Abstract
C16H16O7, monoclinic, P21/n (no. 14), a = 12.830(3) Å, b = 8.1101(16) Å, c = 14.900(3) Å, β = 114.88(3)°, V = 1406.5(6) Å3, Z = 4, Rgt(F) = 0.0578, wRref(F2) = 0.1799, T = 100(2) K.
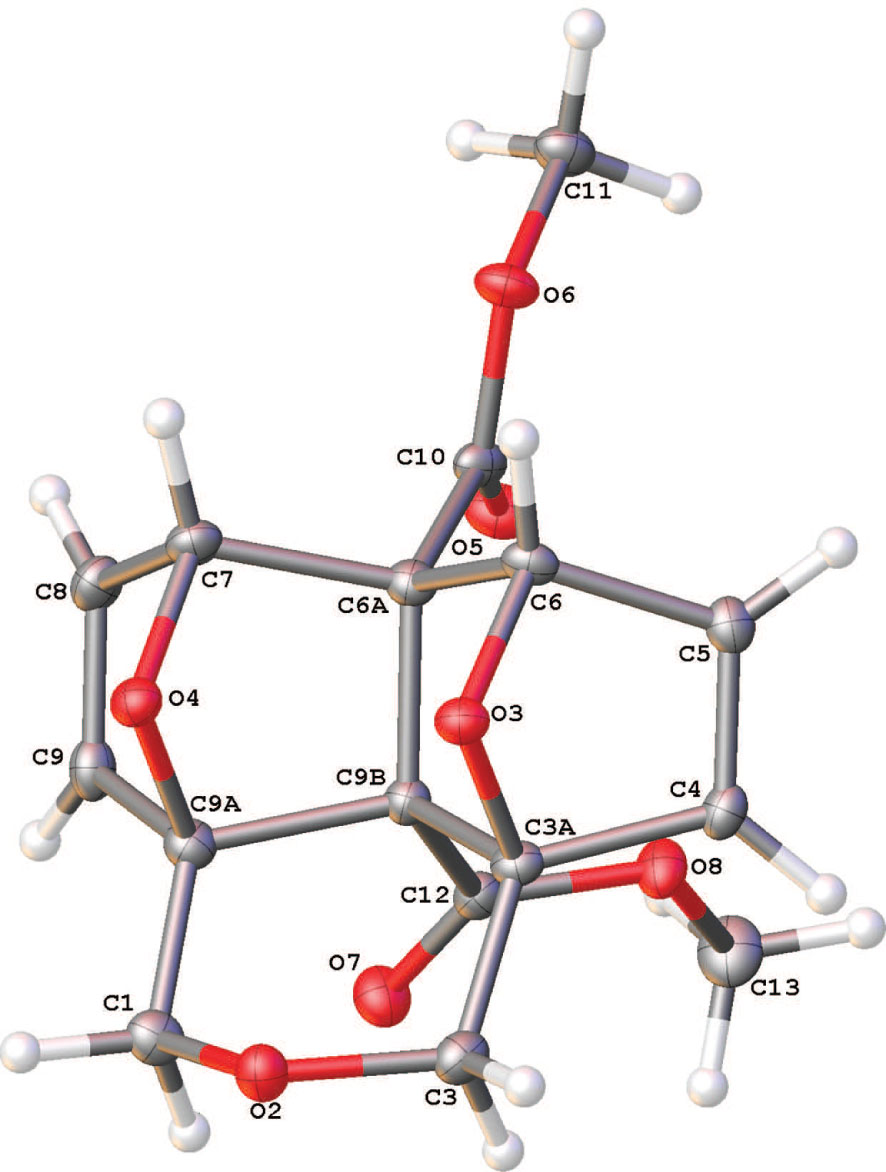
The asymmetric unit of the title crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Crystal collection and handling.
