Abstract
Teucrium polium is a perennial herbaceous plant with a long history of medicinal use in numerous cultures. Silver nanoparticles (AgNPs) using T. polium leaf extract were synthesized and characterized as well as their use in antibacterial and anticancer activities. Ultraviolet–visible spectroscopy, scanning electron microscopy analysis coupled with energy-dispersive X-ray, transmitted electron microscopy (TEM), and Fourier transform infrared spectroscopy analysis validated the effective synthesis. TEM revealed the synthesis of spherical AgNPs ranging in size from 41 to 61 nm. The gas chromatography–mass spectrometry investigation of T. polium leaf extract revealed 10 bioactive components from distinct chemical classes. A test called cytotoxicity showed that AgNPs were toxic to MCF-7 and HepG2 cancer cell lines with the IC50 values = 15 ± 3.18 μg·mL−1 for MCF-7 and 12 ± 2.63 μg·mL−1 for HepG2. It showed high antibacterial activity against Gram-positive and Gram-negative bacteria (minimum inhibitory concentration values ranged from 5.85 ± 2.76 to 31.25 ± 0.00 μg·mL−1). The findings hold promise for developing eco-friendly antibacterial and anticancer agents with enhanced biocompatibility, fostering advancements in both nanotechnology and biomedical sciences, and giving useful insights for future research and development in natural product-based treatments and green nanotechnology.
Abbreviations
- AgNPs
-
silver nanoparticles
- ATCC
-
American Type Culture Collection
- DMSO
-
dimethyl sulfoxide
- EDX
-
energy-dispersive X-ray
- FTIR
-
Fourier transform infrared spectroscopy
- GC–MS
-
gas chromatography–mass spectrometry
- KSU
-
King Saud University
- MBC
-
minimum bactericidal concentration
- MIC
-
minimum inhibitory concentration
- MTT
-
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide
- PBS
-
phosphate-buffered saline
- rRT-PCR
-
real-time reverse transcription–polymerase chain reaction
- SEM
-
scanning electron microscopy
- TEM
-
transmitted electron microscopy
- TFC
-
total flavonoid content
- TPC
-
total phenolic content
- ZOIs
-
zones of inhibition
1 Introduction
Teucrium polium, sometimes known as “felty germander,” is a perennial herbaceous plant from the Lamiaceae family [1]. T. polium, known for its potent therapeutic characteristics, has been utilized in many herbal medicines throughout civilizations for ages [2]. T. polium’s biological activities are due to its rich phytochemical makeup, which contains terpenoids, flavonoids, phenolic compounds, and essential oils [3]. T. polium’s bioactive ingredients provide a wide range of pharmacological effects, making it an interesting subject for ethnopharmacological and scientific research. For example, T. polium has strong antioxidant qualities that scavenge free radicals, protecting cells from oxidative damage and lowering the risk of many chronic diseases, including cardiovascular disease and cancer [4]. T. polium also has anti-inflammatory qualities, which can help treat inflammatory disorders like arthritis, gastritis, and dermatitis. Its anti-inflammatory properties are ascribed to regulating pro-inflammatory mediators and pathways, reducing inflammation and its associated symptoms [5].
One of T. polium’s noteworthy properties is its ability to exhibit antimicrobial action against various pathogens, including bacteria, fungi, and viruses [6]. Its bioactive components have antibacterial properties by disrupting microbial cell membranes, limiting microbial growth, and interfering with microbial metabolic activities, suggesting possible therapeutic efficacy against infectious illnesses [7,8]. T. polium has anti-cancer action, with studies showing cytotoxic effects against numerous cancer cell lines [8,9,10]. Its bioactive ingredients have anti-cancer properties by inducing apoptosis, reducing cell proliferation, and lowering tumor growth, indicating its potential as a therapeutic agent in cancer treatment [11,12]. These different pharmacological effects show their therapeutic potential in preventing and treating numerous ailments, emphasizing their importance in both traditional medicine and modern pharmacology.
In the field of modern science, the search for long-lasting and effective antimicrobial drugs has encouraged researchers to pursue novel approaches, such as the synthesis of nanoparticles from natural sources. AgNPs have received a lot of attention for their amazing biological characteristics. Green synthesis methods, which use plant extracts as reducing and stabilizing agents, have emerged as a viable approach, providing environmentally benign and cost-effective alternatives to traditional chemical synthesis approaches [13]. The utilization of plant extracts in nanoparticle synthesis not only facilitates the reduction of metal ions but also imparts inherent therapeutic properties to the resulting nanoparticles, enhancing their biomedical applications [14]. For instance, a previous study conducted by Mosleh-Shirazi et al. showed that the green-synthesized CoFe2O4 and ZnFe2O4 nanoparticles showed strong photothermal activity against MCF-7 breast cancer cells, particularly CoFe2O4, while causing minimal harm to normal cells, indicating their potential for safe and effective cancer therapy [15]. Another study indicated that MXenes, graphene-like two-dimensional transition nitrogen compounds, showed potent antimicrobial activity against bacteria, fungi, and viruses, with promising applications in wound healing, medical devices, and photothermal therapies [16]. Another study reported that the green synthesized silver-bromelain (Ag-BL) nanoparticles had considerable antibacterial activity against fungi and bacteria, and tolerable cytotoxicity, making them a suitable candidate for medicinal applications [17]. These findings demonstrate the enormous potential of green-synthesized nanoparticles as multifunctional agents in biomedical applications, providing sustainable, effective, and safer solutions to global health concerns.
Notably, the biological properties of the AgNPs using T. polium leaf extract have only been elucidated by a limited number of exploratory studies. The synthesized T. polium AgNPs leaf extract showed significant anticancer efficacy against the human gastric cancer cell line MNK45 [8]. A recent study also found that AgNPs using T. polium extract demonstrated 60% cytotoxicity against MCF-7 breast cancer cell lines but less cytotoxicity against normal breast cell lines (MCF-10A). They were highly effective against six Gram-positive and Gram-negative bacteria [18].
As an alternate use in biotechnology with therapeutic applications, the study’s novelty is in synthesizing and characterizing AgNPs using T. polium leaf extract as a bio-reducing agent, which produces new bioactive compounds or increased therapeutic activity. So, the current study addresses these gaps by conducting a thorough chemical analysis of T. polium leaf extract using gas chromatography–mass spectrometry (GC–MS) to identify their phytochemical contents. The study investigates its anticancer and antibacterial properties, and this study contributes to the growing body of research on sustainable nanomaterials with therapeutic applications. The findings hold promise for developing eco-friendly antimicrobial agents with enhanced biocompatibility, fostering advancements in both nanotechnology and biomedical sciences.
2 Materials and methods
The breast cancer (MCF-7) (ATCC HTB-22) and human hepatoma HepG2 (ATCC HB-8065) cell lines were obtained from the American Type Culture Collection (ATCC). Dulbecco’s modified Eagle’s medium with 4.5 g·L−1 glucose, l-glutamine, and fetal bovine serum were obtained from Gibco, Invitrogen (Grand Island, NY, USA). Penicillin/streptomycin, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT), cisplatin, methanol, Folin–Ciocalteu reagent, AlCl3, gallic acid, ciprofloxacin, dimethyl sulfoxide (DMSO), potassium persulfate, and Mueller Hinton media were obtained from Sigma-Aldrich Ltd. (Ayrshire, UK).
2.1 Preparation of plant extract
T. polium plant leaves were collected from the market in Riyadh, Saudi Arabia (https://maps.app.goo.gl/HpzkHw8PEzBttyew8?g_st=aw). The plant was identified by Professor Dr. Mohammed Fasil of the Department of Botany and Microbiology at King Saud University in Riyadh, Saudi Arabia, and was deposited at the Herbarium with the reference number King Saud University (KSU) NO-11425. T. polium plant leaves were cleaned with distilled water after being dust-washed multiple times under the water tap. The leaves were then pulverized using a mortar and pestle after being shadow-dried for a week. One day at room temperature was spent with 5 g of leaf powder suspended in 100 mL of distilled water in a beaker. Using Whatman No. 1 filter paper, the extract was filtered in a conical flask and chilled at 4°C. AgNPs were synthesized using this solution.
2.2 Green synthesis of AgNPs from the extract
For the preparation of T. polium AgNPs, 2 mL of the extract from the previous step was mixed with 100 mL of a freshly prepared aqueous AgNO3 solution at a concentration of 2 mM, using a 250 mL Erlenmeyer flask, following the methodology outlined by Melkamu et al. [19]. The prepared reaction mixture was left undisturbed under the sunlight. The reaction was monitored visually by observing a color change from pale yellow to brown, indicating the formation of nanoparticles (Figure 1). The bioactive compounds in the T. polium extract act as reducing agents, reducing the silver ions (Ag⁺) to form AgNPs. The mixture was centrifuged and filtered to separate the AgNPs from any remaining plant extract or impurities.

Color change during the biosynthesis of T. polium AgNPs.
2.3 Characterization of AgNPs
2.3.1 Ultraviolet–visible (UV–vis) spectroscopy
The UV–vis spectrophotometer (Shimadzu, Tokyo, Japan) was employed to characterize the T. polium AgNPs. The reduction of pure Ag+ ions was examined within the 250–800 nm range and plot interval of 1.0 nm by using the UV-2450 double-beam instrument, following the manufacturer’s guidelines, as outlined in prior studies [20]. The experiment was conducted in triplicate.
2.3.2 Scanning electron microscopy (SEM) analysis coupled with energy-dispersive X-ray (EDX)
That was used to detect the elemental analysis and chemical composition of the synthesized AgNPs. Briefly, 5 μL of 0.1 mg·mL−1 concentration of the purified AgNPs solution was deposited on the carbon tape of the SEM integrated with EDX. The samples were air-dried for 15 min at room temperature. SEM imaging was conducted by the JSM-6380 LA scanning electron microscope (JEOL Ltd., Tokyo, Japan), Following the manufacturer’s instructions, as described before [21]. JSM-6380 is supplied with a customizable GUI interface that allows the instrument to be intuitively operated, and Smile Shot™ software ensures optimum operation settings and switching between SEM and EDX.
2.3.3 Transmitted electron microscopy (TEM)
The JEM-1011 transmission electron microscope (JEOL Ltd.) employing the TEM technique was utilized to validate the crystalline structure of the synthesized nanoparticles. The preparation and processing of slides followed the manufacturer’s instructions, as detailed in prior research [22].
