Abstract
C98H84Cl6N8O8, triclinic, P1̄ (no. 2), a = 8.3431(3) Å, b = 16.1109(6) Å, c = 18.1405(7) Å, α = 109.242(4)°, β = 95.119(3)°, γ = 103.798(3)°, V = 2197.92(16) Å3, Z = 1, Rgt(F) = 0.0595, wRref(F2) = 0.1689, T = 293(2) K.
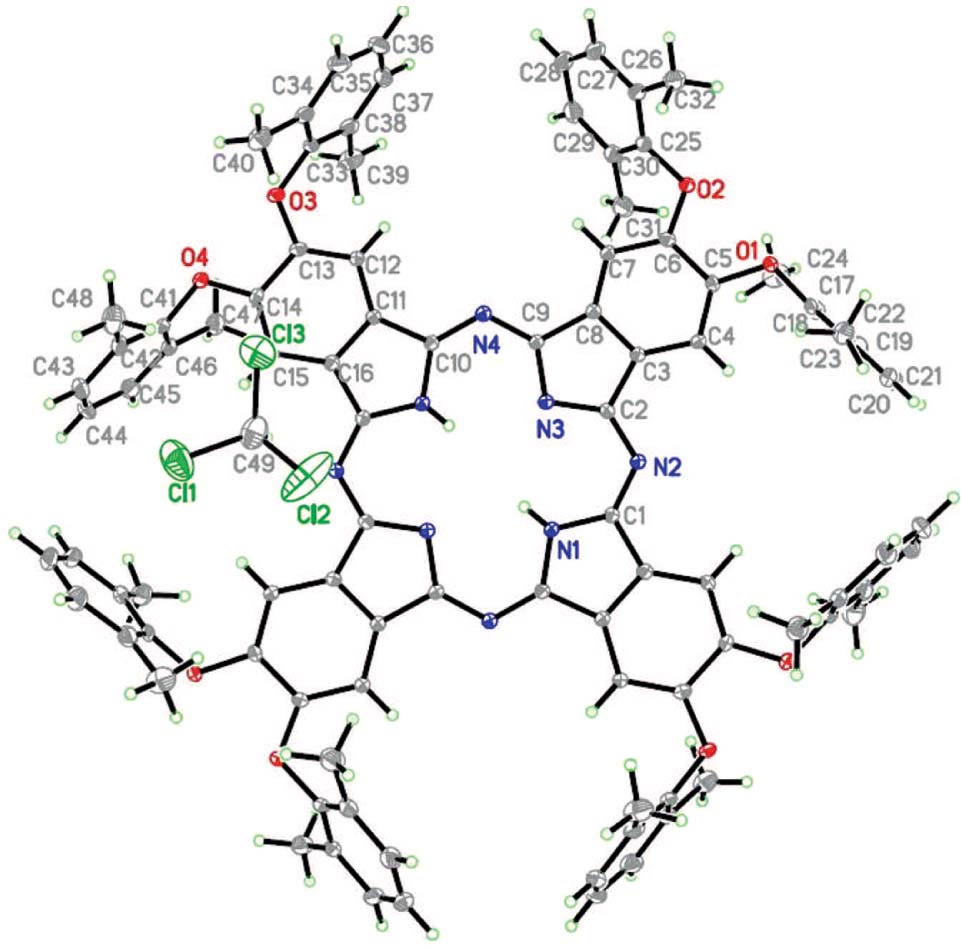
The crystal structure is shown in the figure. The non-hydrogen atoms of the asymmetric unit are labeled. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green block |
| Size: | 0.10 × 0.08 × 0.06 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 2.28 mm−1 |
| Diffractometer, scan mode: | APEX, ω and ϕ scans |
| θmax, completeness: | 63.5°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 14028, 7069, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5641 |
| N(param)refined: | 554 |
| Programs: | Bruker [1, 3] , SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | −0.1555(2) | 0.29448(12) | 0.72314(11) | 0.0247(4) |
| N1 | 0.5431(3) | 0.60934(16) | 0.60052(15) | 0.0235(5) |
| C1 | 0.4743(3) | 0.60477(18) | 0.66491(16) | 0.0221(6) |
| H1 | 0.522(4) | 0.570(2) | 0.558(2) | 0.031(9)* |
| O2 | −0.2205(2) | 0.15334(13) | 0.59084(11) | 0.0282(5) |
| N2 | 0.3595(3) | 0.53648(15) | 0.67115(13) | 0.0230(5) |
| C2 | 0.2965(3) | 0.45500(18) | 0.61243(16) | 0.0214(6) |
| O3 | 0.1971(3) | 0.04385(13) | 0.12218(11) | 0.0287(5) |
| N3 | 0.3465(3) | 0.42391(15) | 0.54137(13) | 0.0223(5) |
| C3 | 0.1606(3) | 0.38431(18) | 0.61993(16) | 0.0220(6) |
| O4 | 0.3897(3) | 0.14863(13) | 0.06637(11) | 0.0294(5) |
| N4 | 0.2539(3) | 0.27817(15) | 0.43062(13) | 0.0233(5) |
| C4 | 0.0650(3) | 0.38415(18) | 0.67917(16) | 0.0228(6) |
| H4 | 0.0835 | 0.4359 | 0.7248 | 0.027* |
| C5 | −0.0576(3) | 0.30472(19) | 0.66749(16) | 0.0218(6) |
| C6 | −0.0888(3) | 0.22660(18) | 0.59694(16) | 0.0233(6) |
| C7 | 0.0069(3) | 0.22754(18) | 0.53856(16) | 0.0236(6) |
| H7 | −0.0122 | 0.1764 | 0.4924 | 0.028* |
| C8 | 0.1330(3) | 0.30782(18) | 0.55144(16) | 0.0216(6) |
| C9 | 0.2500(3) | 0.33499(18) | 0.50298(16) | 0.0219(6) |
| C10 | 0.3475(3) | 0.30574(18) | 0.38343(16) | 0.0212(6) |
| C11 | 0.3473(3) | 0.25114(18) | 0.30185(16) | 0.0218(6) |
| C12 | 0.2582(4) | 0.16098(18) | 0.25422(16) | 0.0238(6) |
| H12 | 0.1880 | 0.1231 | 0.2744 | 0.029* |
| C13 | 0.2783(4) | 0.13045(18) | 0.17634(17) | 0.0248(6) |
| C14 | 0.3835(4) | 0.18815(19) | 0.14503(16) | 0.0229(6) |
| C15 | 0.4729(3) | 0.27647(18) | 0.19220(16) | 0.0229(6) |
| H15 | 0.5434 | 0.3143 | 0.1721 | 0.028* |
| C16 | 0.4535(3) | 0.30713(18) | 0.27161(16) | 0.0226(6) |