| Crystal: | Prism, colourless |
| Size: | 0.25 × 0.15 × 0.10 mm |
| Wavelength: | synchrotron radiation (λ = 0.9699 Å) |
| μ: | 0.282 mm−1 |
| Diffractometer, scan mode: | synchrotron source |
| θmax, completeness: | 38.5°, >97% |
| N(hkl)measured, N(hkl)unique, Rint: | 13125, 3051, 0.0965 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2293 |
| N(param)refined: | 211 |
| Programs: | SHELX [1] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.49596(18) | 0.7828(3) | 0.74001(16) | 0.0197(5) |
| H1A | 0.557699 | 0.735234 | 0.800019 | 0.024 |
| H1B | 0.532417 | 0.853153 | 0.706970 | 0.024 |
| O2 | 0.42176(12) | 0.88305(19) | 0.76850(11) | 0.0203(4) |
| C3 | 0.32894(19) | 0.9602(3) | 0.68657(17) | 0.0197(5) |
| H3A | 0.361011 | 1.033878 | 0.651500 | 0.024 |
| H3B | 0.283276 | 1.028560 | 0.711962 | 0.024 |
| C3A | 0.25123(18) | 0.8339(3) | 0.61457(15) | 0.0156(5) |
| C4 | 0.14311(18) | 0.8870(3) | 0.52386(16) | 0.0169(5) |
| H4 | 0.128009 | 0.991704 | 0.492368 | 0.020 |
| C5 | 0.07493(18) | 0.7548(3) | 0.49870(16) | 0.0175(5) |
| H5 | 0.001691 | 0.745159 | 0.444519 | 0.021 |
| C6 | 0.13864(17) | 0.6225(3) | 0.57473(15) | 0.0164(5) |
| H6 | 0.088478 | 0.535838 | 0.583817 | 0.020 |
| C6A | 0.24101(17) | 0.5543(3) | 0.55197(16) | 0.0157(5) |
| C7 | 0.32232(18) | 0.4288(3) | 0.63400(16) | 0.0180(5) |
| H7 | 0.282245 | 0.331857 | 0.646628 | 0.022 |
| C8 | 0.42161(18) | 0.3862(3) | 0.60684(17) | 0.0201(6) |
| H8 | 0.432604 | 0.286106 | 0.578856 | 0.024 |
| C9 | 0.48936(19) | 0.5183(3) | 0.63049(16) | 0.0197(5) |
| H9 | 0.559281 | 0.531507 | 0.623734 | 0.024 |
| C9A | 0.43087(18) | 0.6452(3) | 0.67109(16) | 0.0175(5) |
| C9B | 0.32100(17) | 0.7098(3) | 0.57792(15) | 0.0150(5) |
| O3 | 0.20060(12) | 0.72308(18) | 0.66095(10) | 0.0165(4) |
| O4 | 0.37938(12) | 0.53564(18) | 0.71785(11) | 0.0176(4) |
| C10 | 0.20355(18) | 0.4746(3) | 0.45164(17) | 0.0173(5) |
| O5 | 0.24502(13) | 0.4945(2) | 0.39265(12) | 0.0242(4) |
| O6 | 0.11832(12) | 0.36485(18) | 0.43728(11) | 0.0199(4) |
| C11 | 0.07643(19) | 0.2713(3) | 0.34575(16) | 0.0214(5) |
| H11A | 0.048904 | 0.347361 | 0.289415 | 0.032 |
| H11B | 0.013231 | 0.199667 | 0.342040 | 0.032 |
| H11C | 0.138885 | 0.203744 | 0.344019 | 0.032 |
| C12 | 0.35819(18) | 0.7856(3) | 0.50283(15) | 0.0158(5) |
| O7 | 0.45792(12) | 0.80065(19) | 0.51634(11) | 0.0226(4) |
| O8 | 0.27094(12) | 0.84531(19) | 0.42136(11) | 0.0207(4) |
| C13 | 0.3039(2) | 0.9123(3) | 0.34665(18) | 0.0280(6) |
| H13A | 0.235775 | 0.955633 | 0.291368 | 0.042 |
| H13B | 0.338625 | 0.825145 | 0.322623 | 0.042 |
| H13C | 0.359680 | 1.001489 | 0.375553 | 0.042 |
Source of materials
Synthesis and their characterization by 1H and 13C-NMR, IR and HRMS methods are reported [21], [22].
Experimental details
H atoms were located in the difference Fourier map, but refined with fixed individual displacement parameters, using a riding model with C—H distances of 0.95–1.0 Å (for aromatic rings), and C—H distances 0.99, 0.98 Å for methylene and methyl groups, with U(H) values of 1.2Ueq(CAr and CH2) and 1.5Ueq(C) (for CH3).
Discussion
Weak interactions, such as hydrogen, halogen, chalcogen, pnicogen, tetrel and icosagen bonds were extensively used in the synthesis, catalysis, crystal engineering, drug delivery, etc. [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Among those, hydrogen bonding has turned out to be particularly suitable for design of organic and coordination compounds [13], [14], [15], [16], [17], [18], [19], [20].
The molecule comprises a fused hexacyclic system containing four five-membered rings in the usual envelope conformations with Cremer and Pople parameters [23] ranging from (Q(2) = 0.521(2)–0.581(2) Å; φ2 = 177.6(2)–181.6(3)°) and one six-membered ring adopting a chair conformation with Cremer and Pople parameters Q = 0.536(2) Å, θ = 4.1(2)°, φ = 14(4)°, respectively. In the crystal structure the molecules are linked by one strong C—O⋯H intermolecular hydrogen bonds which links molecules into centrosymmetric dimers with R22(10) graph-set notation [24]. The carboxylate groups are α-oriented. All distances and angles are normal.