2.3.4 Fourier transform infrared spectroscopy (FTIR)
The FTIR analysis was conducted by the Nicolet 6700 FTIR Spectrometer (Thermo Fisher Scientific, MS, USA) equipped with OMNIC software at the range of 500–4,000 cm–1, following the manufacturer’s instructions. The obtained wavelengths were interpreted to suggest the possible functional groups according to the height and shape of peaks by accessing the FTIR Functional Group Database InstaNANO, https://instanano.com/all/characterization/ftir/ftir-functional-group-search/ (accessed May 12, 2024).
2.4 Determination of phytochemicals of the extract by GC–MS
The GC–MS 7890B GC system from Agilent Technologies (Santa Clara, CA, USA) autosampler injection system was used to inject 0.9 µL of the diluted T. polium leaves extract, which had been diluted with acetone (1–9 volume ratio). The connected database software was used to identify the items. GC in conjunction with a mass selective detector allowed for the identification of the sample’s constituent parts (GC–MS). The target compounds were separated using an Agilent Technologies DB-5 MS capillary column (30 m in length, 0.25 mm in internal diameter, and 0.25 μm of phase thickness). Helium was used as the carrier gas, with a flow rate of 1 mL·min−1, an inlet temperature of 250°C with a split mode ratio of 50, and an oven temperature range of 50–250°C with a total analysis time of 73 min. The MS detector was configured with the following parameters: MS source temperature of 230°C, acquisition scan type, mass between 40 and 500 g·mol−1, scan speed of 1.56, and solvent delay of 2 min.
2.5 Analysis of total phenolic content (TPC) and total flavonoid content (TFC)
The TPC was determined using the Folin–Ciocalteu method [23]. The TPC was calculated using the standard curve created from the gallic acid standard. TPC content in the T. polium leaf extract was given as mg GAE g–1 per gram of extract. Each experiment was carried out three times, and the results were reported as mean ± standard deviation (SD).
The amount of TFC was estimated as described previously by using the method aluminum chloride (AlCl3) colorimetric method which is based on the formation of a complex flavonoid-aluminum having the maximum absorption at 415 nm [24]. A standard curve with quercetin concentrations ranging from 0 to 100 mg·mL−1 was created to quantify TFC. The findings were presented as mean ± SD mg QE g–1 per gram of extract.
2.6 Anticancer activity
2.6.1 Cell culture and cytotoxicity analysis
MCF-7 and HepG2 cell lines were chosen in this investigation because they provide a viable in vitro model system for assessing the cytotoxic properties of various plant extracts [25,26]. Using the MTT test (Life Technologies, Eugene, Oregon, USA), the cytotoxicity of T. polium leaf extract and AgNPs in MCF-7 and HepG2 cells was investigated. Briefly, cells were seeded in a 96-well plate (Corning Costar, Lowell, NY, USA) at a density of 1 × 104 per well. Then, they were subsequently treated with different amounts of T. polium leaf extract (0–400 μg·mL−1) with cisplatin (30 µg·mL−1) as the positive control at 37°C for 48 h at 80–90% confluency. Each well received 10 μL of MTT test solution (5 mg·mL−1 phosphate-buffered saline [PBS]) after 2 days of incubation, and the wells were then incubated for an additional 4 h at 37°C. To dissolve the formazan crystals, 100 μL of DMSO was then added. Ultimately, an enzyme-linked immunosorbent assay reader (BioTek Laboratories, LL, Shoreline, WA, USA) was used to detect each well’s absorbance at 570 nm. 100% of the cells in the negative control, which is the optical density of formazan generated in untreated cells, are viable. To analyze the data and obtain the IC50 value, the mean value was considered [27]. Similarly, synthesized AgNP cell cytotoxicity was tested according to earlier instructions [28].
2.6.2 Gene expression analysis
An analysis of the apoptosis-coding genes was conducted. Fresh media containing specific treatment doses of bioactive compounds was added to the culture medium by the minimum inhibitory concentration (MIC) values extract (35 μg·mL−1) and biosynthesized AgNPs (15 μg·mL−1). Following 24 h, the cells underwent trypsin (0.02%) digestion and were then subjected to a 10-min centrifugation using a refrigerated centrifuge (10,000×g). The pellet was used for real-time reverse transcription-polymerase chain reaction (rRT-PCR) analysis after being reconstituted with PCR buffer for 10 min. Using the RNeasy kit and following the manufacturer’s instructions, RNA extraction was carried out to identify the genes that code for apoptosis (Qiagen, Hilden, Germany). We followed the rRT-PCR methodology as described in our recent study [29].
2.7 Screening for antibacterial activity
2.7.1 Disc diffusion method
The antibacterial efficacy of T. polium leaf extract and synthesized AgNPs against Gram-positive and Gram-negative pathogens was assessed using the disc-diffusion susceptibility technique, as recommended by Al-Dhabi et al. with minor modifications [30] with few modifications. The chosen bacteria represent the Gram-positive strains Staphylococcus aureus (MTCC-29213), Bacillus subtilis (MTCC-10400), Staphylococcus epidermidis (MTCC-12228), as well as three Gram-negative strains: Escherichia coli (ATCC-25922), Klebsiella pneumoniae (MTCC-13883), and Pseudomonas aeruginosa (MTCC-27853) were swabbed (0.1 mL, 1 × 106 CFU mL−1 saline) on Mueller Hinton agar at 37°C for 24 h. The positive control employed was 25 µg·mL−1 of ciprofloxacin. After that, 30 μL of T. polium leaf extract and synthesized AgNP suspension with varying concentrations (i.e., 500, 1,000, and 1,500 μg·mL−1) were applied to sterile paper discs and incubated for 24 h at 37°C. Zones of inhibition (ZOIs) were evaluated after incubation [31].
2.7.2 Analysis of MIC and minimum bactericidal concentration (MBC)
The MIC assay for T. polium leaf extract and synthesized AgNPs against Gram-positive and Gram-negative pathogens was performed by the standard broth microdilution method as described earlier by Basri and Sandra [32] with minor modifications. Briefly, a 96-well plate was filled with 100 μL of HMB with various quantities of the investigated substances at a twofold serial dilution (1.95–1,000 μg·mL−1). Next, 10 µL of bacterial solution was added, bringing the total amount of bacteria in each well to 5 × 106 CFU mL–1. The negative control was 0.1% DMSO with HMB, whereas the positive control was 25 μg·mL−1 of ciprofloxacin. The plates were visually inspected for bacterial growth after a 24-h incubation period at 37°C. Triphenyl tetrazolium chloride (2 mg·mL−1 in PBS working solution was added to each well after treatment, and the mixture was allowed to sit at 37°C for 20 min. Wells having a pink appearance similar to the positive control were considered to be positive for bacterial growth, but wells with a colorless solution were considered negative for bacterial growth. Three duplicates of each experimental analysis were carried out. After visual evaluation of the bacterial growth, the minimum concentration that inhibited growth was determined to be the MIC. The MBC was the lowest concentration at which an agar subculture could be observed [33].
2.8 Statistical analysis
One-way analysis of variance was used for statistical analysis and the findings were presented as mean ± SD. A 5% significance threshold (p < 0.05) was used.
3 Results and discussion
3.1 GC–MS analysis of the T. polium leaf extract
The GC–MS analysis of the T. polium leaf extract revealed a variety of phytochemical components. A total of ten compounds were extracted and it was found that the compounds belong to different bioactive chemical classes. The GC–MS results revealed the existence of fatty acid esters (hexanoic acid, 2,7-dimethyloct-7-en-5-yn-4-yl ester), macrolides ((3E,10Z)-oxacyclotrideca-3,10-diene-2,7-dione), monoterpenoids (isolongifolan-8-ol), pyrimidine of barbituric acid derivatives (cyclobarbital), aryl phosphotriesters (1,4-benzenediol,2,6-bis(1,1-dimethylethyl)-), phenylpropanes (1,2-benzenediol, 3,5-bis(1,1-dimethylethyl)-), aromatic heteropolycyclic 2-aminothiophenes (2-amino-4,4,6,6-tetramethylthieno[2,3-c]furan-3-carbonitrile), aromatic monoterpenoids. Steroid esters (pregn-5-ene-3,20-diol-13-carboxylic acid, 3-acetate-18,20-lactone) (56.62%) was found to be the main photochemical component in the aqueous extract; it was followed by sesquiterpenoids of prenol lipids (oplopanone) (16.41%) and (carvacrol, TBDMS derivative) (7.95%) (Table 1 and Figure 2).
