structure of 1-((dimethylamino)(3-nitrophenyl)methyl)naphthalen-2-ol, C19H18N2O3
- Crystal structure of catena-poly[di(μ2-cyanido-κ2C:N)-dicyanido-tetrakis(dimethyl sulfoxide-κO)-manganese(II)-platinum(II)], C12H24MnN4O4PtS4
- Crystal structure of 4-amino-N-(2-pyrimidinyl)benzenesulfonamide–1,4-dioxane (1/1), C14H18N4O4S
- Crystal structure of bis{1-[(benzotriazol-1-yl)methyl]-1H-1,3-(2-methyl-imidazol)}di-chloridomercury(II), [Hg(C11H11N5)2Cl2], C22H22N10Cl2Hg
- Crystal structure of 2, 3-bis((4-methylbenzoyl)oxy) succinic acid–N, N-dimethylformamide (1/1), C23H25NO9
- Crystal structure of catena-poly[bis(4-(4-carboxyphenoxy)benzoato-κ1O)-μ2-(1,4-bis(1-imidazolyl)benzene-κ2N:N′)cobalt(II)], C40H28N4O10Co
- Crystal structure of 1H-imidazol-3-ium poly[aqua-(μ4-glutarato-κ6O,O′:O′:O′′,O′′′:O′′′)-(nitrato-κ2O,O′)strontium(II)], C8H13N3O8Sr
- Crystal structure of (R)-6-(benzo[b]thiophen-5-yl)-2-methyl-2,6-dihydrobenzo [5,6] silino[4,3,2-cd]indole, C23H17NSSi
- Crystal structure of catena-poly[bis(μ2-thiocyanato-κ2N:S)-(2-(5-methyl-1H-pyrazol-3-yl)pyridine-κ2N,N′)cadmium(II)]–dioxane (1/1), C15H17CdN5O2S2
- Crystal structure of poly[aqua-(μ2-1,4-bis(2′-carboxylatophenoxy)benzene-κ2O:O′)-(μ2-4,4′-bipyridione-κ2N:N′)cadmium(II)] monhydrate, C30H22CdN2O7⋅H2O
- Crystal structure of catena-poly[tetraaqua-(μ2-4,4′-bipyridine-k2N:N′)-bis(μ2-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-k4O,O′:O″,O′″)dicadmium(II)] dihydrate, C20H20NO7Cd
- Crystal structure of 1‐tert‐butyl‐3‐(2,6‐diisopropyl‐4‐phenoxyphenyl)‐2-methylisothiourea, C24H34N2OS
- Crystal structure of catena-poly[triaqua-(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ1O)cobalt(II)] — N,N′-dimethylformamide (1/1), C28H34N8O8Co
- Crystal structure of tetraaqua-bis(1,4-di(1H-imidazol-1-yl)benzene-κ1N)manganese(II) 2,3-dihydroxyterephthalate, C32H32MnN8O10

