Abstract
C34H54N10O6S6, monoclinic, P21 (no. 4), a = 11.8434(5) Å, b = 11.2298(6) Å, c = 17.0706(8) Å, β = 94.661(2)°, V = 2262.86(19) Å3, Z = 4, Rgt(F) = 0.0287, wRref(F2) = 0.0809, T = 150(2) K.
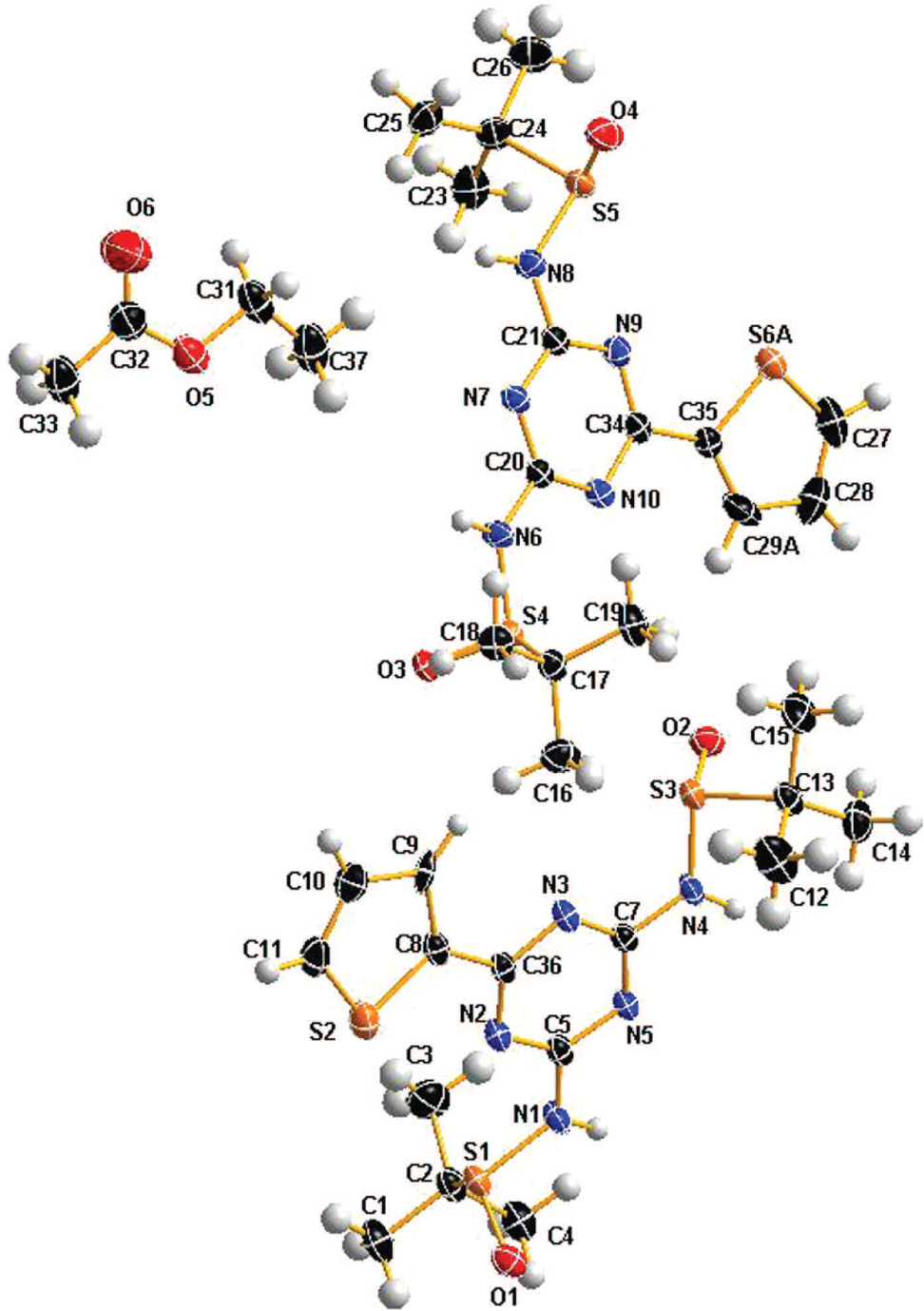
The asymmetric unit of the title crystal structure is shown in the figure (The disorder of the thiophen-2-yl moiety is omitted for clarity). Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Stripe, colorless |
| Size: | 0.13 × 0.05 × 0.04 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 3.22 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| θmax, completeness: | 63.8°, >97% |
| N(hkl)measured, N(hkl)unique, Rint: | 17958, 6789, 0.027 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6602 |
| N(param)refined: | 539 |
| Programs: | Bruker programs [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | −0.1052(2) | −1.5389(2) | −0.42997(13) | 0.0337(5) |
| O2 | −0.61088(19) | −1.0627(2) | −0.18856(13) | 0.0319(5) |
| O3 | −0.35966(17) | −0.6373(2) | −0.31603(13) | 0.0300(5) |
| O4 | −0.8434(2) | −0.1386(3) | −0.06375(16) | 0.0438(6) |
| O5 | −0.2122(2) | −0.1237(3) | −0.16304(16) | 0.0453(7) |
| O6 | −0.1972(4) | 0.0745(4) | −0.1535(3) | 0.0902(14) |
| S1 | −0.09513(6) | −1.44643(7) | −0.36826(4) | 0.02623(18) |
| S2 | −0.09269(7) | −1.04271(9) | −0.44628(5) | 0.0376(2) |
| S3 | −0.59102(6) | −1.05917(7) | −0.27347(4) | 0.02476(17) |
| S4 | −0.48283(6) | −0.64982(7) | −0.30672(4) | 0.02339(17) |
| S5 | −0.76661(6) | −0.23224(8) | −0.02997(5) | 0.02979(19) |
| S6Ba | −0.7619(4) | −0.8060(4) | −0.1217(3) | 0.0329(10) |
| S6Ab | −0.8900(3) | −0.6345(3) | −0.04373(16) | 0.0299(7) |
| N1 | −0.2257(2) | −1.4272(3) | −0.33706(17) | 0.0294(6) |
| H1 | −0.2613 | −1.4884 | −0.3182 | 0.035* |
| N2 | −0.2199(2) | −1.2266(3) | −0.36863(15) | 0.0265(6) |
| N3 | −0.3769(2) | −1.1035(3) | −0.34428(16) | 0.0257(6) |
| N4 | −0.5319(2) | −1.1924(3) | −0.29274(17) | 0.0276(6) |
| H4 | −0.5710 | −1.2578 | −0.2862 | 0.033* |
| N5 | −0.3813(2) | −1.3116(3) | −0.31532(15) | 0.0243(6) |
| N6 | −0.5204(2) | −0.5172(3) | −0.27089(16) | 0.0284(6) |
| H6 | −0.4926 | −0.4530 | −0.2918 | 0.034* |
| N7 | −0.6018(2) | −0.3882(3) | −0.18881(15) | 0.0238(5) |
| N8 | −0.6758(2) | −0.2640(3) | −0.09939(15) | 0.0289(6) |
| H8 | −0.6329 | −0.2069 | −0.1162 | 0.035* |
| N9 | −0.7260(2) | −0.4629(3) | −0.09735(15) | 0.0258(6) |
| N10 | −0.6434(2) | −0.5971(3) | −0.18516(15) | 0.0245(6) |
| C1 | 0.0955(3) | −1.5356(4) | −0.3028(3) | 0.0453(10) |
| H1A | 0.0941 | −1.5903 | −0.3476 | 0.068* |
| H1B | 0.1419 | −1.5698 | −0.2582 | 0.068* |
| H1C | 0.1279 | −1.4592 | −0.3173 | 0.068* |
| C36 | −0.2725(2) | −1.1229(3) | −0.36833(17) | 0.0240(6) |
| C2 | −0.0251(3) | −1.5162(3) | −0.2798(2) | 0.0320(8) |
| C3 | −0.0282(4) | −1.4259(5) | −0.2133(2) | 0.0507(11) |
| H3A | −0.0104 | −1.3466 | −0.2327 | 0.076* |
| H3B | 0.0278 | −1.4481 | −0.1703 | 0.076* |
| H3C | −0.1039 | −1.4252 | −0.1941 | 0.076* |
