A facile biodegradation of polystyrene microplastic by Bacillus subtilis
-
Norah Raqi Al-Otaibi
, Rasha Elsayim
Abstract
The extensive application of polystyrene (PS) in the industry and the release of polystyrene microplastics (PS-MPs) in the environmental compartments has raised global concerns. The ability of microbes to utilize PS as a carbon source has been currently established. This study utilized Bacillus subtilis (ATCC 11774) to break down environmentally relevant sized PS-MPs (5 µm) with and without abiotic (thermal and UV radiations) pretreatment for a period of 4 weeks. The biodegradation rate was validated using UV–visible (UV–Vis) and Fourier transform infrared (FTIR) spectrophotometry, scanning electron microscopy coupled with EDX analysis. After 4 weeks, all inoculated PS-MP samples with and without pretreatment showed marked changes in the UV–vis spectra in comparison to the pristine PS-MPs. Additionally, FTIR spectra displayed surface modifications of functional groups in all inoculated samples linked to chain scission/oxidation were highlighted by a notable increase in the carbonyl index during biodegradation. SEM micrographs confirmed the marked fragility of the particles, and a probable oxidation degree was evaluated as an atomic O/C ratio that corroborates the biodegradative potential of B. subtilis. The core finding underscores that B. subtilis can grow on, alter, and use PS as a carbon source, either with or without abiotic pretreatment, emphasizing the role of biological pathways as a sustainable strategy for plastic waste management.
1 Introduction
Since its discovery in 1833 when Frenchman Henri Braconnot first stumbled upon this new material, plastic has emerged as a popular substitute for traditional materials like glass, metal, and wood [1,2,3]. Plastic pollutants disintegrate in the natural environment by physical, chemical, and biological processes, resulting in vast amounts of microplastics (MPs) (<5 mm in diameter) [1]. Following that, the MPs then disperse into the environment, where they can be transported into all environmental compartments. Humans can then be exposed to MPs through three different routes: skin contact, ingestion, or inhalation [4,5]. Furthermore, being regularly exposed to MPs even in a negligible amount is detrimental to human health. Moreover, when the MPs pervade through the skin from cleaners or foams, it may result in their bioaccumulation in the liver and digestive tract [6,7]. In addition, the MPs that are transmitted into the environment can enter the food chain, resulting in the consumption of contaminated food by humans [8].
Statistical exploration shows that MPs compose about 92.4 of plastic debris and are generally composed of polystyrene (PS), polyethylene (PE), and polypropylene [9]. PS plastic (often known as Styrofoam in its expanded form) is a major environmental concern due to its widespread use, persistence in the environment, and challenges in recycling. PS is a key component of environmental MPs, causing significant ecological and human health hazards at the cellular level. This substance, which is widely used in packaging, insulation, and consumer goods, fragments into smaller particles rather than entirely decomposing, resulting in a pervasive presence of PS MPs in all environmental compartments including the food chain. As a result, there is a pressing need for long-term methods to reduce PS pollution [10]. Currently, plastic in the environment can be degraded via three modes: oxidative photodegradation, thermo-oxidative declination, and biodegradation. Out of the three strategies, biodegradation is the most sustainable since it is both environmentally friendly and cost-effective. Photo-oxidation, which makes use of UV light and microbes, is the second-best option. Thermo-oxidative processing, on the other hand, is undesirable since it uses more energy than nature provides [11,12].