GC–MS analysis results of T. polium leaf extract
| Rt | Width | Area | Area (%) | Start time | End time | Name | Molecular formula | Molecular weight |
|---|---|---|---|---|---|---|---|---|
| 23.056 | 0.167 | 461,590 | 3.15 | 22.897 | 23.334 | Hexanoic acid, 2,7-dimethyloct-7-en-5-yn-4-yl ester | C16H26O2 | 250.38 |
| 26.918 | 0.055 | 478,859 | 3.27 | 26.858 | 26.956 | (3E,10Z)-Oxacyclotrideca-3,10-diene-2,7-dione | C12H16O3 | 208.25 |
| 27.009 | 0.053 | 2,397,622 | 16.41 | 26.956 | 27.197 | Oplopanone | C15H26O2 | 238.37 |
| 27.881 | 0.148 | 355,874 | 2.43 | 27.656 | 27.916 | Isolongifolan-8-ol | C15H26O | 222.37 |
| 28.297 | 0.061 | 247,051 | 1.69 | 28.094 | 28.382 | Cyclobarbital | C12H16N2O3 | 236.27 |
| 31.15 | 0.041 | 80,401 | 0.55 | 31.047 | 31.181 | 1,4-Benzenediol, 2,6-bis(1,1-dimethylethyl)- | C14H22O2 | 710.9 |
| 34.289 | 0.329 | 910,684 | 6.23 | 33.79 | 34.769 | 1,2-Benzenediol, 3,5-bis(1,1-dimethylethyl)- | C14H22O2 | 222.32 |
| 39.802 | 0.087 | 244,482 | 1.67 | 39.68 | 39.915 | 2-Amino-4,4,6,6-tetramethylthieno[2,3-c]furan-3-carbonitrile | C11H14N2OS | 222.31 |
| 40.022 | 0.109 | 1,162,538 | 7.95 | 39.937 | 40.15 | Carvacrol, TBDMS derivative | C16H28OSi | 264.84 |
| 40.253 | 0.137 | 8,277,069 | 56.62 | 40.15 | 40.766 | Pregn-5-ene-3,20-diol-13-carboxylic acid, 3-acetate-18,20-lactone | C23H32O4 | 372.5 |
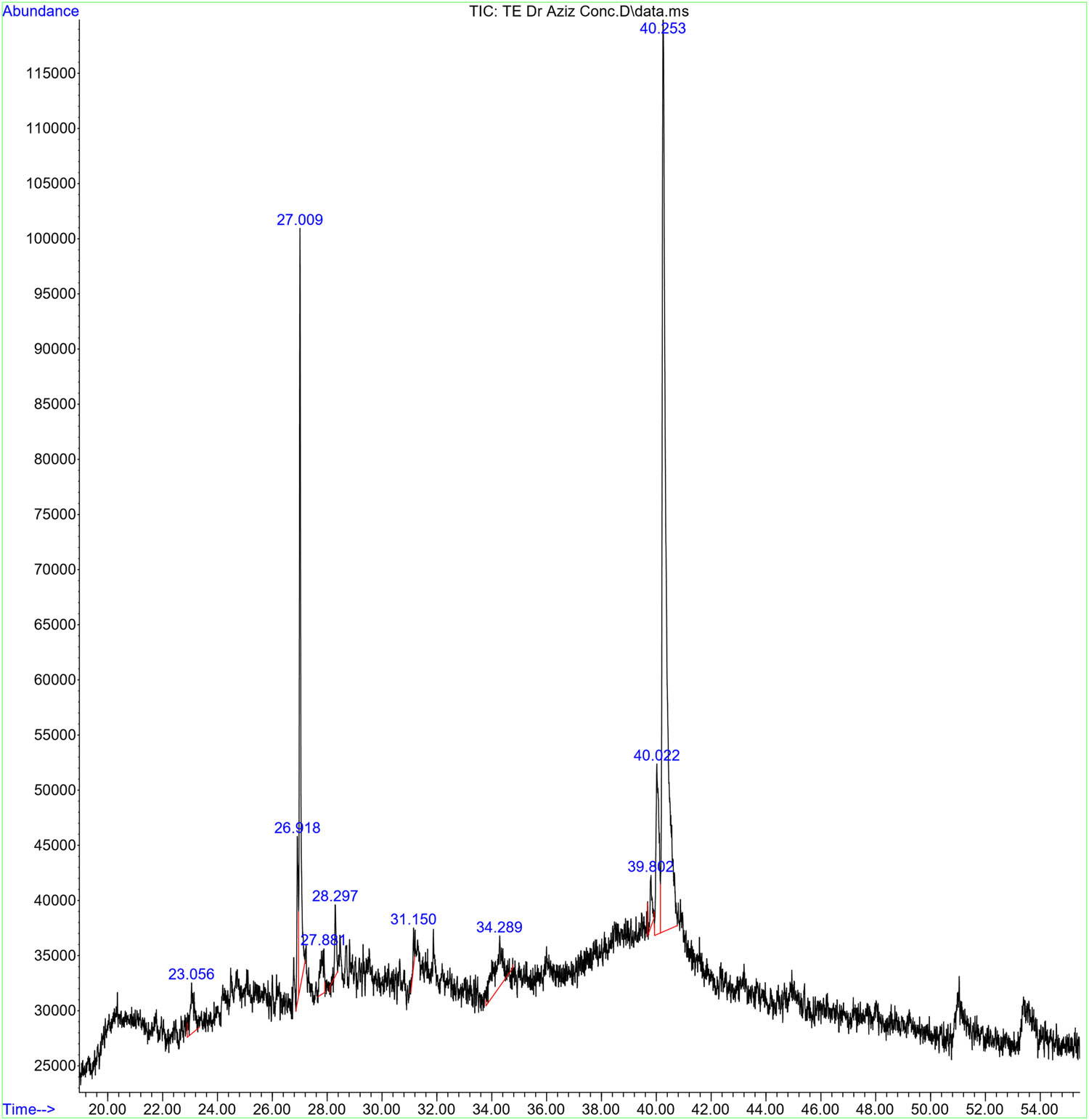
GC–MS spectrum of the aqueous extract of T. polium leaf. The GC–MS apparatus was set for 73 min, and an aqueous extract was used for the study. Every detected peak in the spectrum designates a recognized chemical, and the existence of a sizable peak denotes the extract’s major component.
In agreement with our findings, previous studies showed that the GC–MS analysis of the Ariel parts or root methanolic extracts of T. polium revealed variable phytochemicals including germacrene D, β-pinene, carvacrol, bicyclogermacrene, α-thujene, α-pinene, epi-bicyclosesquiphellandrene, t-cadinol, and limonene were the most abundant [34,35]. Some other studies showed that the GC–MS analysis of T. polium essential oils revealed the existence of sesquiterpenes (germacrene D, trans-caryophyllene, and bicyclogermacrene), monoterpenes (β-pinene and linalool), monoterpenoids (geranial and z-citral) compounds [36]. Another study showed that the phytochemical screening of the T. polium ethanolic extract of the aerial parts revealed the existence of many compounds including saponins, alkaloids, polyphenols, flavonoids, tannins, terpenoids, quinines, and coumarins which might explain its potential antioxidant and antibacterial activity against P. aeruginosa, E. coli, S. aureus, and B. subtilis [37]. Such a rich phytochemical profile suggests the potential of T. polium as a source of bioactive molecules with various pharmacological activities.
Several of these chemicals have been previously examined for their biological effects, indicating intriguing areas for further research. In the study conducted by Atallah et al. [38], they revealed that hexanoic acid derivatives found in the black sand of Egypt had significant antibacterial activity against B. subtilis, Salmonella enteritidis, and Pseudomonas aeruginosa. Macrolides are well-known natural antibiotic, antiviral, antiparasitic, antimycotic, anticancer, or immunosuppressant agents [39]. A previous study revealed that (3E,10Z)-oxacyclotrideca-3,10-diene-2,7-dione induced structural alerts and in vitro mutagenicity, which might be due to the ester and phenol groups exist in its chemical structure [40]. Another study demonstrated that some macrolide antibiotics had significant cytotoxic effects against head and neck squamous cancer cell lines under nutrient-deprived conditions [41]. Oplopanone is one of the sesquiterpenoid ketones, which is known for its biological characteristics such as anti-tuberculosis [42] and anti-diabetes effects [43]. Monoterpenoids like isolongifolan-8-ol possess potent antimicrobial properties, inhibiting the growth of both Gram-positive and Gram-negative bacteria [44]. Cyclobarbital is a pyrimidine derivative, which is a well-known anticonvulsant, and hypnotic agent, and has some anti-proliferative properties against Ehrlich ascites carcinoma cells [45,46]. Carvacrol, TBDMS derivative, is an aromatic monoterpenoid that possesses like carvacrol has shown strong antimicrobial, immune response-modulating, antioxidant, anti-inflammatory, anticancer, antidiabetic, and other biological activities [47]. The existence of these chemicals in T. polium leaf extract shows that it has promise as a natural antibacterial and anticancer agent. Further research is needed to understand the mechanisms of action and therapeutic potential of these substances, both individually and in combination.
3.2 Phenolic and flavonoid content of T. polium leaf extract
TPC and TFC of the aqueous extract of T. polium leaf extract were evaluated. As it is seen the plant is rich in TPC (108.26 ± 1.46 mg GAE g–1 dry weight of the dry extract) [R 2 = 0.997]) and TFC (84 ± 2.14 mg QE g–1 dry weight of the dry extract [R 2 = 0.989]). These phenolic chemicals are essential for scavenging free radicals and avoiding oxidative stress-induced damage to cells and tissues. Also, T. polium extract has a TFC of 84 ± 2.14 mg QE g–1 dry weight of the dry extract (R 2 = 0.989), demonstrating its medicinal potential. Flavonoids have a variety of pharmacological functions, including anticancer, anti-inflammatory, and cardiovascular protection. A previous study showed that T. polium had a TPC of 14.57–157.84 mg of GaA g–1 and TFC of 6.48–139.87 mg of Ru g–1 of extract [48]. Another study showed the total flavonoids (20.78 mg equivalent QE g–1 DW extract) and total polyphenols (227.43 mg equivalent GAE g–1 DW extract) of the ethanolic extract of T. polium Ariel parts which might explain its potent biological activities [37]. Also, in the study conducted by Benchikh et al., they showed that hydroalcoholic extract was rich in flavonoids (24.30 ± 0.44 μg QE mg–1) and phenols (36.35 ± 0.294 μg GAE mg–1), which was reflected in its tested antioxidant, anti-Hyperglycemic, anti-Alzheimer, and anti-inflammatory activities [49]. T. polium’s high TPC value shows that it has the potential to be a natural source of antioxidants, which might help prevent oxidative stress-related disorders. The considerable TFC value emphasizes the presence of flavonoids in the T. polium extract, indicating its potential application in various disease conditions.
3.3 FTIR analysis of the T. polium leaf extract and the synthesized AgNPs
The combined analysis of FTIR spectroscopy and biogenic synthesis of AgNPs using T. polium leaf extract reveals important information about the chemical composition and biophysical properties of this natural extract, as well as its potential applications in nanotechnology and medicine. The FTIR study of T. polium leaf extract revealed four major functional groups: hydroxyl (O–H) stretching at 3,441 cm⁻¹, carbonyl (C═O) stretching at 1,630 cm⁻¹, C–N stretching at 1,115 cm⁻¹, and C–Cl stretching at 595.9 cm⁻¹ (Table 2 and Figure 3). These functional groups indicate the existence of alcohols, alkene compounds, amines, and halogen compounds, respectively. The strong and wide absorption band at 3,441 cm⁻¹ indicates the presence of numerous hydroxyl groups, possibly related to phenolic chemicals in the leaf extract. The identification of these functional groups reveals important information about the chemical composition of T. polium leaf extract, which may be used to guide future research into its biological activity and possible medicinal uses. In the study conducted by Rakhshan et al. [50], the FTIR analysis of T. polium stem extract revealed strong absorption for the functional groups of O–H stretching, C═O asymmetric stretching, and hydroxyl groups of kaolinite. Another study used FTIR spectroscopy and showed that T. polium had seven functional groups for alcohols, alkyl, aliphatic aldehydes, and amides [51].