| C4 | −0.0782(3) | −1.6351(4) | −0.2619(2) | 0.0371(8) |
| H4A | −0.1573 | −1.6228 | −0.2508 | 0.056* |
| H4B | −0.0364 | −1.6711 | −0.2159 | 0.056* |
| H4C | −0.0753 | −1.6882 | −0.3072 | 0.056* |
| C5 | −0.2768(3) | −1.3183(3) | −0.34064(19) | 0.0255(7) |
| C7 | −0.4252(2) | −1.2020(3) | −0.31812(17) | 0.0236(7) |
| C8 | −0.2132(3) | −1.0207(3) | −0.39833(19) | 0.0259(7) |
| C9 | −0.2482(3) | −0.8959(3) | −0.39191(19) | 0.0269(7) |
| H9 | −0.3114 | −0.8659 | −0.3673 | 0.032* |
| C10 | −0.1638(3) | −0.8276(4) | −0.4320(2) | 0.0382(8) |
| H10A | −0.1668 | −0.7433 | −0.4367 | 0.046* |
| C11 | −0.0803(3) | −0.8949(4) | −0.4621(2) | 0.0382(8) |
| H11 | −0.0211 | −0.8613 | −0.4893 | 0.046* |
| C12 | −0.7077(4) | −1.0887(5) | −0.4143(2) | 0.0581(13) |
| H12A | −0.6710 | −1.1649 | −0.4242 | 0.087* |
| H12B | −0.6580 | −1.0232 | −0.4275 | 0.087* |
| H12C | −0.7795 | −1.0833 | −0.4469 | 0.087* |
| C13 | −0.7302(3) | −1.0807(3) | −0.32801(19) | 0.0297(7) |
| C14 | −0.7906(3) | −1.1882(4) | −0.2998(3) | 0.0438(9) |
| H14A | −0.7981 | −1.1812 | −0.2433 | 0.066* |
| H14B | −0.7471 | −1.2600 | −0.3101 | 0.066* |
| H14C | −0.8660 | −1.1937 | −0.3278 | 0.066* |
| C15 | −0.7941(3) | −0.9660(4) | −0.3109(3) | 0.0489(10) |
| H15A | −0.8694 | −0.9676 | −0.3393 | 0.073* |
| H15B | −0.7517 | −0.8971 | −0.3281 | 0.073* |
| H15C | −0.8019 | −0.9601 | −0.2543 | 0.073* |
| C16 | −0.5190(3) | −0.7508(4) | −0.4478(2) | 0.0404(9) |
| H16A | −0.5350 | −0.8203 | −0.4158 | 0.061* |
| H16B | −0.4377 | −0.7476 | −0.4549 | 0.061* |
| H16C | −0.5618 | −0.7570 | −0.4993 | 0.061* |
| C17 | −0.5539(3) | −0.6381(3) | −0.40671(18) | 0.0276(7) |
| C18 | −0.5149(3) | −0.5273(3) | −0.4475(2) | 0.0338(8) |
| H18A | −0.5444 | −0.5284 | −0.5028 | 0.051* |
| H18B | −0.4319 | −0.5254 | −0.4442 | 0.051* |
| H18C | −0.5430 | −0.4565 | −0.4218 | 0.051* |
| C19 | −0.6796(3) | −0.6360(4) | −0.3966(2) | 0.0419(9) |
| H19A | −0.6998 | −0.5597 | −0.3736 | 0.063* |
| H19B | −0.6985 | −0.7011 | −0.3618 | 0.063* |
| H19C | −0.7219 | −0.6459 | −0.4480 | 0.063* |
| C20 | −0.5907(3) | −0.5012(3) | −0.21180(18) | 0.0236(6) |
| C21 | −0.6680(3) | −0.3749(3) | −0.12933(18) | 0.0244(6) |
| C23 | −0.5845(4) | −0.2591(4) | 0.0738(2) | 0.0463(10) |
| H23A | −0.6290 | −0.3243 | 0.0939 | 0.069* |
| H23B | −0.5328 | −0.2274 | 0.1165 | 0.069* |
| H23C | −0.5407 | −0.2889 | 0.0317 | 0.069* |
| C24 | −0.6636(3) | −0.1613(4) | 0.04210(19) | 0.0354(8) |
| C25 | −0.6014(3) | −0.0582(4) | 0.0067(2) | 0.0394(8) |
| H25A | −0.5486 | −0.0894 | −0.0296 | 0.059* |
| H25B | −0.5591 | −0.0134 | 0.0488 | 0.059* |
| H25C | −0.6563 | −0.0054 | −0.0218 | 0.059* |
| C26 | −0.7379(4) | −0.1168(5) | 0.1052(2) | 0.0559(12) |
| H26A | −0.7883 | −0.0539 | 0.0833 | 0.084* |
| H26B | −0.6897 | −0.0851 | 0.1498 | 0.084* |
| H26C | −0.7832 | −0.1830 | 0.1231 | 0.084* |
| C27 | −0.9234(3) | −0.7734(4) | −0.0364(3) | 0.0473(10) |
| H27Ab | −0.9838 | −0.7981 | −0.0068 | 0.057* |