During bioremediation, microorganisms, especially bacteria, can alter the manifestation of MPs along with their functional group structures and other features [13,14]. There have been several studies [15,16,17] that have reported the biodeterioration of plastics. For example, Anabaena spiroides, Bacillus sp., Lysinibacillus sp., and Pseudomonas sp. were identified as ideal candidate strains [3]. Previous studies have also highlighted that Bacillus subtilis and Bacillus licheniformis, the most common bacteria in soil, can be potential candidates for microbial remediation of plastic contamination in the environment [3,18]. One of the most promising microbes used in MP degradation is B. subtilis. It has been effectively utilized in combination with other microbes in wastewater treatment attributed to its high degradative capacity for organic compounds including plastic. Biodegradation by B. subtilis used alone has been reported on PE films [18,19,20,21]. PS comprises a straight carbon backbone, and phenyl moieties are attached to alternate backbone atoms. There have been very few reports on the biodegradation of PS due to its distinct structure, which makes it difficult to break down. A comprehensive review by Ho et al. [22] reported findings of previous research on the biodegradability of various PS materials. PS materials employed in these various studies were pure PS, modified PS, or PS combined with other polymers. Pseudomonas spp. and Bacillus spp. have shown potential in degrading PS, particularly when pretreated, to make the material more accessible. The pretreatment approach is a crucial initial step before considering the biological strategy. Most prior studies involved pretreatments of MPs prior to biodegradation procedures. The pretreatment methods used range from heat treatment, γ-irradiation, and UV. Pretreatments are necessary primarily to reduce the hydrophobicity of the polymer, making them susceptible to microbial action [23]. Furthermore, the major research gap that needs to be addressed is the developing strategies focused on degrading PS micro- and nano-plastics in marine, freshwater, and terrestrial environments. Most of the previous studies have used PS plastic products or composites and have not focused much on the microplastic that adds up as a major environmental concern.
The concept served as a framework for the design of the present study to investigate the biodegradative potency of B. subtilis (ATCC 11774) on PS-MPs with and without pretreatment with heat and UV radiations. PS-MPs are frequently discovered as microplastic contaminant in ecosystems, making them an important material for environmental and biological impact research. The particle size (5 µm) used in the study is characteristic of MPs commonly detected in environmental samples. Colony-forming units (CFU) were used as the response parameter to identify the drivers of biodegradation after 30 days of testing. The ideal conditions for biodegradation of PS-MP by the designated bacterial strain were then ascertained using biodegradation tests.
2 Materials and methods
2.1 Materials
Commercially available, ready-to-use PS-MP beads (Alpha Nanotech Inc., Vancouver, Canada) 5 μm in size, colorless to pale white in color, and spherical in shape. PS-MPs were commercially obtained in wet form in solution at an initial concentration of 10 mg·ml−1 and a density of 1.01–1.06 g·cm−3. PS-MPs were delivered in Milli-Q-Water medium and were used as received. Figure 1(a) and (b) shows the SEM micrographs and zeta distribution data of the PS-MPs prior to the study.
The bacterial strain, B. subtilis (ATCC 11774), used in the present study was obtained from the Department of Microbiology at Vision College, Riyadh, Saudi Arabia. The strain was transported within 30 min to the laboratory in a nutrient-rich medium such as Luria-Bertani (LB) broth in a cold storage bag containing ice to maintain a low temperature to ensure that the strain remained viable during transport. Bacterial cells were grown in LB broth, which contained sodium chloride (10 g·l−1), tryptone (10 g·l−1), and yeast extract (5 g·l−1). NaOH was used to alter the pH of the LB broth to 7.4 (this is close to the optimal pH for several bacterial species, and many bacterial enzymes function optimally at or near physiological pH, which is 7.4). The mineral salt medium, Bushnell-Haas (BH) broth, was utilized in the experiment for the bacterial decomposition of PS-MPs. The BH broth was composed of K2HPO4 (1 g·l−1), KH2PO4 (1 g·l−1), NH4NO3 (1 g·l−1), CaCl2 (0.02 g·l−1), MgSO4 (0.20 g·l−1), and FeCl3 (0.05 g·l−1); NaOH was used to adjust the pH to 7 [24]. The BH medium contains only essential inorganic salts and no carbon sources. This causes bacteria to use plastic as their only carbon source, allowing researchers to directly examine the microorganisms’ capacity to break down the polymer. In addition, it provides the necessary minerals and trace elements to support bacterial growth.