| C17 | −0.1900(4) | 0.37306(19) | 0.77348(17) | 0.0268(6) |
| C18 | −0.3363(4) | 0.3907(2) | 0.7497(2) | 0.0354(7) |
| C19 | −0.3789(5) | 0.4641(2) | 0.8012(2) | 0.0469(9) |
| H19 | −0.4774 | 0.4771 | 0.7869 | 0.056* |
| C20 | −0.2756(5) | 0.5178(2) | 0.8733(2) | 0.0515(10) |
| H20 | −0.3047 | 0.5670 | 0.9074 | 0.062* |
| C21 | −0.1293(5) | 0.4991(2) | 0.8950(2) | 0.0431(9) |
| H21 | −0.0603 | 0.5366 | 0.9435 | 0.052* |
| C22 | −0.0826(4) | 0.4253(2) | 0.84595(17) | 0.0318(7) |
| C23 | 0.0716(5) | 0.4022(2) | 0.87151(19) | 0.0419(8) |
| H23A | 0.1452 | 0.4545 | 0.9139 | 0.063* |
| H23B | 0.1282 | 0.3853 | 0.8274 | 0.063* |
| H23C | 0.0401 | 0.3518 | 0.8895 | 0.063* |
| C24 | −0.4450(5) | 0.3325(3) | 0.6696(2) | 0.0513(10) |
| H24A | −0.5593 | 0.3330 | 0.6718 | 0.077* |
| H24B | −0.4381 | 0.2707 | 0.6555 | 0.077* |
| H24C | −0.4066 | 0.3566 | 0.6304 | 0.077* |
| C25 | −0.2718(4) | 0.0798(2) | 0.51717(18) | 0.0288(7) |
| C26 | −0.2391(4) | −0.0020(2) | 0.51448(19) | 0.0316(7) |
| C27 | −0.2946(4) | −0.0757(2) | 0.4425(2) | 0.0402(8) |
| H27 | −0.2753 | −0.1314 | 0.4384 | 0.048* |
| C28 | −0.3779(4) | −0.0676(2) | 0.3771(2) | 0.0451(9) |
| H28 | −0.4121 | −0.1173 | 0.3292 | 0.054* |
| C29 | −0.4102(4) | 0.0138(2) | 0.3826(2) | 0.0419(8) |
| H29 | −0.4679 | 0.0182 | 0.3384 | 0.050* |
| C30 | −0.3583(4) | 0.0899(2) | 0.45322(19) | 0.0343(7) |
| C31 | −0.3983(5) | 0.1777(2) | 0.4590(2) | 0.0447(8) |
| H31A | −0.4425 | 0.1997 | 0.5065 | 0.067* |
| H31B | −0.4800 | 0.1668 | 0.4136 | 0.067* |
| H31C | −0.2979 | 0.2228 | 0.4606 | 0.067* |
| C32 | −0.1521(4) | −0.0109(2) | 0.5857(2) | 0.0406(8) |
| H4A1 | −0.2048 | 0.0115 | 0.6302 | 0.061* |
| H4A2 | −0.0363 | 0.0242 | 0.5976 | 0.061* |
| H4A3 | −0.1592 | −0.0742 | 0.5752 | 0.061* |
| C33 | 0.1221(4) | −0.02066(18) | 0.15373(16) | 0.0260(6) |
| C34 | 0.2211(4) | −0.0691(2) | 0.17681(17) | 0.0305(7) |
| C35 | 0.1459(5) | −0.1343(2) | 0.2065(2) | 0.0426(8) |
| H35 | 0.2080 | −0.1686 | 0.2219 | 0.051* |
| C36 | −0.0207(5) | −0.1488(2) | 0.2134(2) | 0.0461(9) |
| H36 | −0.0689 | −0.1920 | 0.2345 | 0.055* |
| C37 | −0.1170(4) | −0.1007(2) | 0.1897(2) | 0.0430(9) |
| H37 | −0.2294 | −0.1120 | 0.1947 | 0.052* |
| C38 | −0.0472(4) | −0.0348(2) | 0.15812(19) | 0.0332(7) |
| C39 | −0.1476(5) | 0.0175(3) | 0.1289(2) | 0.0507(10) |
| H39A | −0.2575 | 0.0044 | 0.1420 | 0.076* |
| H39B | −0.1571 | −0.0004 | 0.0724 | 0.076* |
| H39C | −0.0923 | 0.0821 | 0.1537 | 0.076* |