Acknowledgements
X-ray crystallographic studies using synchrotron radiation were performed at the unique scientific facility Kurchatov Synchrotron Radiation Source supported by the Ministry of Education and Science of the Russian Federation (project code RFMEFI61917X0007). This work has also been partially supported by Universidad de Antofagasta, and Baku State University.
References
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G.: The halogen bond. Chem. Rev. 116 (2016) 2478–2601.10.1021/acs.chemrev.5b00484Search in Google Scholar PubMed PubMed Central
Kvyatkovskaya, E. A.; Zaytsev, V.; Zubkov, F. I.; Dorovatovskii, P. V.; Zubavichus, Y. V.; Khrustalev, V. N.: Interaction between maleic acid and N-R-furfurylamines: crystal structure of 2-methyl-N-[(5-phenylfuran-2-yl)methyl]propan-2-aminium(2Z)-3-carboxyacrylate and N-[(5-iodofuran-2-yl)methyl]-2-methylpropan-2-aminium(2Z)-3-carboxyprop-2-enoate. Acta Crystallogr. E 73 (2017) 515–519.10.1107/S2056989017003541Search in Google Scholar PubMed PubMed Central
Mahmudov, K. T.; Kopylovich, M. N.; Guedes da Silva, M. F. C.; Pombeiro, A. J. L.: Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 46 (2017) 10121–10138.10.1039/C7DT01685ASearch in Google Scholar PubMed
Scheiner, S.: The pnicogen bond: its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 46 (2013) 280–288.10.1021/ar3001316Search in Google Scholar PubMed
Jlassi, R.; Ribeiro, A. P. C.; Guedes da Silva, M. F. C.; Mahmudov, K. T.; Kopylovich, M. N.; Anisimova, T. B.; Naïli, H.; Tiago, G. A. O.; Pombeiro, A. J. L.: Polynuclear copper(II) complexes as catalysts for the peroxidative oxidation of cyclohexane in a room-temperature ionic liquid. Eur. J. Inorg. Chem. 2014 (2014) 4541–4550.10.1002/ejic.201402352Search in Google Scholar
Politzer, P.; Murray, J. S.; Clark, T.; Resnati, G.: The σ-hole revisited. Phys. Chem. Chem. Phys. 19 (2017) 32166–32178.10.1039/C7CP06793CSearch in Google Scholar PubMed
Akbari, A. F.; Mahmoudi, G.; Gurbanov. A. V.; Zubkov, F. I.; Qu, F.; Gupta, A.; Safin, D. A.: Solvent-driven azide-induced mononuclear discrete versus one-dimensional polymeric aromatic Mobius cadmium(II) complexes of an N6 tetradentate helical ligand. Dalton Trans. 46 (2017) 14888–14896.10.1039/C7DT02952GSearch in Google Scholar
Shixaliyev, N. Q.; Ahmadova, N. E.; Gurbanov, A. V.; Maharramov, A. M.; Mammadova, G. Z.; Nenajdenko, V. G.; Zubkov, F. I.; Mahmudov, K. T.; Pombeiro, A. J. L.: Tetrel, halogen and hydrogen bonds in bis(4-((E)-(2,2-dichloro-1-(4-substitutedphenyl)vinyl)diazenyl)phenyl)methane dyes. Dyes Pigments 150 (2018) 377–381.10.1016/j.dyepig.2017.12.033Search in Google Scholar
Gurbanov, A. V.; Mahmudov, K. T.; Sutradhar, M.; Guedes da Silva, M. F. C.; Mahmudov, T. A.; Guseinov, F. I.; Zubkov, F. I.; Maharramov, A. M.; Pombeiro, A. J. L.: Copper(II) complexes with carboxylic- or sulfonic-functionalized arylhydrazones of acetoacetanilide and their application in cyanosilylation of aldehydes. J. Organomet. Chem. 834 (2017) 22–27.10.1016/j.jorganchem.2017.02.006Search in Google Scholar
Ma, Z.; Gurbanov, A. V.; Sutradhar, M.; Kopylovich, M. N.; Mahmudov, K. T.; Maharramov, A. M.; Guseinov, F. I.; Zubkov, F. I.; Pombeiro, A. J. L.: Effective cyanosilylation of aldehydes with copper(II)-based polymeric catalysts. J. Mol. Catal. A: Chem. 428 (2017) 17–23.10.1016/j.molcata.2016.11.036Search in Google Scholar
Shetnev, A. A.; Zubkov, F. I.: The latest advances in chemistry of 1,2,4-oxadiazines. Chem. Heterocycl. Compd. 53 (2017) 495–497.10.1007/s10593-017-2081-1Search in Google Scholar