| H27a | −0.9875 | −0.7900 | −0.0082 | 0.057* |
| C28 | −0.8621(4) | −0.8522(4) | −0.0738(3) | 0.0481(10) |
| H28b | −0.8786 | −0.9349 | −0.0716 | 0.058* |
| H28Aa | −0.8705 | −0.9363 | −0.0745 | 0.058* |
| C29Ba | −0.8705(16) | −0.6535(17) | −0.0478(12) | 0.040(6) |
| H29Ba | −0.8929 | −0.5812 | −0.0248 | 0.048* |
| C29Ab | −0.7815(11) | −0.7838(14) | −0.1124(9) | 0.046(4) |
| H29Ab | −0.7310 | −0.8200 | −0.1458 | 0.055* |
| C31 | −0.3151(3) | −0.1191(5) | −0.1233(2) | 0.0471(10) |
| H31A | −0.3764 | −0.0810 | −0.1574 | 0.057* |
| H31B | −0.3029 | −0.0725 | −0.0741 | 0.057* |
| C32 | −0.1605(4) | −0.0196(4) | −0.1728(2) | 0.0460(10) |
| C33 | −0.0528(3) | −0.0354(5) | −0.2107(3) | 0.0536(11) |
| H33A | 0.0061 | −0.0645 | −0.1717 | 0.080* |
| H33B | −0.0294 | 0.0411 | −0.2317 | 0.080* |
| H33C | −0.0643 | −0.0932 | −0.2536 | 0.080* |
| C34 | −0.7123(2) | −0.5695(3) | −0.12818(17) | 0.0235(6) |
| C35 | −0.7806(3) | −0.6656(3) | −0.09926(19) | 0.0271(7) |
| C37 | −0.3462(4) | −0.2460(5) | −0.1054(3) | 0.0557(12) |
| H37A | −0.3566 | −0.2913 | −0.1545 | 0.084* |
| H37B | −0.4167 | −0.2468 | −0.0791 | 0.084* |
| H37C | −0.2853 | −0.2821 | −0.0709 | 0.084* |
Occupancies: a = 0.417(6), b = 0.583(6).
Source of material
Under the protection of N2, Mg (4.80 g, 0.20 mol) and I2 (1.02 g, 0.004 mol) were added to the solution of tetrahydrofuran (150 mL). After 10 min 2-bromothiophene (32.60 g, 0.20 mol) was added to the suspension mixture dropwisely. When Mg disappeared, the reaction mixture was cooled to −15 °C. A solution of 2,4,6-trichloro-1,3,5-triazine (36.88 g, 0.20 mol) in tetrahydrofuran was added to the above solution of thiophen-2-yl magnesium bromide within 5 min. After 5 h, the mixture was filtered and the filtrate was evaporated to get a yellow solid, which was purified by chromatography on silica gel to get 2,4-dichloro-6-(thiophen-2-yl)-1,3,5-triazine (26.0 g, yield 56%) as a yellow solid. To a suspension of NaH (1.92 g, 0.08 mol) in 60 mL of tetrahydrofuran was added tert-butanesulfinamide (9.70 g, 0.08 mol). The mixture was stirred at room temperature for 30 min. Then a solution of 2,4-dichloro-6-(thiophen-2-yl)-1,3,5-triazine (4.64 g, 0.02 mol) in 20 mL of tetrahydrofuran was added slowly (about in 5 min). After stirring at reflux for 12 h, the reaction was quenched with 2 mL of MeOH and evaporated to get a yellow solid which was dissolved in the mixture of CH2Cl2 and water. The organic layer was separated, washed with water, dried by anhydrous Na2SO4 and then filtered. The filtrate was evaporated to get a yellow solid, which was purified by chromatography on silica gel (PE:EA = 6:1) to afford 2,4-bis(R-tertbutylsulfonamido)-6-(thiophen-2-yl)-1,3,5-triazine (3.05 g, yield 38%). 1H NMR: (400 MHz, chloroform-d) δ9.99 (s, 2H), 8.13 (s, 1H), 7.60 (d, J = 8 Hz, 1H), 7.17 (t, J = 3.4 Hz, 1H), 1.37 (s, 18H). Crystals were obtained by recrystallization with ethyl acetate at room temperature.