Functional groups produced by FTIR analysis
| Treatment | Spectra | Functional group | Compound class | Shape and strength |
|---|---|---|---|---|
| T. polium leaf extract | 3,441 | O–H stretching | Alcohol | Strong, broad |
| 1,630 | C═C stretching | Alkene | Medium, sharp | |
| 1,115 | C–N stretching | Amine | Medium, broad | |
| 595.9 | C–Cl stretching | Halo compound | Medium, broad | |
| T. polium AgNPs | 3,427 | O–H stretching | Alcohol | Strong, broad |
| 2,925 | C–H stretching | Alkane | Medium, sharp | |
| 3,427 | C═C stretching | Alkene | Medium, sharp | |
| 1,384 | O–H bending | Alcohol | Medium, sharp | |
| 1,050 | C–N stretching | Amine | Medium, sharp | |
| 601 | C–Cl stretching | Halo compound | Medium, broad |
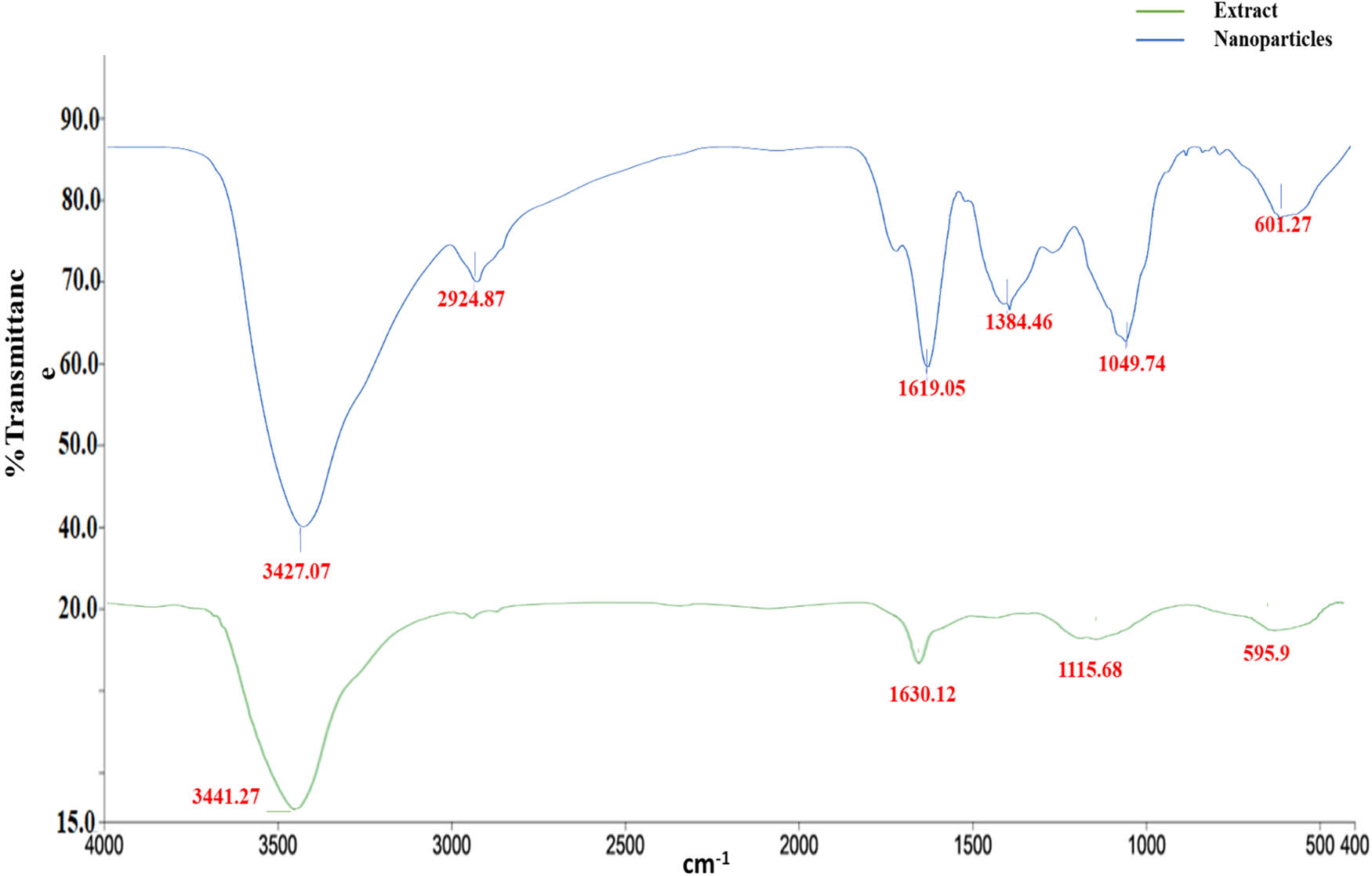
FTIR spectra of T. polium leaf extract and the synthesized AgNPs.
3.4 The biogenic attributes of AgNPs synthesized from aqueous extract of T. polium leaf extract
In the present study, the synthesis of AgNPs occurred through the reduction of Ag+ to Ag0, resulting in a noticeable change in the color of the reaction mixture from light yellow to dark brown. To analyze and identify the synthesized AgNPs, various spectroscopic techniques (UV–vis and EDX) and TEM were employed. In the characterization experiments of the synthesized AgNPs, the prominent peak observed (∼442 nm) in the UV–vis spectrum is characteristic of the surface plasmon resonance (SPR) band of AgNPs, which further confirms their formation. This peak indicates the collective oscillation of conduction electrons in the nanoparticles upon interaction with light, which is a defining feature of metallic nanoparticles (Figure 4). EDX analysis and SEM imaging were used to assess the qualitative and quantitative status of all elements that participated in the synthesis process. As shown in Figure 5a, b, the SEM image showed the morphological characteristics of the synthesized AgNPs. EDX analysis showed a peak of 2.983 keV in the silver region with 8.92% of the mass. That is the typical condition for silver crystal absorption due to their remarkable SPR phenomena. Notably, the two peaks of carbon and oxygen atoms had very weak signals at 0.227 and 0.525 keV, respectively. The TEM analysis in this study confirms the spherical morphology of the AgNPs and provides insight into their size distribution. The average diameter of the AgNPs ranging from 61 to 41 nm suggests the uniformity of particle size and the absence of significant aggregation, which is crucial for various applications requiring precise control over nanoparticle size (Figure 6). In the study conducted by Amini et al., UV–vis spectroscopy of synthesized AgNPs from aqueous extract of T. polium revealed a prominent SPR peak around 430 nm. In the same study, TEM analysis showed that AgNPs were predominantly spherical and homogeneous in size, with an average diameter of 14.3 ± 9.7 nm [52]. In the study conducted by Hashemitabar et al., the AgNPs biosynthesized from the leaf extract of T. polium had a spherical shape and an estimated size of 8–28 nm which was confirmed by the Dynamic light scattering, zeta potential analysis, and TEM [18]. The hydrodynamic diameter in the current study was slightly larger, possibly due to the presence of larger particles impacting light scattering during measurement. Another study showed that AgNPs synthesized from the aqueous extract of T. polium had an SPR of 420 nm. At the same time, the XRD analysis confirmed the presence of pure silver metal, indicating a successful reduction of silver ions to metallic silver by the T. polium leaf extract under the specified reaction conditions, besides, TEM images revealed that the majority of AgNPs observed were spherical, with a small proportion being elongated and ranged in size from 5 to 30 nm [53]. Overall, the combination of spectroscopic and microscopic techniques employed in this study provides a comprehensive characterization of the synthesized AgNPs, offering valuable insights into their optical, elemental, and morphological properties. These findings contribute to the growing body of knowledge in nanotechnology and hold potential for various applications in areas such as catalysis, biomedicine, and environmental remediation.
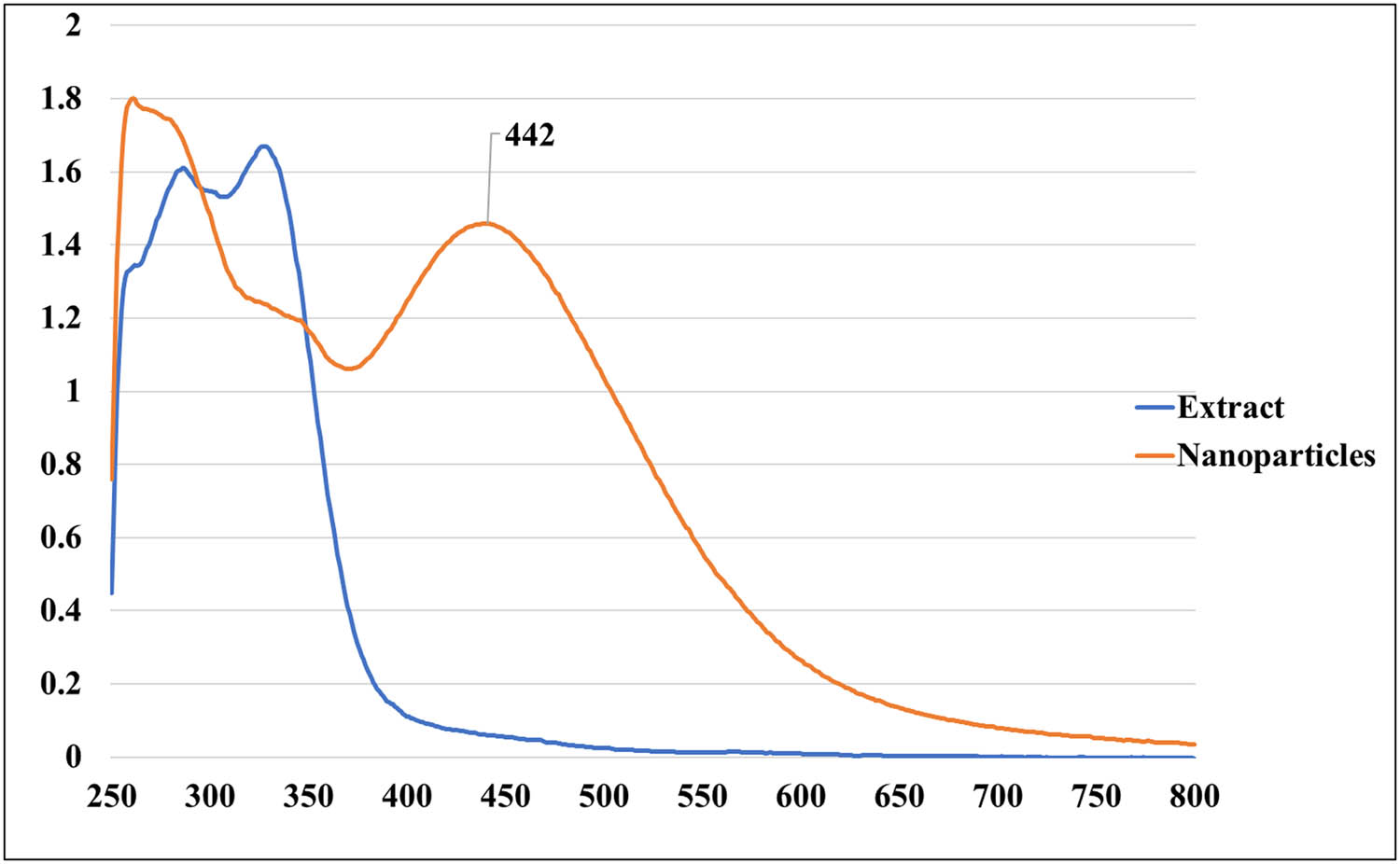
UV–vis spectroscopy of the synthesized T. polium AgNPs compared to the crude extract.