The strain was inoculated directly in nutrient broth media and left overnight at 37°C. Initially, the bacterial culture was transferred into the medium by adding a loopful of bacterial colonies (the loop used was a 10 µl in size, and the bacterial count was approximately 107–108 cells). Bacterial strains are ideally sub-cultured every 2–4 weeks to maintain viability. The plate count method and microscopic examination are used to assess the viability of the culture. The bacterial cells were kept stored at 4°C on a slant of LB agar. This form of storage can keep bacterial cells alive for up to 2 months.
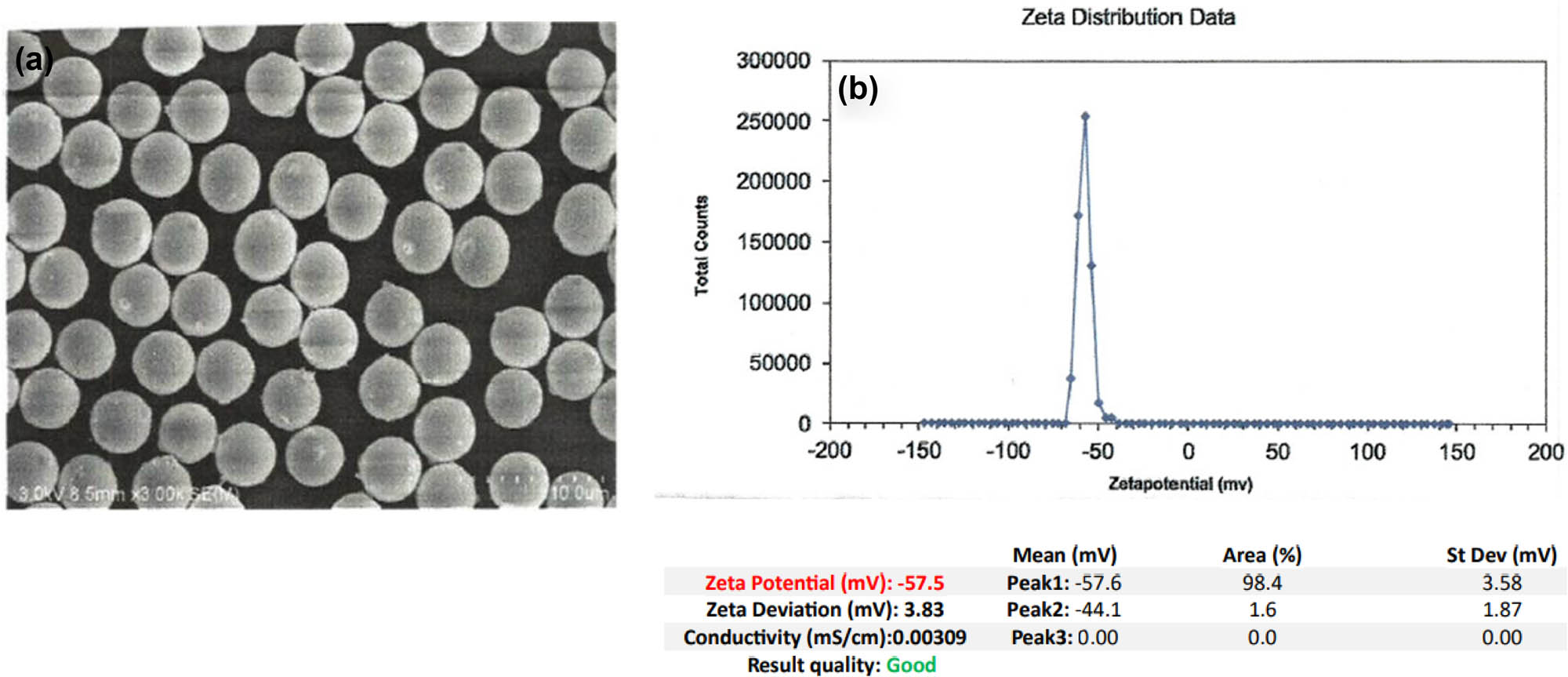
(a) SEM micrograph and (b) zeta distribution data of the PS-MPs prior to the study.