Experimental details
The data were scaled and corrected for absorption using SADABS-2016/2 (Bruker, APEX-II). The hydrogen atoms were placed at calculated positions and refined as riding atoms with isotropic displacement parameters. The structure was refined as an inversion twin giving a Flack parameter of 0.024(14). The disorder of the thiophen-2yl moiety is handled with an aproximately 1/1 ratio.
Discussion
As an important aromatic unit, thiophene ring was widely used in constructing many novel heterocyclic compounds. Due to its unique pharmacological activity [3], [4] , high catalytic activity [5] and good photoelectric property [6] thiophene derivatives were widely used in the areas of pharmacy, chemical industry, agriculture [7] and organic semiconductor materials [8], [9] . Developing novel chiral compounds containing thiophene rings and studying its properties have drawn more and more attentions of researchers in recent years. In this paper, 2,4-bis(R-tertbutylsulfonamido)-6-(thiophen-2-yl)-1,3,5- triazine was synthesized using 1,3,5-triazine as starting material. Its structure was characterized by 1H NMR and X-ray diffraction.
There are two cystallographically independent molecules (2(C15H23N5O2S3)) and one solvent molecule (C4H8O2) in the asymmetric unit, in which all bond lengths are in normal ranges [10]. In one of independent molecule (C15H23N5 O2 S3), the typical bond length of S1—N1, S1—O1, N1—C5 and S2—C8 are 1.690(3) Å, 1.477(3) Å, 1.364(5) Å, 1.720(3) Å respectively. The angle of C11—S2—C8 is 91.23(18)°, which is smaller than that of C11—C10—C9 is 115.0(4)°. The torsion angle of C9—C8—C36—N3 is 11.318°, which demonstrated that thiophene ring and the 1,3,5-triazine ring were not coplanar. In molecular packing, classical (i) and non-classical (ii) hydrogen bonds were observed as following: (i) N1—H1⋯O3′ hydrogen bond (d(H1⋯O3′) = 2.04 Å), N4—H4⋯N7′ hydrogen bond (d(H4⋯N7′) = 2.27 Å), N6—H6⋯N5′′ hydrogen bond (d(H6⋯N5′′) = 2.12 Å), N8—H8⋯O2′′ hydrogen bond (d(H8⋯O2′′) = 2.07 Å); (ii) C4—H4A⋯O3′ hydrogen bond (d(H4A⋯O3′) = 2.56 Å), C10—H10A⋯O1′′ hydrogen bond (d(H10A⋯O1′′) = 2.41 Å), C12—H12C⋯O1′′′ hydrogen bond (d(H12C⋯O1′′′) = 2.46 Å), C28—H28A⋯O4′ hydrogen bond (d(H28A⋯O4′) = 2.30 Å), (′ = x, y − 1, z; ′′ = x, y + 1, z; ′′′ = −x − 1, y + 0.5, −z − 1).
Acknowledgements
We acknowledge the supports by Key project of Shaanxi Provincial Education Department (17JS029), Natural Science Basic Research Program of Shaanxi (2017JM8070). China Postdoctoral Science Foundation funded project (2016M602994).
References
1. Bruker. APEX2, SAINT and SADABS. Brucker AXS Inc., Madison, WI, USA (2009).Search in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
3. Zhou, J.; Klaβ, T.; Zhang, A.; Johnson, K. M.; Wang, C. Z.; Ye, Y.; Kozikowski, A. P.: Synthesis and pharmacological evaluation of (Z)-9-(heteroarylmethylene)-7-azatricyclo[4.3.1.03,7]decanes: ThiopheneAnalogues as potent norepinephrine transporter inhibitors. Bioorg. Med. Chem. Lett. 13 (2003) 3565–3569.10.1016/S0960-894X(03)00786-8Search in Google Scholar
4. Pillai, A. D.; Rathod, P. D.; Xavier, F. P.; Vasu, K. K.; Padh, H.; Sudarsanam, V.: Design, synthesis, and pharmacological evaluation of some2-[4-morpholino]-3-aryl-5-substituted thiophenes as novel anti-inflammatory agents: generation of a novel anti-inflammatory pharmacophore. Bioorg. Med. Chem. 12 (2004) 4667–4671.10.1016/j.bmc.2004.06.028Search in Google Scholar PubMed
5. Gao, M. Z.; Kong, D.; Clearfield, A.; Zingaro, R. A.: Novel structure-defined chiral bis(oxazolinyl)thiophenes for Ru-catalyzed asymmetric cyclopropanation. Tetrahedron. Lett. 45 (2004) 5649–5652.10.1016/j.tetlet.2004.05.120Search in Google Scholar
6. Han, J.; Yin, H.; Liu, C.; Wang, J.; Jian, X.: Construction of donor-acceptor polymers containing thiophenephthalazinonemoiety via classic Ullmann C—N couplingpolymerization and their optical-electrical properties. Polymer 101 (2016) 241–256.10.1016/j.polymer.2016.08.090Search in Google Scholar