| C40 | 0.4037(4) | −0.0495(2) | 0.1696(2) | 0.0436(8) |
| H5A1 | 0.4507 | −0.0924 | 0.1835 | 0.065* |
| H5A2 | 0.4631 | 0.0117 | 0.2047 | 0.065* |
| H5A3 | 0.4135 | −0.0554 | 0.1159 | 0.065* |
| C41 | 0.4579(4) | 0.20785(19) | 0.02816(16) | 0.0247(6) |
| C42 | 0.6239(4) | 0.2188(2) | 0.01890(17) | 0.0318(7) |
| C43 | 0.6856(4) | 0.2739(2) | −0.0232(2) | 0.0424(8) |
| H43 | 0.7963 | 0.2828 | −0.0307 | 0.051* |
| C44 | 0.5850(5) | 0.3156(2) | −0.0540(2) | 0.0440(9) |
| H44 | 0.6281 | 0.3522 | −0.0820 | 0.053* |
| C45 | 0.4200(4) | 0.3030(2) | −0.04332(19) | 0.0382(8) |
| H45 | 0.3529 | 0.3311 | −0.0646 | 0.046* |
| C46 | 0.3529(4) | 0.2492(2) | −0.00138(17) | 0.0294(7) |
| C47 | 0.1736(4) | 0.2340(2) | 0.0111(2) | 0.0397(8) |
| H47A | 0.1129 | 0.2557 | −0.0225 | 0.059* |
| H47B | 0.1714 | 0.2668 | 0.0656 | 0.059* |
| H47C | 0.1222 | 0.1697 | −0.0018 | 0.059* |
| C48 | 0.7296(5) | 0.1712(3) | 0.0518(2) | 0.0532(10) |
| H48A | 0.8373 | 0.1822 | 0.0361 | 0.080* |
| H48B | 0.6749 | 0.1064 | 0.0316 | 0.080* |
| H48C | 0.7443 | 0.1944 | 0.1087 | 0.080* |
| C49 | 0.9318(6) | 0.3612(3) | 0.3093(3) | 0.0631(12) |
| H49 | 0.8332 | 0.3791 | 0.2942 | 0.076* |
| Cl1 | 1.0711(2) | 0.37556(13) | 0.24635(12) | 0.1074(6) |
| Cl2 | 1.0213(3) | 0.42992(13) | 0.40712(9) | 0.1453(10) |
| Cl3 | 0.86685(17) | 0.24623(10) | 0.29942(9) | 0.0835(4) |
Source of material
n-Pentanol and n-octanol were distilled from sodium under nitrogen. Column chromatography was carried out on silica gel columns (Merck, Kieselgel 60, 70–230 mesh) with the indicated eluents. All other reagents and solvents were used as received. 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyaninate was prepared according to the literature procedures with slight modification [4]. 4,5-Dichlorophthalonitrile (4.0 g, 20 mmol) and 2,6-dimethylphenol (14.6 g, 120 mmol) in dry DMSO (40 mL) were strirred under dry nitrogen at 95 °C. Dry potassium carbonate (60 g) was added (in 5 min) and the solution was stirred at 95 °C for an additional 1.5 h, then cooled and poured into ice-water (400 mL). The sticky precipitate was filtered, washed with cold water, ethanol, and then dissolved in trichloromethane and precipitated with ethanol with subsequent evaporation of dichloromethane. The precipitate was filtered and vacuum-dried to get the 4,5-bis(2,6-dimethylphenoxy)phthalonitrile. Then the bis(diiminoisoindoline) (prepared from the 101 mg (0.57 mmol) 1,2,4,5-tetra-cyanobenzene) and 4,5-bis(2,6-dimethylphenoxy)phthalonitrile (1.4 g, 4 mmol) were added to the n-BuOH (40 mL) solution and the mixture was heated under reflux for 48 h. After the reaction mixture had been cooled, methanol (200 mL) was added and the resulting mixture was stirred for 1 h. The precipitate was collected by the centrifugation, washed with methanol, dried and then extracted with toluene. After evaporation of toluene the solid was dissolved in trifluoroacetic acid (25 mL) and stirred in the dark for 1 h. The solution was poured into ice-water (100 mL) the precipitate collected, washed successively with water, 5% NaHCO3, water and methanol, then dried under vacumm, extracted with toluene/hexanes (4:1 v/v) and subjected to chromatography on silica gel eluting with toluene/hexanes gradually reducing the hexanes content from 20 to 50 vol%. From the first green fraction containing 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyaninate the solvents were evaporated and the resulting solid was recrystallised from the toluene and trichloromethane. (0.36 g, 24%). 1H NMR (200 MHz, CDCl3, 25 °C, TMS): δ = 8.18(s, 8H), 7.30−7.41(brm, 24H), 2.43(s, 48H) – 0.76 ppm(brs, 2H); IR(KBr): 3296(N—H), 3024, 2952, 2922, 2854, 1612, 1588, 1442, 1396, 1328, 1276, 1222, 1188, 1092, 1016, 920, 878, 834, 800, 762, 708, 696 cm−1.
Experimental details
Hydrogen atoms were added using a riding model implemented in the crystallographic software [2], [3].
Comment
Some of the most important applications of phthalocyanines (Pcs) are based on the interaction of visible light with the chromophores of these molecules: dyes and pigments, photoconductors in laser printers, photosensitisers in the photodynamic therapy of cancer and degradation of pollutants of waste and natural water [5], [6], [7], [8], [9], [10], [11], [12]. Pcs as thin films or adsorbed on inorganic semiconductors have potential applications in photovoltaic or dye-sensitised solar cells. Based on these applications and taking into account the increasing interest in near-infrared (NIR) absorbing systems, it is important to sythesize compounds with absorptions over a broad region of visible light down to the NIR region. The similar phthalocyanine 2,3,9,10,16,17,23,24-octakis(2-tetrahydropyran)phthalocyanine has been reported [13].
The figure depicts a perspective drawing of the compound showing the atom numbering. The atoms of the phthalocyaninate ring are in the same plane, the 2,6-dimethylphenoxy group is almost vertical to the phthalocyaninate ring.