Desiraju, G. R.: Supramolecular synthons in crystal engineering – a new organic synthesis. Angew. Chem. Int. Ed. 34 (1995) 2311–2327.10.1002/anie.199523111Search in Google Scholar
Mahmudov, K. T.; Pombeiro, A. J. L.: Resonance–assisted hydrogen bonding as a driving force in synthesis and a synthon in the design of materials. Chem. Eur. J. 22 (2016) 16356–16398.10.1002/chem.201601766Search in Google Scholar PubMed
Mahmudov, K. T.; Kopylovich, M. N.; Sabbatini, A.; Drew, M. G. B.; Martins, L. M. D. R. S.; Pettinari, C.; Pombeiro, A. J. L.: Cooperative metal–ligand assistedE/Zisomerization and cyano activation at CuII and CoII complexes of arylhydrazones of active methylene nitriles. Inorg. Chem. 53 (2014) 9946–9958.10.1021/ic501704gSearch in Google Scholar PubMed
Gurbanov, A. V.; Maharramov, A. M.; Zubkov, F. I.; Saifutdinov, A. M.; Guseinov, F. I.: Cyanosilylation of aldehydes catalyzed by iron(III) arylhydrazone-beta-diketone complexes. Aust. J. Chem. 71 (2018) 190–194.10.1071/CH17595Search in Google Scholar
Mahmudov, K. T.; Kopylovich, M. N.; Guedes da Silva, M. F. C.; Pombeiro, A. J. L.: Non-covalent interactions in the synthesis of coordination compounds: recent advances. Coord. Chem. Rev. 345 (2017) 54–72.10.1016/j.ccr.2016.09.002Search in Google Scholar
Hazra, S.; Martins, N. M. R.; Mahmudov, K. T.; Zubkov, F. I.; Guedes da Silva, M. F. C.; Pombeiro, A. J. L.: A tetranuclear diphenyltin(IV) complex and its catalytic activity in the aerobic Baeyer-Villiger oxidation of cyclohexanone. J. Organomet. Chem. 867 (2018) 197–200.10.1016/j.jorganchem.2017.12.040Search in Google Scholar
Mahmoudi, G.; Zangrando, E.; Mitoraj, M. P.; Gurbanov, A. V.; Zubkov, F. I.; Moosavifar, M.; Konyaeva, I. A.; Kirillov, A. M.; Safin, D. A.: Extended lead(II) architectures engineered via tetrel bonding interactions. New J. Chem. 42 (2018) 4959–4971.10.1039/C8NJ00525GSearch in Google Scholar
Mahmudov, K. T.; Guedes da Silva, M. F. C.; Sutradhar, M.; Kopylovich, M. N.; Huseynov, F. E.; Shamilov, N. T.; Voronina, A. A.; Buslaeva, T. M.; Pombeiro, A. J. L.: Lanthanide derivatives comprising arylhydrazones of β-Diketones: cooperative E/Z isomerization and catalytic activity in nitroaldol reaction. Dalton Trans. 44 (2015) 5602–5610.10.1039/C4DT03788JSearch in Google Scholar
Borisova, K. K.; Kvyatkovskaya, E. A.; Nikitina, E. V.; Aysin, R. R.; Novikov, R. A.; Zubkov, F. I.: Classical example of total kinetic and thermodynamic control: the Diels–Alder reaction between DMAD and bis-furyl dienes. J. Org. Chem. 83 (2018) 4840–4850.10.1021/acs.joc.8b00336Search in Google Scholar PubMed
Borisova, K. K.; Nikitina, E. V.; Novikov, R. A.; Khrustalev, V. N.; Dorovatovskii, P. V.; Zubavichus, Y. V.; Kuznetsov, M. L.; Zaytsev, V. P.; Varlamov, A. V.; Zubkov, F. I.: Diels–Alder reactions between hexafluoro-2-butyne and bis-furyl dienes: kinetic versus thermodynamic control. Chem. Commun. 54 (2018) 2850–2853.10.1039/C7CC09466CSearch in Google Scholar PubMed
Cremer, D.; Pople, J. A.: General definition of ring puckering coordinates. J. Am. Chem. Soc. 97 (1975) 1354–1358.10.1021/ja00839a011Search in Google Scholar
Bernstein, J.; Davis, R. E.; Shimoni, L.; Chang, N.-L.: Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 34 (1995) 1555–1573.10.1002/anie.199515551Search in Google Scholar
©2018 Pavel V. Dorovatovskii et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Articles in the same Issue
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S