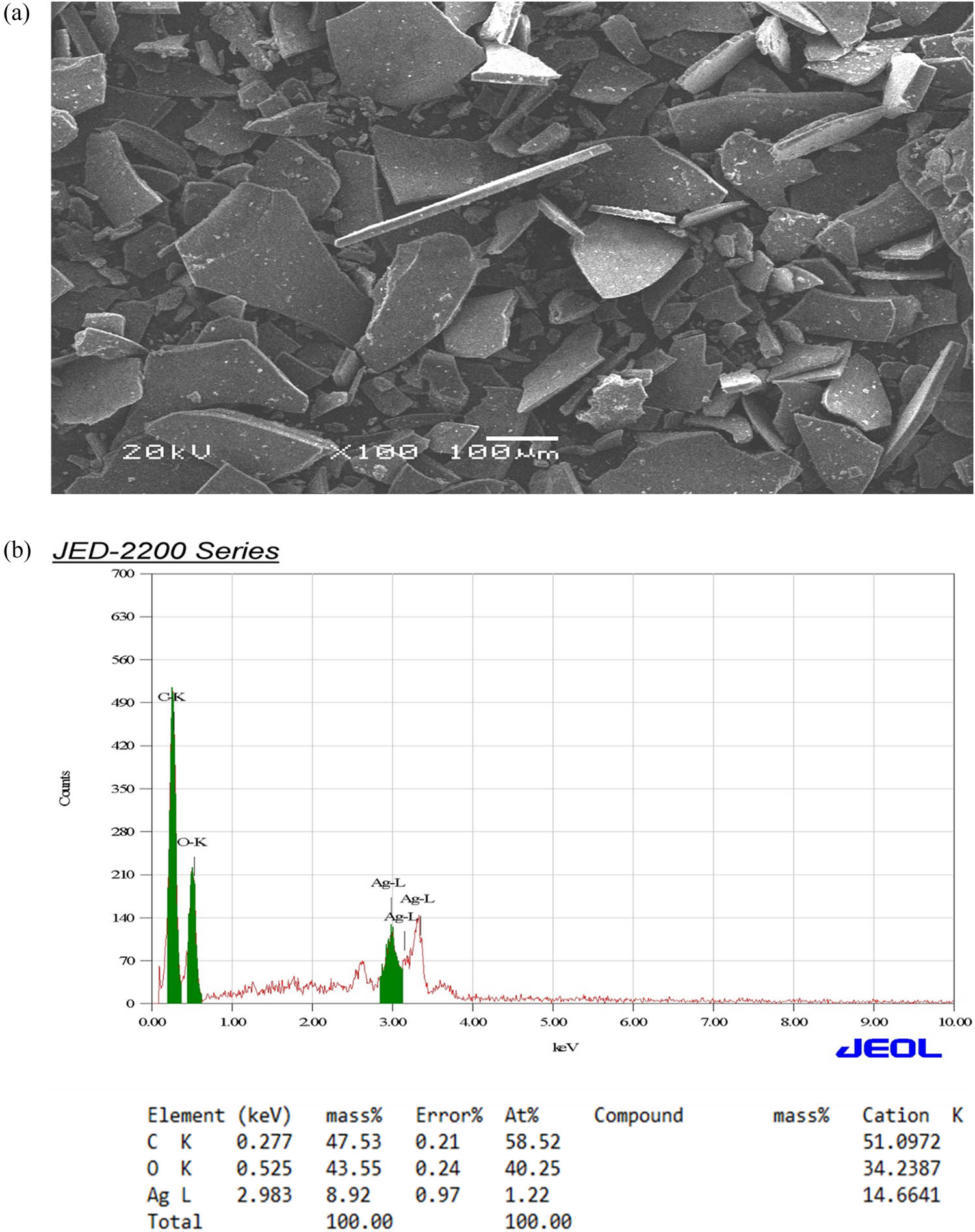
SEM image (a) and EDX (b) of the synthesized AgNPs.
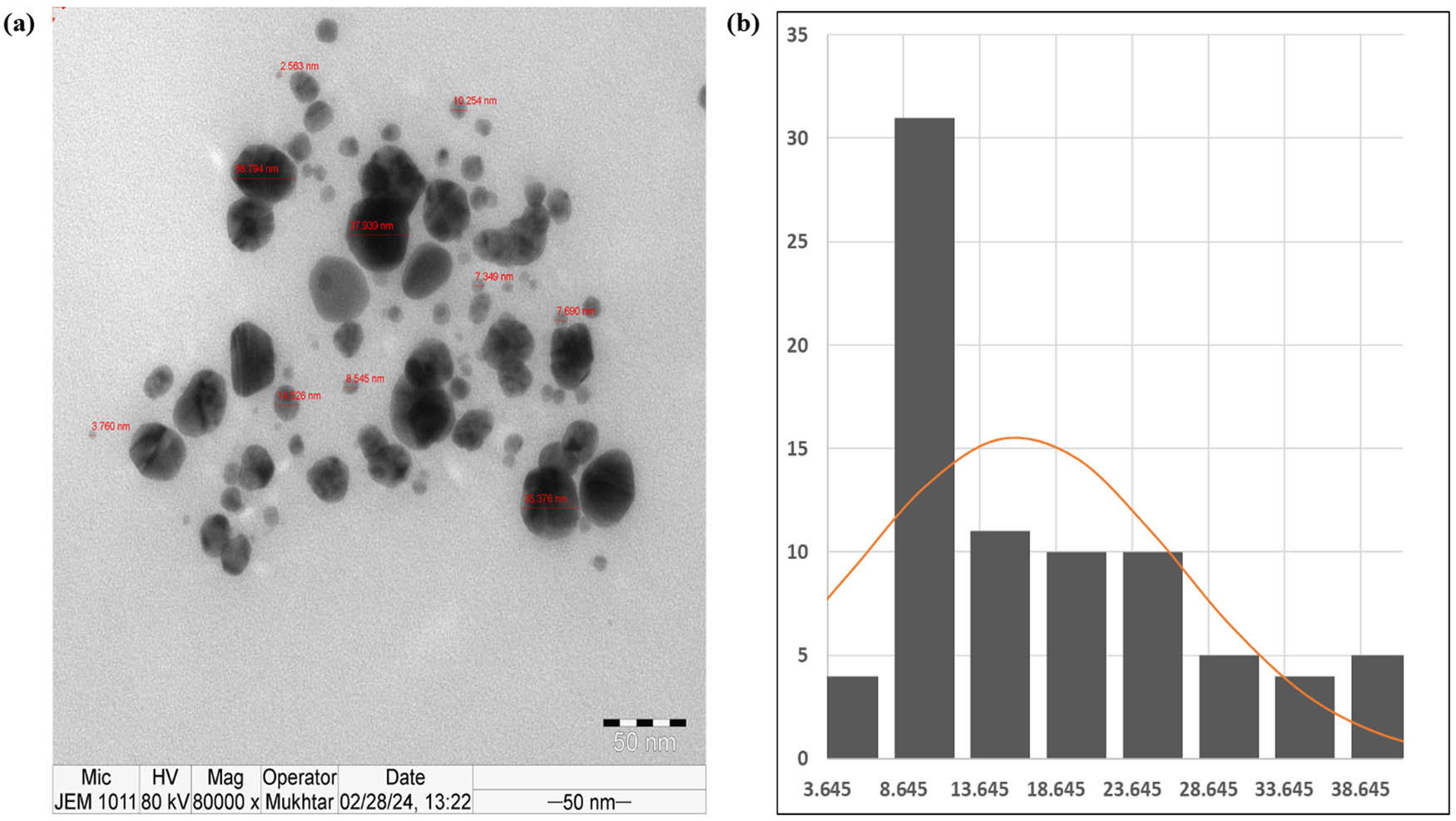
TEM of the synthesized AgNPs. (a) TEM image and (b) particle size distribution for the sample fitted with a log-normal distribution function.
3.5 Cell cytotoxicity and apoptotic analysis
The toxicity of aqueous T. polium leaf extract and synthesized AgNPs on human cancer cell lines MCF-7 and HepG2 was investigated. The MTT assay results revealed a dose- and time-dependent decrease in cell viability, compared to untreated cells. The IC50 values were 15 ± 3.18 μg·mL−1 and 34.5 ± 3.16 μg·mL−1 in MCF-7 and 12 ± 2.63 μg·mL−1 and 26.5 ± 2.23 μg·mL−1 in HepG2 induced by synthesized AgNPs and the crude extract, respectively (Figure 7a and b). The apoptosis analysis supported these findings. Caspase 3, 8, and 9 activity was considerably higher in cells treated with T. polium leaf extract or synthesized AgNPs than in the control group. The treated cells expressed decreased amounts of anti-apoptotic genes Bcl-xL and Bcl-2 compared to untreated controls (Figure 8a and b). This shows that both therapies induce apoptosis in cancer cells, contributing to their lethal effects. The morphological evaluation of treated cells supported these findings, where the treated cells showed obvious ultrastructural changes. The lethal effects of T. polium and AgNPs on cancer cells are consistent with research demonstrating the promise of plant extracts and nanoparticles in cancer therapy. Previous studies showed the significant anticancer potential of T. polium and AgNPs by triggering apoptosis of MNK45 human gastric cancer cell line [8], NALM-6 acute lymphoblastic leukemia lymphocyte-like cells [52], and VERO (African Green Monkey Kidney) cell lines [6] through elevation of ROS, protein damage, and oxidative stress mechanisms. T. polium AgNPs were found to induce cytotoxic activity for Ntra-2 cancer cells with an IC50 of 106.58 µg·mL−1; besides, the upregulation of caspase 3 and caspase 9 and downregulation of Bcl-2 and genes involved in angiogenesis [10]. An interesting study showed that T. polium L. was able to reduce the carcinogenesis of the liver cells in an animal model, with a remarkable increase in its total antioxidant status [54]. Another study showed that the green-synthesized zinc oxide nanoparticles (TP-ZnO-NPs) from T. polium extract demonstrated significant antiproliferative and anticancer activities against HGC-27 gastric cancer cells, highlighting their potential as a novel therapeutic agent [55]. These studies and our results showed that the observed dose-dependent cytotoxicity and apoptosis demonstrate the therapeutic potential of T. polium and AgNPs in cancer cells.