7. Luo, Z.; Liu, Z.; Yang, Z.: The synthesis and photoactivated cytotoxicity of novel5-phenyl-3-(2,2?:5?,2?-terthien-5-yl)-4,5-dihydro-1H-pyrazolines. Chinese. Chem. Lett. 25 (2014) 333–336.10.1016/j.cclet.2013.11.007Search in Google Scholar
8. Qian, X.; Lan, X.;Yan, R.; He, Y.; Huang, J.; Hou, L.: T-shaped (D)2–A–p–A type sensitizers incorporating indoloquinoxaline and triphenylamine for organic dye-sensitized solar cells. Electrochim. Acta. 232 (2017) 377–386.10.1016/j.electacta.2017.02.166Search in Google Scholar
9. Taniguchi, T.; Fukui, K.; Asahi, R.; Urabe, Y.; Ikemoto, A.; Nakamoto, J.; Inada, Y.; Yamao, T.; Hotta, S.: Enhanced performance of organic solar cells based on thiophene/phenyleneco-oligomers. Synthetic. Met. 227 (2017) 156—162.10.1016/j.synthmet.2017.04.007Search in Google Scholar
10. Zuo, Z.; Lei, F.; Dai, Y.; Wang, L.: The crystal structure of 2-phenyl-4,6-bis(R-tert-butylsulfonamido)-1,3,5-triazine – ethyl acetate (2/1), C38H58N10O6S4. Z. Kristallogr. NCS 233 (2018) 555–557.10.1515/ncrs-2017-0292Search in Google Scholar
©2019 Zhenyu Zuo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 1H-indole-5-carboxylic acid – 4,4′-bipyridine (2/1), C14H11N2O2
- Crystal structure of ethyl 2-amino-4-(4-ethoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO6
- Crystal structure of ethyl 2-amino-4-(4-bromothiophen-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C16H16BrNO4S
- The crystal structure of 6-amino-2-methyl-8-(4-(methylthio)phenyl)-2,3,8,8a-tetrahydroisoquinoline-5,7,7(1H)-tricarbonitrile – ethanol (1/1), C20H19N5S
- Crystal structure of 6-amino-8-(4-isopropylphenyl)-2-methyl-2,3,8,8a-tetrahydroisoquinoline-5,7,7(1H)-tricarbonitrile-ethanol (1/1), C24H29N5O
- Crystal structure of 1,1′-(ethane-1,2-diyl)bis(3-ethyl-1H-imidazol-3-ium)bis(hexafluorido phosphate), C12H20F12N4P2
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-2-pivaloyl-2,3-dihydro-1H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isoquinoline-3a1,6a-dicarboxylate, C21H25NO8
- Crystal structure of methyl 4-(4-bromothiophen-2-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H20BrNO3S
- Hydrothermal synthesis and crystal structure of catena-poly[bis(4-((pyridin-4-ylmethyl)amino)benzoato-κ3N:O,O′)zinc(II) – 1,2-di(pyridin-4-yl)ethene – water (1/1/1), C38H34N6O5Zn
- The crystal structure of 1,2-dimethyl-3,4-dinitrobenzene, C8H8N2O4
- Synthesis and crystal structure of trans-tetraaqua-bis(3-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)propanoato-κO)zinc(II) tetrahydrate, C38H48N2O26S2Zn
- Crystal structure of diaqua-bis(μ2-6-chloropyridin-2-olato-κ3N,O:O)-tetrakis(chloropyridin-2-olato-κ1O)-bis(penanthroline-κ2N,N′)diterbium(III), C54H38Cl6Tb2N10O8
- Crystal structure of oxidobis(piperidine-1-carbodithioato-κ2S,S′)vanadium(IV), C12H20N2OS4V
- Crystal structure of 2-((tert-butyldimethylsilyl)oxy)-5-methylisophthalaldehyde, C15H22O3Si
- Crystal structure of catena-poly[tetraiodido-(μ2-1,4-bis(2-methyl-1H-imidazol-1-yl)benzene-κ2N:N′)dimercury(II)], C14H14Hg2I4N4
- Crystal structure of tetrakis(n-butyl)-(μ2-1,2-bis(2-oxidobenzoyl)hydrazine-1,2-diido-κ6N,O,O′:N′,O′′,O′′′)ditin(IV), C30H44N2O4Sn2
- Crystal structure of ethyl 2-amino-4-(4-hydroxy-3-methoxyphenyl)-7-methyl-5-oxo-4H,5H-pyrano-[4,3-b]pyran-3-carboxylate, C19H19NO7
- Crystal structure of 3-aminopyrazine-2-carbohydrazide, C5H7N5O
- Crystal structure of ethanol-bis(N-((5-(ethoxycarbonyl)-3,4-dimethyl-1H-pyrrol-2-yl)methylene)benzohydrazonato-κ2N,O)copper(II), C36H42N6O7Cu
- Crystal structure of 3-methyl-2-oxo-2H-chromen-7-yl propionate, C13H12O4
- Crystal structure of 2-(dimethylamino)ethyl 4-aminobenzoate, C11H16N2O2
- Crystal structure of 3-(benzo[d]thiazol-2-ylamino)isobenzofuran-1(3H)-one, C15H10N2O2S
- Crystal structure of 3-((1H-benzo[d]imidazol-2-yl)amino)-2-(1H-benzo[d]imidazol-2-yl)isoindolin-1-one, C22H16N6O
- Crystal structure of (2,2′-bipyridine-κ2N,N′)bis(4-(dimethylamino)phenyldiphenylphosphane-κP)copper(I) tetrafluoroborate, C50H48BCuF4N4P2