Acknowledgements
This work was supported by Henan science and technology project (Nos. 172102210161, 172102210160) and the Research Fund of Anyang Institute of Technology (No. YJJ2016014).
References
Bruker. SMART and SAINT for Windows NT Software Reference Manuals, Version 5.0. Bruker Analytical X-Ray Systems, Madison, WI (1997).Suche in Google Scholar
Sheldrick, G. M.: SADABS, a software for empirical absorption correction. University of Göttingen, Göttingen, Germany (1997).Suche in Google Scholar
SHELXL Reference Manual, version 5.1. Bruker Analytical X-Ray Systems, Madison, WI (1997).Suche in Google Scholar
Sergey, M.; Christian, L.; Eugeny, A. E.; Olga, S.; Beate, R.; Dieter, W.: Synthesis and photophysical properties of annulated dinuclear and trinuclear phthalocyanines. Chem. Eur. J. 12 (2006) 1468–1474.10.1002/chem.200500617Suche in Google Scholar PubMed
Bian, Y. Z.; Chen, X. H.; Wang, D. Y.; Choi, C. F.; Zhou, Y.; Zhu, P. H.; Ng, D. K. P.; Jiang, J. Z.; Weng, Y. X.; Li, X. Y.: Porphyrin-appended europium(III) bis(phthalocyaninato) complexes: synthesis, characterization, and photophysical properties. Chem. Eur. J. 13 (2007) 4169–4177.10.1002/chem.200601668Suche in Google Scholar PubMed
Sheng, N.; Zhu, P. H.; Ma, C. Q.; Jiang, J. Z.: The synthesis, spectroscopy, electrochemistry and photophysical properties of novel, sanwich europium(III) complexes with a porphyrin ligand bearing four pyrenyl groups in meso-positions. Dyes Pigments 81 (2009) 91–96.10.1016/j.dyepig.2008.09.009Suche in Google Scholar
Gao, Y. N.; Ma, P.; Chen, Y. L.; Zhang, Y.; Bian, Y. Z.; Li, X. Y.; Jiang, J. Z.; Ma, C. Q.: Design, synthesis, characterization, and OFET properties of amphiphilic heteroleptic tis(phthalocyaninato) europium(III) complexes. The effect of crown ether hydrophilic substituents. Inorg. Chem. 48 (2009) 45–54.10.1021/ic801040zSuche in Google Scholar
Lu, G. F.; Zhang, X. M.; Cai, X.; Jiang, J. Z.: Tuning the morphology of self-assembled nanostructures of amphiphilic tetra(p-hydroxyphenyl)prophyrins with hydrogen and metal-ligand coordination bonding. J. Mater. Chem. 19 (2009) 2417–2424.10.1039/b820127gSuche in Google Scholar
Zhang, L. Y.; Chen, M. X.; Jiang, Y. L.; Chen, M. M.; Ding, Y. N.; Liu, Q. Y.: A facile preparation of montmorillonite-supported copper sulfide nanocompositesand their application in the detection of H2O2. Sens. Actuators, B 239 (2017) 28–35.10.1016/j.snb.2016.07.168Suche in Google Scholar
Sun, L.; Ding, Y. Y.; Jiang, Y. L.; Liu, Q. Y.: Montmorillonite-loaded ceria nanocomposites with superior peroxidase-like activity for rapid colorimetric detection of H2O2. Sens. Actuators, B 239 (2017) 848–856.10.1016/j.snb.2016.08.094Suche in Google Scholar
Cao, W.; Gao, C.; Zhang, Y. Q.; Qi, D. D.; Liu, T.; Wang, K.; Duan, C. Y.; Gao, S.; Jiang, J. Z.: Rational enhancement of the energy barrier of bis(tetrapyrrol) dysprosium SMMs via replacing atom of prophyrin core. Chem. Sci. 6 (2015) 5947–5954.10.1039/C5SC02314ASuche in Google Scholar PubMed PubMed Central
Wang, H. L.; Wang, B. W.; Bian, Y. Z.; Gao, S.; Jiang, J. Z.: Single-molecule magnetism of tetrapyrrole lanthanide compounds with sandwich multiple-decker structures. Coord. Chem. Rev. 306 (2016) 195–216.10.1016/j.ccr.2015.07.004Suche in Google Scholar