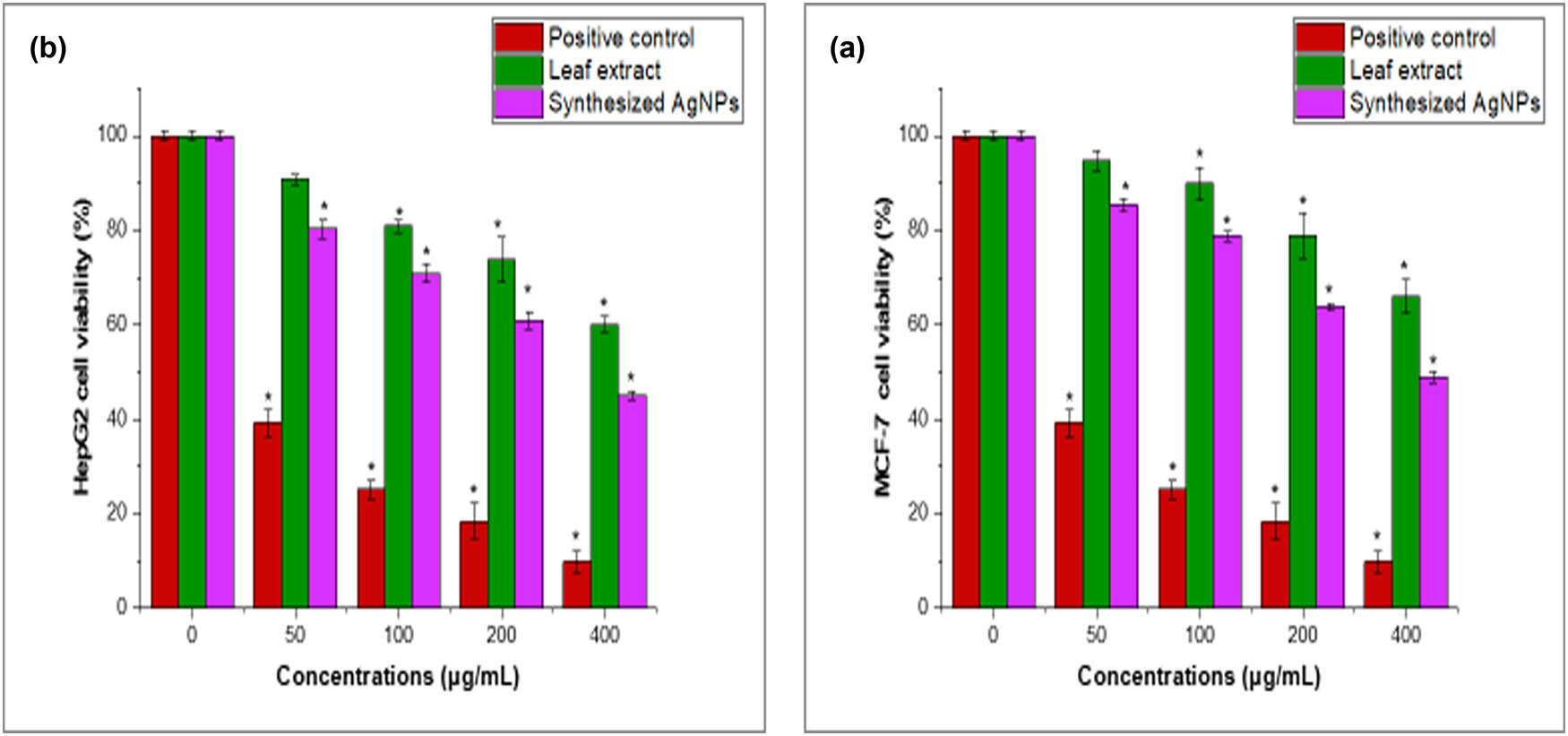
The effect of aqueous T. polium leaf extract and synthesized AgNPs on the viability of MCF-7 (a) and HepG2 cells (b) was examined using the MTT assay. Cells were treated with T. polium leaf extract and synthesized AgNPs (0–400 μg·mL−1) for 24 h. Mean ± SD is presented from three independent experiments (*p < 0.05 compared to non-treated cells (control)).
![Figure 8
Effect of aqueous T. polium leaf extract (35 μg·mL−1) (a) and synthesized AgNPs (15 μg·mL−1) (b) on MCF-7 and HepG2 cell lines. Synthesized AgNPs treated MCF-7 and HepG2 cells showed an increased percentage of the apoptotic population (caspase 3, 8, and 9 and Bax) after 24 h incubation. The results are represented as the mean ± SD of three independent experiments (*p < 0.05 compared to non-treated cells [control]).](/document/doi/10.1515/gps-2024-0227/asset/graphic/j_gps-2024-0227_fig_008.jpg)
Effect of aqueous T. polium leaf extract (35 μg·mL−1) (a) and synthesized AgNPs (15 μg·mL−1) (b) on MCF-7 and HepG2 cell lines. Synthesized AgNPs treated MCF-7 and HepG2 cells showed an increased percentage of the apoptotic population (caspase 3, 8, and 9 and Bax) after 24 h incubation. The results are represented as the mean ± SD of three independent experiments (*p < 0.05 compared to non-treated cells [control]).
3.6 Antibacterial effects
In the current study, the antibacterial effect of T. polium leaf extract and synthesized AgNPs against bacterial strains was investigated against Gram-positive bacteria (S. aureus, S. epidermidis, and B. subtilis) and Gram-negative bacteria (K. pneumoniae and P. aeruginosa). In comparison to the positive control of 25 µg·mL−1 ciprofloxacin, the synthesized AgNPs demonstrated robust antibacterial efficacy. The MIC values for T. polium leaf extract ranged from 31.25 ± 0.00 and 62.5 ± 0.00 μg·mL−1, while the MIC values for synthesized AgNPs ranged from 5.85 ± 2.76 to 31.25 ± 0.00 μg·mL−1 (Tables 3 and 4). In agreement with these findings, a previous study revealed that the 4-hydroxybenzoic acid and catechol fractions of the T. polium ethyl acetate and ethanolic extracts had significant antibacterial activities against S. aureus, S. typhi, P. aeruginosa, K. pneumoniae, and E. coli with ZOI ranged from 19 to 23 mm [56]. In the study conducted by Alreshidi et al. [6], the methanolic extract of T. polium had high zoon ranged from 11 to 19.33 mm for Acinetobacter baumannii, Proteus mirabilis, P. aeruginosa, Streptococcus pyogens, and Enterobacter cloacae and lowered effects against E. coli, K. pneumoniae, Staphylococcus sciuri, P. aeruginosa, S. aureus, and Sphingomonas paucimobilis with ZOIs ranged from 6 to 7 mm. In multiple studies of the essential oil extracted from T. polium, the GC–MS analysis revealed that the phytochemical composition of 3-carene, γ-muurolene, α-pinene, α-phellandrene, carvacrol, and caryophyllene increased its antibacterial activity against S. aureus and A. baumannii [57,58,59]. Another study showed that the methanolic extracts of T. polium aerial parts and root had more antibacterial activities against Gram-positive bacteria (B. cereus and S. aureus) than Gram-negative bacteria (Shigella flexneri and E. coli) with an MBC value ranged from 150 to 600 µg·mL−1 in the extracts from the root parts [60]. A recent study demonstrated that the AgNPs fabricated from T. polium extracts had stronger antibacterial activities than the crude extracts [18]. In a clinical trial, a toothpaste containing T. polium extract significantly reduced the number and growth of Streptococcus mutans in the mouth [61]. Overall, these results support the potential use of T. polium and synthesized AgNPs as effective antibacterial agents.
The ZOIs, MIC, and MBC of aqueous T. polium leaf extract
| Bacterium/dilution | Positive control | 500 μg·mL−1 | 250 μg·mL−1 | 125 μg·mL−1 | 62.5 μg·mL−1 | MIC (μg·mL−1) | MBC (μg·mL−1) |
|---|---|---|---|---|---|---|---|
| S. aureus | 23 ± 1.67 | 17 ± 2.68 | 15 ± 1.33 | 11 ± 2.37 | 9 ± 0.89 | 62.5 ± 0.00 | 125 ± 0.00 |
| S. epidermidis | 23 ± 1.17 | 21 ± 1.98 | 19 ± 1.38 | 14 ± 1.93 | 10 ± 1.73 | 31.25 ± 0.00 | 62.5 ± 0.00 |
| B. subtilis | 25 ± 0.46 | 19 ± 1.08 | 15 ± 1.93 | 13 ± 1.64 | 11 ± 1.32 | 62.5 ± 0.00 | 125 ± 0.00 |
| E. coli | 26 ± 2.17 | 21 ± 2.59 | 18 ± 1.26 | 14 ± 1.43 | 12 ± 2.35 | 62.5 ± 0.00 | 125 ± 0.00 |
| K. pneumoniae | 27 ± 1.38 | 20 ± 1.93 | 18 ± 2.84 | 14 ± 1.32 | 11 ± 1.13 | 31.25 ± 0.00 | 62.5 ± 0.00 |
| P. aeruginosa | 27 ± 2.28 | 21 ± 0.35 | 16 ± 1.34 | 13 ± 1.23 | 12 ± 2.28 | 31.25 ± 0.00 | 62.5 ± 0.00 |
The ZOIs, MIC, and MBC of the synthesized AgNPs
| Bacterium/dilution | Positive control | 500 μg·mL−1 | 250 μg·mL−1 | 125 μg·mL−1 | 62.5 μg·mL−1 | MIC (μg·mL−1) | MBC (μg·mL−1) |
|---|---|---|---|---|---|---|---|
| S. aureus | 23 ± 1.67 | 19 ± 2.61 | 15 ± 0.33 | 13 ± 2.36 | 11 ± 1.69 | 31.25 ± 0.00 | 62.5 ± 0.00 |
| S. epidermidis | 23 ± 1.17 | 25 ± 0.98 | 22 ± 1.33 | 19 ± 1.93 | 13 ± 0.72 | 11.71 ± 5.52 | 15.3 ± 0.00 |
| B. subtilis | 25 ± 0.46 | 23 ± 1.34 | 19 ± 1.92 | 16 ± 0.74 | 14 ± 0.36 | 23.43 ± 11.04 | 31.25 ± 0.00 |
| E. coli | 26 ± 2.17 | 21 ± 0.53 | 19 ± 1.25 | 15 ± 1.45 | 11 ± 0.35 | 7.81 ± 0.00 | 15.63 ± 0.00 |
| K. pneumoniae | 27 ± 1.38 | 24 ± 0.92 | 20 ± 0.81 | 15 ± 1.39 | 10 ± 0.21 | 11.71 ± 5.52 | 15.63 ± 0.00 |
| P. aeruginosa | 27 ± 2.28 | 27 ± 2.61 | 23 ± 0.33 | 14 ± 2.36 | 9 ± 1.69 | 5.85 ± 2.76 | 7.81 ± 0.00 |
4 Conclusion
The current work exhibits the extensive phytochemical, biogenic, and biological features of Teucrium polium leaf extract and its synthesized AgNPs. The GC–MS analysis revealed a broad profile of bioactive chemicals, including steroid esters, sesquiterpenoids, and monoterpenoids, emphasizing the extract’s antibacterial and anticancer properties. Notably, the extract’s high phenolic and flavonoid content demonstrates its great antioxidant ability, which supports its involvement in fighting oxidative stress-related illnesses. The FTIR examination revealed the presence of essential functional groups involved in bioactivity, whereas UV–vis, EDX, SEM, and TEM investigations corroborated the effective synthesis of AgNPs. The AgNPs had a spherical morphology and an average size of 41–61 nm, indicating their potential for precision biological applications. The cytotoxicity results showed that the extract and AgNPs killed MCF-7 and HepG2 cancer cells in a dose- and time-dependent manner, with considerable apoptosis induction via caspase activation and reduction of anti-apoptosis gene expression. These data demonstrate the therapeutic efficacy of T. polium and its AgNPs in cancer treatment. Furthermore, AgNPs have antibacterial action against both Gram-positive and Gram-negative bacteria, with lower MIC values, indicating their potential as natural antimicrobial agents. Overall, our study sheds new light on the bioactive components and multifunctional capabilities of T. polium and its synthesized AgNPs, highlighting their potential applications in nanotechnology, biomedicine, and disease treatment. The findings provide a solid platform for future study into the mechanisms of action and clinical applications of these natural bioresources.