- Crystal structure of citric acid–acetonitrile (1/1), C8H11NO7
- Crystal structure of diethyl 2-(4-methoxyphenyl)-1-phenyl-1,2-dihydropyridine-3,5-dicarboxylate, C24H25NO5
- The crystal structure of poly[triaqua-bis(μ3-2,5-dihydroxyterephthalato-κ4O,O′:O′′:O′′′)-(μ4-oxalato-κ4O,O′:O′′,O′′′)cerium(III)], C9H10CeO11
- Crystal structure of 1-(5-(anthracen-9-yl)-3-(4-hydroxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)propan-1-one, C26H22N2O2
- Synthesis and crystal structure of 5-(8-(((2-carboxyethyl)ammonio)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate trihydrate, C19H23NO12S
- Crystal structure of rac-trans-6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromophenolato-κ4N,N′,O,O′)-bis(methanol)cobalt(III) chloride, C22H25Br2Co8N2O4Cl
- Crystal structure of 1-((R)-(2′-(dimethylamino)-[1,1′-binaphthalen]-2-yl))-3-((S)-2-hydroxy-1-phenylethyl)thiourea, C31H29N3OS
- Crystal structure and photochemical property of 1,8-bis(p-tolylthio)pyrene, C30H22S2
- Crystal structure of 2-(2-(2-amino-6-chloro-9H-purin-9-yl)ethyl)propane-1,3-diyl diacetate, C14H18ClN5O4
- Crystal structure of ethyl 5-amino-1-(pyridin-2-yl)-1H-pyrazole-4-carboxylate, C11H12N4O2
- Crystal structure of trichloro-(4-chloro-2,6-bis(diphenylmethyl)-N-((pyridin-2-yl)methylene)aniline)-aluminum dichloromethane solvate, C39H31AlCl6N2
- Bis(ethanol-κO)-bis(6-aminopicolinato-κ2N,O)magnesium(II), C16H22O6N4Mg
- Crystal structure of catena-poly[aqua-(μ2-1,7-dicarba-closo-dodecaborane-1,7-dicarboxylato-κ2O:O′)-(1,10-phenanthrolin-κ2N,N′)copper(II)], C16H20B10CuN2O5
- Crystal structure of (1,2-dicarba-closo-dodecaborane-1,2-dithiolato κ2S,S′)-bis(1,10-phenanthroline κ2N,N′)zinc(II), C26H26B10Zn4S2
- Crystal structure of diaqua-bis(1,10-phenanthroline-κ2N,N′)-bis(1,7-dicarba-closo-dodecaborane-1,7-dicarboxylato-κ3O,O′:O′′) dicobalt(II) — ethanol (1/1), C34H46B20Co2N4O11
- Crystal structure of ((5,5′-dimethoxy-2,2′-(1,2-phenylenebis(nitrilomethylidyne)))diphenolato-κ4O,N,O′,N′)copper(II), C22H18N2CuO4
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethan-1-ol, C17H14BrNO2
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one, C34H34N5O2Br
- Crystal structure of (Z)-2-((adamantan-1-ylimino)methyl)-5-methoxyphenol, C18H23NO2
- Crystal structure of bis((E)-2-ethoxy-6-(((2-hydroxyethyl)imino)methyl)phenolato-κ2N,O)copper(II), C22H28N2CuO6
- Crystal structure of 2,3-diphenyl-5,6-bis(4-methoxyphenyl)pyrazine, C30H24N2O2
- Crystal structure of dichlorido bis[1-((2,4-dimethyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN]cadmium(II), Cd(C12H13N5)2Cl2
- The crystal structure of 1,5-di(naphthalen-2-yl)-3-(pyridin-2-yl)pentane-1,5-dione, C30H23NO2
- The crystal structure of 2-((3-methylthiophen-2-yl)methylene)malononitrile, C9H6N2S
- The crystal structure of 1,4-dinitroso-2,3,5,6-tetraacetoxy-piperazine, C12H16N4O10
- Crystal structure of bis(2,4,6-trichlorophenyl) malonate, C15H6Cl6O4
- The crystal structure of trans-dichlorido-bis(pyridine-2-carboxylato-κ2N,O)platinum(IV), C12H8Cl2N2O4Pt
- Crystal structure of 3-nitroquinoline 1-oxide, C9H6N2O3
- Crystal structure of 2-(piperidin-1-ium-4-yl)-1H-benzo[d]imidazol-3-ium dichloride dihydrate, C12H21Cl2N3O2
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene, C30H50O
- Crystal structure of (E)-4-((2-fluoro-3-(trifluoromethyl)benzylidene)amino)-3-methyl-1H-1,2,4-triazole-5(4H)-thione, C11H8F4N4S
- Crystal structure of 5-(4-fluorophenyl)-4-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C9H8FN3S
- Crystal structure of catena-poly[(1-(4-fluorophenyl)-N–(5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)methanimine], (C12H14FN3S2Sn)n
- The crystal structure of 4-(methoxycarbonyl)benzoic acid, C9H8O4
- The crystal structure of N,N′-(6-(thiophen-2-yl)-1,3,5-triazine-2,4-diyl)bis(2-methylpropane-2-sulfonamide) – ethyl acetate(2/1), C34H54N10O6S6
- Crystal structure of N′-(1-(2-hydroxyphenyl)ethylidene)-5-methyl-1-phenyl-1H-1,2,3-triazole-4-carbohydrazide, C18H17N5O2