Andrew, S. C.; D. Bradley, G. W.; Andrew, J. P. W.; David, J. W.; Steven, J. L.; Anthony, G. M. B.; Brian, M. H.: Enantiomerically pure ”winged” spirane porphyrazinoctaols. Angew. Chem. Int. Ed. Engl. 7 (1997) 760–761.10.1002/anie.199707601Suche in Google Scholar
©2018 Wang Xin et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of di-μ2-aqua-tetraaqua-bis(4-(1H-1,2,4-triazol-1-yl)benzoato-κN)disodium(I) C18H24N6Na2O10
- Crystal structure of diaqua-bis(2-bromo-4-chloro-6-formylphenolato-κ2O,O′)cobalt(II), C16H16Cl2CrN3O7
- Crystal structure of catena-poly[(μ2-1-(4-(1H-pyrazol-1-yl)phenyl)ethan-1-one-κ2N:O)-bis(1,1,1-trifluoro-4-oxo-4-(thiophen-2-yl)but-2-en-2-olato-κ2O,O′)copper(II)], C27H18CuF6N2O5S2
- Crystal structure of ethyl 2-amino-4-(3,5-difluorophenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C18H15F2NO5
- Crystal structure of 5,5′-dimethoxy-2,2′-[1,1′-(ethylenedioxydinitrilo)diethylidyne]diphenol, C20H24N2O6
- Crystal structure of (E)-1-(4-(((E)-3,5-dichloro-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-methyl oxime, C16H14Cl2N2O2
- Crystal structure of 2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyanine - trichloromethane (1/2), C98H84Cl6N8O8
- Crystal structure of methyl 2-((1-(2-(methoxycarbonyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-1-naphthoate, C24H21N3O5
- Crystal structure of catena-poly[(μ2-3,3′-thiodipropionato-κ2O:O′)-(bipyridine-κ2N,N′)copper(II)] C16H16CuN2O4S
- Crystal structure of [4-chloro-2-(((2-((3-ethoxy-2-oxidobenzylidene)amino)phenyl)imino)(phenyl)methyl)phenolato-κ4N,N′,O,O′}nickel(II) - ethyl acetate (1/1), C32H29ClN2NiO5
- Crystal structure of (4-(4-chlorophenyl)-5-ethyl-1,3-dioxane-5-carboxylato-κ2O,O′)-(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′)nickel(II) perchlorate monohydrate, C29H52Cl2N4NiO9
- Crystal structure of ethyl 2-amino-4-(3,4-dimethylphenyl)-7-methyl-5-oxo-4H,5H-pyrano[4,3-b]pyran-3-carboxylate, C20H21NO5
- Structure and photochromism of 1,2-bis[2-methyl-5-(3-quinolyl)-3-thienyl]-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C33H20F6N2S2
- Crystal structure of catena-poly[diaqua-bis(μ2-3,5-di(1H-1,2,4-triazol-1-yl)benzoate-κ2N:N′)cobalt(II))] 2.5 hydrate, C22H23CoN12O8.50
- The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd
- Crystal structure of catena-poly[bis(4,4′-dipyridylaminium-kN)-(μ2-germanowolframato-κ2O:O′)-(2,2′-bipyridine-κ2N,N′)copper(II)] with a Keggin-type heteropolyoxoanion, [Cu(C10H8N2)(C10H10N3)2][GeW12O40] ⋅ H2O
- Crystal structure of diaqua-(N-(1-(pyrazin-2-yl)ethylidene)pyridin-1-ium-4-carbohydrazonate-κ3N,N′,O)-tris[nitrato-κ2O,O′)lanthanum(III), C12H15N8O12La
- The crystal structure of 2-hydroxy-4-((2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy)-3,6-dimethylbenzoic acid–methanol (1/1), C20H24O8
- Crystal structure of guanidinium tetrapropylammonium bis(hydrogencarbonate) dihydrate, C15H40N4O8
- Crystal structure of (Z)-2-bromo-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-phenylprop-2-en-1-one, C23H27BrO2