Acknowledgment
The authors thank the Researchers Supporting Project number (RSP2025R418), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: The research was supported by the Researchers Supporting Project number (RSP2025R418), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Conceptualization, R.M.A. and I.M.A.; methodology, I.M.A.; software, I.M.A.; validation, R.M.A. and I.M.A.; formal analysis, I.M.A.; investigation, R.M.A.; resources, R.M.A.; data curation, R.M.A.; writing – original draft preparation, I.M.A.; writing – review and editing, R.M.A.; visualization, R.M.A.; supervision, R.M.A.; project administration, R.M.A.; funding acquisition, R.M.A. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Bahramikia S, Gavyar PHH, Yazdanparast R. Teucrium polium L: An updated review of phytochemicals and biological activities. Avicenna J Phytomed. 2022;12:224. 10.22038/AJP.2021.19155.Suche in Google Scholar PubMed PubMed Central
[2] Rahmouni F, Saoudi M, Rebai T. Therapeutics studies and biological properties of Teucrium polium (Lamiaceae). BioFactors. 2021;47:952–63. 10.1002/biof.1782.Suche in Google Scholar PubMed
[3] Toplan GG, Göger F, Taşkin T, Genç GE, Civaş A, Işcan G, et al. Phytochemical composition and pharmacological activities of Teucrium polium L. collected from eastern Turkey. Turk J Chem. 2022;46:269–82. 10.3906/kim-2107-13.Suche in Google Scholar PubMed PubMed Central
[4] Ait Chaouche FS, Mouhouche F, Hazzit M. Antioxidant capacity and total phenol and flavonoid contents of Teucrium polium L. grown in Algeria. Mediterr J Nutr Metab. 2018;11:135–44. 10.3233/MNM-17189.Suche in Google Scholar
[5] Al-Naemi HA, Alasmar RM, Al-Ghanim K. Alcoholic extracts of Teucrium polium exhibit remarkable anti-inflammatory activity: In vivo study. Biomol Biomed. 2024;24:82. 10.17305/bb.2023.9239.Suche in Google Scholar PubMed PubMed Central
[6] Alreshidi M, Noumi E, Bouslama L, Ceylan O, Veettil VN, Adnan M, et al. Phytochemical screening, antibacterial, antifungal, antiviral, cytotoxic, and anti-quorum-sensing properties of Teucrium polium L. aerial parts methanolic extract. Plants. 2020;9:1418. 10.3390/plants9111418.Suche in Google Scholar PubMed PubMed Central
[7] Ben Othman M, Bel Hadj Salah-Fatnassi K, Ncibi S, Elaissi A, Zourgui L. Antimicrobial activity of essential oil and aqueous and ethanol extracts of Teucrium polium L. subsp. gabesianum (LH) from Tunisia. Physiol Mol Biol Plants. 2017;23:723–9. 10.1007/s12298-017-0444-9.Suche in Google Scholar PubMed PubMed Central
[8] Hashemi SF, Tasharrofi N, Saber MM. Green synthesis of silver nanoparticles using Teucrium polium leaf extract and assessment of their antitumor effects against MNK45 human gastric cancer cell line. J Mol Struct. 2020;1208:127889. 10.1016/j.molstruc.2020.127889.Suche in Google Scholar
[9] Elmasri WA, Hegazy M-EF, Mechref Y, Paré PW. Structure-antioxidant and anti-tumor activity of Teucrium polium phytochemicals. Phytochem Lett. 2016;15:81–7. 10.1016/j.phytol.2015.11.007.Suche in Google Scholar
[10] Roumi S, Tabrizi MH, Eshaghi A, Abbasi N. Teucrium polium extract‐loaded solid lipid nanoparticles: A design and in vitro anticancer study. J Food Biochem. 2021;45:e13868. 10.1111/jfbc.13868.Suche in Google Scholar PubMed
[11] Guesmi F, Tyagi AK, Prasad S, Landoulsi A. Terpenes from essential oils and hydrolate of Teucrium alopecurus triggered apoptotic events dependent on caspases activation and PARP cleavage in human colon cancer cells through decreased protein expressions. Oncotarget. 2018;9:32305. 10.18632/oncotarget.25955.Suche in Google Scholar PubMed PubMed Central
[12] Panicker NG, Balhamar SOMS, Akhlaq S, Qureshi MM, Rizvi TS, Al-Harrasi A, et al. Identification and characterization of the caspase-mediated apoptotic activity of Teucrium mascatense and an isolated compound in human cancer cells. Molecules. 2019;24:977. 10.3390/molecules24050977.Suche in Google Scholar PubMed PubMed Central
[13] Dhaka A, Mali SC, Sharma S, Trivedi R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023;6:101108. 10.1016/j.rechem.2023.101108.Suche in Google Scholar
[14] Kulkarni D, Sherkar R, Shirsathe C, Sonwane R, Varpe N, Shelke S, et al. Biofabrication of nanoparticles: Sources, synthesis, and biomedical applications. Front Bioeng Biotechnol. 2023;11:1159193. 10.3389/fbioe.2023.1159193.Suche in Google Scholar PubMed PubMed Central
[15] Mosleh-Shirazi S, Kasaee SR, Dehghani F, Kamyab H, Kirpichnikova I, Chelliapan S, et al. Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceram Int. 2023;49:11293–301. 10.1016/j.ceramint.2022.11.329.Suche in Google Scholar
[16] Amani AM, Rahbar A, Vafa E, Tayebi L, Abbasi M, Kamyab H, et al. Exploring the functionality of MXenes as promising versatile antimicrobial agents and their novel applications. Mater Today Commun. 2024;41:110774. 10.1016/j.mtcomm.2024.110774.Suche in Google Scholar
[17] Gheisari F, Kasaee SR, Mohamadian P, Chelliapan S, Gholizadeh R, Zareshahrabadi Z, et al. Bromelain-loaded silver nanoparticles: Formulation, characterization and biological activity. Inorg Chem Commun. 2024;161:112006. 10.1016/j.inoche.2023.112006.Suche in Google Scholar
[18] Hashemitabar G, Aflakian F, Sabzevar AH. Assessment of antibacterial, antioxidant, and anticancer effects of biosynthesized silver nanoparticles using Teucrium polium extract. J Mol Struct. 2023;1291:136076. 10.1016/j.molstruc.2023.136076.Suche in Google Scholar
[19] Melkamu WW, Bitew LT. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon. 2021;7:1–11. 10.1016/j.heliyon.2021.e08459.Suche in Google Scholar PubMed PubMed Central
[20] Quevedo AC, Guggenheim E, Briffa SM, Adams J, Lofts S, Kwak M, et al. UV-Vis spectroscopic characterization of nanomaterials in aqueous media. J Vis Exp. 2021;176:e61764. 10.3791/61764. PMID: 34747394.Suche in Google Scholar PubMed
[21] Singh P, Mijakovic I. Strong antimicrobial activity of silver nanoparticles obtained by the green synthesis in Viridibacillus sp. extracts. Front Microbiol. 2022;13:820048. 10.3389/fmicb.2022.820048.Suche in Google Scholar PubMed PubMed Central
[22] Al-Otibi FO, Alrumaizan GI, Alharbi RI. Evaluation of anticandidal activities and phytochemical examination of extracts prepared from Vitex agnus-castus: A possible alternative in treating candidiasis infections. BMC Complement Med Ther. 2022;22:69. 10.1186/s12906-022-03552-x.Suche in Google Scholar PubMed PubMed Central
[23] Wolfe KL, Liu RH. Apple peels as a value-added food ingredient. J Agric Food Chem. 2003;51:1676–83. 10.1021/jf025916z.Suche in Google Scholar PubMed
[24] Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. 10.38212/2224-6614.2748.Suche in Google Scholar
[25] Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. 10.1016/0022-1759(83)90303-4.Suche in Google Scholar PubMed
[26] Alkhudhayri AA, Wahab R, Siddiqui MA, Ahmad J. Selenium nanoparticles induce cytotoxicity and apoptosis in human breast cancer (MCF-7) and liver (HEPG2) cell lines. Nanosci Nanotechnol Lett. 2020;12:324–30. 10.1166/nnl.2020.3115.Suche in Google Scholar
[27] Al-Dhabi NA, Valan Arasu M. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evid-Based Complement Altern Med. 2016;2016:1–13. 10.1155/2016/7631864.Suche in Google Scholar PubMed PubMed Central
[28] Ahn S, Siddiqi MH, Aceituno VC, Simu SY, Zhang J, Jimenez Perez ZE, et al. Ginsenoside Rg5: Rk1 attenuates TNF-α/IFN-γ-induced production of thymus-and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell Dev Biol-Animal. 2016;52:287–95. 10.1007/s11626-015-9983-y.Suche in Google Scholar PubMed
[29] Aziz IM, Alshalan RM, Rizwana H, Alkhelaiwi F, Almuqrin AM, Aljowaie RM, et al. Chemical composition, antioxidant, anticancer, and antibacterial activities of roots and seeds of Ammi visnaga L. methanol extract. Pharmaceuticals. 2024;17:121. 10.3390/ph17010121.Suche in Google Scholar PubMed PubMed Central
[30] Al-Dhabi NA, Valan Arasu M, Vijayaraghavan P, Esmail GA, Duraipandiyan V, Kim YO, et al. Probiotic and antioxidant potential of Lactobacillus reuteri LR12 and Lactobacillus lactis LL10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms. 2020;8:1461. 10.3390/microorganisms8101461.Suche in Google Scholar PubMed PubMed Central
[31] Singh P, Singh H, Kim YJ, Mathiyalagan R, Wang C, Yang DC. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzyme Microb Technol. 2016;86:75–83. 10.1016/j.enzmictec.2016.02.005.Suche in Google Scholar PubMed