- Crystal structure of 3-(4-methoxyphenyl)-1-phenylprop-2-yn-1-one, C16H12O2
- Crystal structure of N′-(1-(benzofuran-2-yl)ethylidene)-2-cyanoacetohydrazide, C13H11N3O2
- Crystal structure of hexa-μ2-chlorido-μ4-oxido-tetrakis(1-vinyl-1H-imidazole-κN)tetracopper(II), C20H24Cu4Cl6N8O
- Crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-4-methylbenzenesulfonohydrazide, C22H22O5N2S
- Crystal structure of 2-acetyl pyrene, C18H12O
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of 1H-indole-5-carboxylic acid – 4,4′-bipyridine (2/1), C14H11N2O2
- Crystal structure of ethyl 2-amino-4-(4-ethoxyphenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C23H21NO6
- Crystal structure of ethyl 2-amino-4-(4-bromothiophen-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C16H16BrNO4S
- The crystal structure of 6-amino-2-methyl-8-(4-(methylthio)phenyl)-2,3,8,8a-tetrahydroisoquinoline-5,7,7(1H)-tricarbonitrile – ethanol (1/1), C20H19N5S
- Crystal structure of 6-amino-8-(4-isopropylphenyl)-2-methyl-2,3,8,8a-tetrahydroisoquinoline-5,7,7(1H)-tricarbonitrile-ethanol (1/1), C24H29N5O
- Crystal structure of 1,1′-(ethane-1,2-diyl)bis(3-ethyl-1H-imidazol-3-ium)bis(hexafluorido phosphate), C12H20F12N4P2
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-2-pivaloyl-2,3-dihydro-1H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isoquinoline-3a1,6a-dicarboxylate, C21H25NO8
- Crystal structure of methyl 4-(4-bromothiophen-2-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H20BrNO3S
- Hydrothermal synthesis and crystal structure of catena-poly[bis(4-((pyridin-4-ylmethyl)amino)benzoato-κ3N:O,O′)zinc(II) – 1,2-di(pyridin-4-yl)ethene – water (1/1/1), C38H34N6O5Zn
- The crystal structure of 1,2-dimethyl-3,4-dinitrobenzene, C8H8N2O4
- Synthesis and crystal structure of trans-tetraaqua-bis(3-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)propanoato-κO)zinc(II) tetrahydrate, C38H48N2O26S2Zn
- Crystal structure of diaqua-bis(μ2-6-chloropyridin-2-olato-κ3N,O:O)-tetrakis(chloropyridin-2-olato-κ1O)-bis(penanthroline-κ2N,N′)diterbium(III), C54H38Cl6Tb2N10O8
- Crystal structure of oxidobis(piperidine-1-carbodithioato-κ2S,S′)vanadium(IV), C12H20N2OS4V
- Crystal structure of 2-((tert-butyldimethylsilyl)oxy)-5-methylisophthalaldehyde, C15H22O3Si
- Crystal structure of catena-poly[tetraiodido-(μ2-1,4-bis(2-methyl-1H-imidazol-1-yl)benzene-κ2N:N′)dimercury(II)], C14H14Hg2I4N4
- Crystal structure of tetrakis(n-butyl)-(μ2-1,2-bis(2-oxidobenzoyl)hydrazine-1,2-diido-κ6N,O,O′:N′,O′′,O′′′)ditin(IV), C30H44N2O4Sn2
- Crystal structure of ethyl 2-amino-4-(4-hydroxy-3-methoxyphenyl)-7-methyl-5-oxo-4H,5H-pyrano-[4,3-b]pyran-3-carboxylate, C19H19NO7
- Crystal structure of 3-aminopyrazine-2-carbohydrazide, C5H7N5O
- Crystal structure of ethanol-bis(N-((5-(ethoxycarbonyl)-3,4-dimethyl-1H-pyrrol-2-yl)methylene)benzohydrazonato-κ2N,O)copper(II), C36H42N6O7Cu
- Crystal structure of 3-methyl-2-oxo-2H-chromen-7-yl propionate, C13H12O4
- Crystal structure of 2-(dimethylamino)ethyl 4-aminobenzoate, C11H16N2O2
- Crystal structure of 3-(benzo[d]thiazol-2-ylamino)isobenzofuran-1(3H)-one, C15H10N2O2S
- Crystal structure of 3-((1H-benzo[d]imidazol-2-yl)amino)-2-(1H-benzo[d]imidazol-2-yl)isoindolin-1-one, C22H16N6O
- Crystal structure of (2,2′-bipyridine-κ2N,N′)bis(4-(dimethylamino)phenyldiphenylphosphane-κP)copper(I) tetrafluoroborate, C50H48BCuF4N4P2
- Crystal structure of citric acid–acetonitrile (1/1), C8H11NO7
- Crystal structure of diethyl 2-(4-methoxyphenyl)-1-phenyl-1,2-dihydropyridine-3,5-dicarboxylate, C24H25NO5
- The crystal structure of poly[triaqua-bis(μ3-2,5-dihydroxyterephthalato-κ4O,O′:O′′:O′′′)-(μ4-oxalato-κ4O,O′:O′′,O′′′)cerium(III)], C9H10CeO11
- Crystal structure of 1-(5-(anthracen-9-yl)-3-(4-hydroxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)propan-1-one, C26H22N2O2
- Synthesis and crystal structure of 5-(8-(((2-carboxyethyl)ammonio)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate trihydrate, C19H23NO12S