- Crystal structure of 2-(4-(4H-1,2,4-triazol-4-yl)phenyl)acetic acid, C10H9N3O4
- Crystal structure of 4,4′-(1,4-phenylene)bis(1H-imidazol-3-ium)bis(2-carboxybenzoate), C30H26N4O8
- Crystal structure of 4,4′-(4,10-diphenyl-4,10-dihydropyreno[4,5-d:9,10-d′]diimidazole-5,11-diyl)bis(N,N-diphenylaniline), C66H44N6
- Crystal structure of catena-poly[diaqua-bis(μ2-5-(3-(1H-imidazol-5-yl)phenyl)tetrazol-2-ido-κ2N:N′)cobalt(II)], C20H18CoN12O2
- Crystal structure of 1,3-dimethyl-2-(p-tolyl)-1H-perimidin-3-ium iodide 1.5 hydrate, C20H22IN2O1.5
- Crystal structure of 2-(4-methoxyphenyl)chromane, C16H16O2
- Crystal structure of poly[(μ2-2-carboxy-5-nitroisophthalato-κ2O:O′)-(μ2-4-((1H-imidazol-1-yl)methyl)pyridine-κ2N:N′)zinc(II)], C18H12N4O8Zn
- Crystal structure of bis(1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)tetraiodidodicadmium(II), [Cd2(C13H15N5)2I4]
- Crystal structure of tetramethylammonium bis(acetato-κ1O)-tetrakis(μ3-3-((hydroxyimino)methyl)-5-methoxy-2-oxidobenzoate-κ5O,O′:O′,N:O′′)tetrazinc(II) — N,N′-dimethylformamide — water (1/2/2), C62H96Zn4N10O28
- Crystal structure of poly[(μ4-5-tert-butylisophthalato-κ4O:O′:O′′:O′′′)-(1,3-dimethyl-2-imidazolidinone-κO)zinc(II)] C17H22N2O5Zn
- Crystal structure of [tris(2-benzimidazolylmethyl)amine-κ4N,N′,N′′,N′′′]-[(pyridine-2,6-dicarboxylato-κ2O,N)]cadmium(II)–methanol (1:3) C34H36CdN8O7
- The crystal structure of bis(1H-benzo[d]imidazol-2-amine-κN)-diiodidocadmium(II), C14H14CdI2N6
- Crystal structure of tetrakis(1H-benzimidazol-2-amine)-κN)-bis(μ2-sulfonato-κ2O:O′)dizinc(II) - methanol (1/1), C30H36N12O10S2Zn2
- Crystal structure of 3β-methoxy-20α-dimethylamino-pregn-5-ene, C24H41NO
- Crystal structure of dimethyl 4,4′-oxydibenzoate, C16H14O5
- Crystal structure of catena-poly[diiodido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-3-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)zinc(II)], C17H16I2N4OZn
- Crystal structure of 4-((E)-((E)-5-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-4-oxopiperidin-3-ylidene)methyl)benzonitrile, C26H18F2N2O3S
- Crystal structure of bis(acetato-κ1O)-bis(1-(pyridin-2-yl)ethan-1-one oxime-κ2N,N′)zinc(II), C18H22N4O6Zn
- The crystal structure of 9-butoxy-2-(hydroxymethyl)-2H-imidazo[1,5-a]quinolin-10-ium bromide, C17H21O2N2Br
- Crystal stucture of 2-(tert-butyl)-6-(hydroxymethyl)-4-methylphenol, C12H18O2
- Crystal structure of catena-poly[(2-(5-chloroquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethan-1-one-κ3N,O,O′)-(dinitrato-κ2O,O′)mercury(II)], C15H15N4O8ClHg
- Crystal structure of dimethyl (3aS,6R,6aS,7S)-1H,3H,6H,7H-3a,6:7,9a-diepoxybenzo[de]isochromene-3a1,6a-dicarboxylate, C16H16O7
- The crystal structure of 2-(dimethoxymethyl)-4-(4-methylphenyl)-1H-imidazole—petroleum ether-chloroform (3/1), C27H33Cl3N4O4
- Crystal structure of 8-(trifluoromethyl)imidazo[1,2-a]pyridine-3-carbaldehyde, C9H5F3N2O
- The crystal structure of N,N-diethyl-4,6-bis(naphthalen-2-yloxy)-1,3,5-triazin-2-amine, C27H24N4O2
- Crystal structure of 5-bromo-7-chloro-3,3a-dihydrocyclopenta[b]chromen-1(2H)-one, C12H8BrClO2
- Crystal structure of 2-(bis(4-fluorophenyl)methylene)hydrazine-1-carbothioamide, C14H11F2N3S