[32] Basri DF, Sandra V. Synergistic interaction of methanol extract from Canarium odontophyllum Miq. Leaf in combination with oxacillin against methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591. Int J Microbiol. 2016;2016:1–7. 10.1155/2016/5249534.Suche in Google Scholar PubMed PubMed Central
[33] Aljeldah MM, Yassin MT, Mostafa AA-F, Aboul-Soud MA. Synergistic antibacterial potential of greenly synthesized silver nanoparticles with fosfomycin against some nosocomial bacterial pathogens. Infect Drug Resist. 2022;16:125–42. 10.1155/2016/5249534.Suche in Google Scholar
[34] Sharifi-Rad J, Quispe C, Herrera-Bravo J, Akram M, Abbaass W, Semwal P, et al. Phytochemical constituents, biological activities, and health-promoting effects of the Melissa officinalis. Oxid Med Cell Longev. 2021;2021:1–20.10.1155/2021/6584693Suche in Google Scholar PubMed PubMed Central
[35] Boutefaha Z, Diab KA, Gheraibia S, El-Nekeety AA, Belattar N, Hassan ME, et al. Screening of the phytochemical constituents of Teucrium polium extract and evaluation of their prophylactic role against the oxidative damage and cytotoxicity of Aflatoxin B1 in rats. Toxicon. 2023;233:107252. 10.1016/j.toxicon.2023.107252.Suche in Google Scholar PubMed
[36] Benali T, Habbadi K, Bouyahya A, Khabbach A, Marmouzi I, Aanniz T, et al. Phytochemical analysis and study of antioxidant, anticandidal, and antibacterial activities of Teucrium polium subsp. polium and Micromeria graeca (Lamiaceae) essential oils from Northern Morocco. Evid-Based Complement Altern Med. 2021;2021:1–10. 10.1155/2021/6641720.Suche in Google Scholar PubMed PubMed Central
[37] Timizar Z, Boutemak K, Hadj Ziane-Zafour A, Touzout N, Tahraoui H, Jaouadi B, et al. Comprehensive analysis of phytochemical composition, antioxidant potential, and antibacterial activity of T. polium. Separations. 2024;11:90. 10.3390/separations11040090.Suche in Google Scholar
[38] Atallah BM, Haroun SA, El-Mohsnawy E. Antibacterial activity of two actinomycetes species isolated from black sand in North Egypt. S Afr J Sci. 2023;119:1–8.10.17159/sajs.2023/14509Suche in Google Scholar
[39] Lenz KD, Klosterman KE, Mukundan H, Kubicek-Sutherland JZ. Macrolides: From toxins to therapeutics. Toxins. 2021;13:347. 10.3390/toxins13050347.Suche in Google Scholar PubMed PubMed Central
[40] EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Bampidis V, Azimonti G, Bastos MDL, Christensen H, Durjava M, et al. Safety and efficacy of feed additives consisting of essential oils derived from the flower buds or the leaves of Syzygium aromaticum (L.) Merr. & LM Perry (clove bud oil and clove leaf oils) for all animal species (FEFANA asbl). EFSA J. 2023;21:e08183. 10.2903/j.efsa.2023.8183.Suche in Google Scholar PubMed PubMed Central
[41] Hirasawa K, Moriya S, Miyahara K, Kazama H, Hirota A, Takemura J, et al. Macrolide antibiotics exhibit cytotoxic effect under amino acid-depleted culture condition by blocking autophagy flux in head and neck squamous cell carcinoma cell lines. PLoS One. 2016;11:e0164529. 10.1371/journal.pone.0164529.Suche in Google Scholar PubMed PubMed Central
[42] Ren H-C, Zhang J, Liang H. Two new p-coumaroylated sesquiterpenoids from Pilea cavaleriei. J Asian Nat Prod Res. 2018;20:109–16. 10.1080/10286020.2017.1320990.Suche in Google Scholar PubMed
[43] Calway T, Du G-J, Wang C-Z, Huang W-H, Zhao J, Li S-P, et al. Chemical and pharmacological studies of Oplopanax horridus, a North American botanical. J Nat Med. 2012;66:249–56. 10.1007/s11418-011-0602-2.Suche in Google Scholar PubMed PubMed Central
[44] Mahizan NA, Yang S-K, Moo C-L, Song AA-L, Chong C-M, Chong C-W, et al. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019;24:2631. 10.3390/molecules24142631.Suche in Google Scholar PubMed PubMed Central
[45] Maitra S, De A, Das B, Roy SN, Chakraborty R, Samanta A, et al. Seasonal variation of phyto-constituents of tea leaves affects antiproliferative potential. J Am Coll Nutr. 2019;38:415–23. 10.1080/07315724.2018.1538829.Suche in Google Scholar PubMed
[46] Chohan TA, Sarfraz M, Rehman K, Muhammad T, Ghori MU, Khan KM, et al. Phytochemical profiling, antioxidant and antiproliferation potential of Euphorbia milii var.: Experimental analysis and in-silico validation. Saudi J Biol Sci. 2020;27:3025–34. 10.1016/j.sjbs.2020.08.003.Suche in Google Scholar PubMed PubMed Central
[47] Chen Y, Ba L, Huang W, Liu Y, Pan H, Mingyao E, et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur J Pharmacol. 2017;796:90–100. 10.1016/j.ejphar.2016.11.053.Suche in Google Scholar PubMed
[48] Stankovic MS, Niciforovic N, Mihailovic V, Topuzovic M, Solujic S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc Bot Pol. 2012;81:117–22. 10.5586/asbp.2012.010.Suche in Google Scholar
[49] Benchikha N, Messaoudi M, Larkem I, Ouakouak H, Rebiai A, Boubekeur S, et al. Evaluation of possible antioxidant, anti-hyperglycaemic, anti-alzheimer and anti-inflammatory effects of Teucrium polium aerial parts (Lamiaceae). Life. 2022;12:1579. 10.3390/life12101579.Suche in Google Scholar PubMed PubMed Central
[50] Rakhshan N, Mansournia M, Kashi FJ. Plant extract-strategy using Teucrium Polium stems to green synthesize Ag/AgCl bionanocomposite imprinted on Fe3O4/kaolinite and potentials in catalytic and chemosensor applications. Arab J Chem. 2022;15:103719. 10.1016/j.arabjc.2022.103719.Suche in Google Scholar
[51] Al-Aoh HA, Alamrani NA. Chemically modified Teucrium polium (Lamiaceae) plant act as an effective adsorbent tool for potassium permanganate (KMnO4) in wastewater remediation. Open Chem. 2022;20:736–47. 10.1515/chem-2022-0196.Suche in Google Scholar
[52] Amini SM, Samareh Salavati Pour M, Vahidi R, Kouhbananinejad SM, Sattarzadeh Bardsiri M, Farsinejad A, et al. Green synthesis of stable silver nanoparticles using Teucrium polium extract: in-vitro anticancer activity on NALM-6. Nanomed Res J. 2021;6:170–8. 10.22034/nmrj.2021.02.008.Suche in Google Scholar
[53] Mohammadi F, Yousefi M, Ghahremanzadeh R. Green synthesis, characterization and antimicrobial activity of silver nanoparticles (AgNPs) using leaves and stems extract of some plants. Adv J Chem-Sect A. 2019;2:266–75. 10.33945/SAMI/AJCA.2019.4.1.Suche in Google Scholar
[54] Movahedi A, Basir R, Rahmat A, Charaffedine M, Othman F. Remarkable anticancer activity of Teucrium polium on hepatocellular carcinogenic rats. Evid‐Based Complement Altern Med. 2014;2014:726724. 10.1155/2014/726724.Suche in Google Scholar PubMed PubMed Central
[55] Bozgeyik I, Ege M, Temiz E, Erdal B, Koyuncu I, Temiz C, et al. Novel zinc oxide nanoparticles of Teucrium polium suppress the malignant progression of gastric cancer cells through modulating apoptotic signaling pathways and epithelial to mesenchymal transition. Gene. 2023;853:147091. 10.1016/j.gene.2022.147091.Suche in Google Scholar PubMed
[56] Mohammed MA, Saeed YS, Ali JF. Antibacterial activity of phenolic compounds of Teucrium polium L. Pak J Pharm Sci. 2023;36:1435–42.Suche in Google Scholar
[57] El Atki Y, Aouam I, El Kamari F, Taroq A, Lyoussi B, Oumokhtar B, et al. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab J Chem. 2020;13:3866–74. 10.1016/j.arabjc.2019.04.001.Suche in Google Scholar
[58] Saleh I, Abd-ElGawad A, El Gendy AE-N, Abd El Aty A, Mohamed T, Kassem H, et al. Phytotoxic and antimicrobial activities of Teucrium polium and Thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants. 2020;9:716. 10.3390/plants9060716.Suche in Google Scholar PubMed PubMed Central
[59] Ghavam M, Markabi FS. Evaluation of yield, chemical profile, and antimicrobial activity of Teucrium polium L. essential oil used in Iranian folk medicine. Appl Biochem Biotechnol. 2024;20:1–17. 10.1007/s12010-023-04847-6.Suche in Google Scholar PubMed
[60] Sharifi-Rad M, Pohl P, Epifano F, Zengin G, Jaradat N, Messaoudi M. Teucrium polium (L.): phytochemical screening and biological activities at different phenological stages. Molecules. 2022;27:1561. 10.3390/molecules27051561.Suche in Google Scholar PubMed PubMed Central
[61] Khoramian Tusi S, Jafari A, Marashi SMA, Faramarzi Niknam S, Farid M, Ansari M. The effect of antimicrobial activity of Teucrium Polium on Oral Streptococcus Mutans: A randomized cross-over clinical trial study. BMC Oral Health. 2020;20:1–8. 10.1186/s12903-020-01116-4.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Artikel in diesem Heft
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