- Crystal structure of rac-trans-6,6′-((cyclohexane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-bromophenolato-κ4N,N′,O,O′)-bis(methanol)cobalt(III) chloride, C22H25Br2Co8N2O4Cl
- Crystal structure of 1-((R)-(2′-(dimethylamino)-[1,1′-binaphthalen]-2-yl))-3-((S)-2-hydroxy-1-phenylethyl)thiourea, C31H29N3OS
- Crystal structure and photochemical property of 1,8-bis(p-tolylthio)pyrene, C30H22S2
- Crystal structure of 2-(2-(2-amino-6-chloro-9H-purin-9-yl)ethyl)propane-1,3-diyl diacetate, C14H18ClN5O4
- Crystal structure of ethyl 5-amino-1-(pyridin-2-yl)-1H-pyrazole-4-carboxylate, C11H12N4O2
- Crystal structure of trichloro-(4-chloro-2,6-bis(diphenylmethyl)-N-((pyridin-2-yl)methylene)aniline)-aluminum dichloromethane solvate, C39H31AlCl6N2
- Bis(ethanol-κO)-bis(6-aminopicolinato-κ2N,O)magnesium(II), C16H22O6N4Mg
- Crystal structure of catena-poly[aqua-(μ2-1,7-dicarba-closo-dodecaborane-1,7-dicarboxylato-κ2O:O′)-(1,10-phenanthrolin-κ2N,N′)copper(II)], C16H20B10CuN2O5
- Crystal structure of (1,2-dicarba-closo-dodecaborane-1,2-dithiolato κ2S,S′)-bis(1,10-phenanthroline κ2N,N′)zinc(II), C26H26B10Zn4S2
- Crystal structure of diaqua-bis(1,10-phenanthroline-κ2N,N′)-bis(1,7-dicarba-closo-dodecaborane-1,7-dicarboxylato-κ3O,O′:O′′) dicobalt(II) — ethanol (1/1), C34H46B20Co2N4O11
- Crystal structure of ((5,5′-dimethoxy-2,2′-(1,2-phenylenebis(nitrilomethylidyne)))diphenolato-κ4O,N,O′,N′)copper(II), C22H18N2CuO4
- Crystal structure of 1-(5-bromo-2-(4-methoxyphenyl)-1H-indol-7-yl)ethan-1-ol, C17H14BrNO2
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one, C34H34N5O2Br
- Crystal structure of (Z)-2-((adamantan-1-ylimino)methyl)-5-methoxyphenol, C18H23NO2
- Crystal structure of bis((E)-2-ethoxy-6-(((2-hydroxyethyl)imino)methyl)phenolato-κ2N,O)copper(II), C22H28N2CuO6
- Crystal structure of 2,3-diphenyl-5,6-bis(4-methoxyphenyl)pyrazine, C30H24N2O2
- Crystal structure of dichlorido bis[1-((2,4-dimethyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN]cadmium(II), Cd(C12H13N5)2Cl2
- The crystal structure of 1,5-di(naphthalen-2-yl)-3-(pyridin-2-yl)pentane-1,5-dione, C30H23NO2
- The crystal structure of 2-((3-methylthiophen-2-yl)methylene)malononitrile, C9H6N2S
- The crystal structure of 1,4-dinitroso-2,3,5,6-tetraacetoxy-piperazine, C12H16N4O10
- Crystal structure of bis(2,4,6-trichlorophenyl) malonate, C15H6Cl6O4
- The crystal structure of trans-dichlorido-bis(pyridine-2-carboxylato-κ2N,O)platinum(IV), C12H8Cl2N2O4Pt
- Crystal structure of 3-nitroquinoline 1-oxide, C9H6N2O3
- Crystal structure of 2-(piperidin-1-ium-4-yl)-1H-benzo[d]imidazol-3-ium dichloride dihydrate, C12H21Cl2N3O2
- Crystal structure of (4S,4aS,6aR,6bR,12aS,12bR,14aS,14bR)-3,3,6a,6b,9,9,12a-heptamethyloctadecahydro-1H,3H-4,14b-ethanophenanthro[1,2-h]isochromene, C30H50O
- Crystal structure of (E)-4-((2-fluoro-3-(trifluoromethyl)benzylidene)amino)-3-methyl-1H-1,2,4-triazole-5(4H)-thione, C11H8F4N4S
- Crystal structure of 5-(4-fluorophenyl)-4-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C9H8FN3S
- Crystal structure of catena-poly[(1-(4-fluorophenyl)-N–(5-((trimethylstannyl)thio)-1,3,4-thiadiazol-2-yl)methanimine], (C12H14FN3S2Sn)n
- The crystal structure of 4-(methoxycarbonyl)benzoic acid, C9H8O4
- The crystal structure of N,N′-(6-(thiophen-2-yl)-1,3,5-triazine-2,4-diyl)bis(2-methylpropane-2-sulfonamide) – ethyl acetate(2/1), C34H54N10O6S6
- Crystal structure of N′-(1-(2-hydroxyphenyl)ethylidene)-5-methyl-1-phenyl-1H-1,2,3-triazole-4-carbohydrazide, C18H17N5O2
- Crystal structure of 3-(4-methoxyphenyl)-1-phenylprop-2-yn-1-one, C16H12O2
- Crystal structure of N′-(1-(benzofuran-2-yl)ethylidene)-2-cyanoacetohydrazide, C13H11N3O2
- Crystal structure of hexa-μ2-chlorido-μ4-oxido-tetrakis(1-vinyl-1H-imidazole-κN)tetracopper(II), C20H24Cu4Cl6N8O
- Crystal structure of N′-((1E,2E)-4-(7-methoxy-2-oxo-2H-chromen-8-yl)-2-methylbut-2-en-1-ylidene)-4-methylbenzenesulfonohydrazide, C22H22O5N2S
- Crystal structure of 2-acetyl pyrene, C18H12O