2.2 Pretreatments
A volume of 500 μl of PS-MPs was poured on glass slides in an imaginary square (3 × 3 cm) and given a pretreatment; the first was treated with UV radiation (Purifier Vertical Clean Bench, LABCONCO, 254 nm UV lamp) for 24 h [25]. The distance between the UV lamp and the glass slides was 70 cm, ensuring consistent exposure across samples. Temperature was monitored throughout the treatment to avoid excessive heating that could influence plastic degradation rates, maintaining an ambient temperature of approximately 25°C inside the laminar flow cabinet. These conditions were controlled to optimize the intensity of UV radiation for effective PS biodegradation. In addition, the other abiotic treatment was temperature treatment, where the PS-MPs were subjected to 200°C for 15 min in an air frying oven [26].
2.3 Biodegradation experiments
B. subtilis was cultivated in 250 ml flasks with 100 ml of BH broth in addition to pretreated PS-MPs after dissolving them in the BH broth to assess the bacterial ability to degrade the PS-MPs. PS-MPs were added to the media after which the bacterial suspension. The percentage of the PS-MPs relative to the volume of the medium was calculated as follows:
Volume of BH media: 100 ml
Volume of bacterial suspension: 10 ml
Volume of plastic: 500 µl (which is equivalent to 0.5 ml)
Total volume = volume of BH media + volume of bacterial suspension + volume of PS-MP = 110.5 ml. Thus, the percentage of PS-MPs was approximately 0.45%.
Thereafter, 10 ml of Bacillus cells cultured overnight were collected, resuspended in distilled water, and centrifuged at 12,000 rpm for 2 min at 4°C. The procedure was frequented twice to remove the LB medium. Before inoculating the finished cells into the BH medium, the cell density was adjusted to an OD600 of 0.5 McFarland standard to ensure consistency across experiments. The finished cells were inoculated into 100 ml BH broth and cultured for 30 days at 37°C with continual shaking at 135 rpm on thermostatic rotary shakers (Excella® E24 Series Refrigerated Benchtop Incubator Shaker). The flasks contained mineral medium, bacterial suspension, and PSMPs without and with abiotic pretreatment (in separate flasks). Furthermore, the control flasks, which contain mineral medium and bacterial suspension without PS-MPs particles, were set to observe bacterial growth. The PS-MPs in BH broth with no pretreatments and no bacterial inoculation were employed as a negative control and incubated under the same conditions as the sample groups. To ensure that the PS-MPs without bacteria did not undergo abiotic degradation, periodic measurements were conducted throughout the experimental period; the control vials were visually inspected at regular intervals for any signs of physical degradation, such as changes in color, texture, or the presence of micro fragments. Fourier transform infrared (FTIR) analysis was performed that assessed the chemical changes in the PS-MPs, which would suggest degradation. All experiments were carried out separately in triplicate.
2.3.1 Determination of B. subtilis cell count
Through the experimental period of 30 days, once a week, the colony counting method was employed to determine the amount of viable bacterial cells. Using the pour plate technique, 100 μl of culture broth was put onto plates in addition to LB to determine colony counts [27]. The plates were then incubated for 24 h at 37°C. The number of viable cells per milliliter was expressed as CFU.
2.3.2 Biodegradability tests
Changes in the chemical characteristics of the PS-MP samples, including the appearance or elimination of functional groups with subsequent bacterial incubation with pretreatment and without were analyzed by FTIR spectroscopy (Prestige-21, Shimadzu, Columbia, MD, US). Potassium bromide (KBr) pellets were used for sample preparation to obtain clear spectral data. The FTIR spectrometer was configured to transmittance (%) mode, with a total of 16 accumulated scans to improve signal quality and reduce noise. A spectral resolution of 16.0 was selected to balance sensitivity and clarity in the measurements. The wavenumber range was set from 280 to 4,400 cm⁻¹, covering the essential absorption regions for analysis. Prior to analysis, all PS-MP specimens, including the blank, negative control, and samples, were washed successively with 2% sodium dodecyl sulphate (SDS), 70% ethanol, and distilled water. SDS was utilized at 2% because it is an anionic detergent that removes organic residues, grease, and surface-bound proteins from plastic. It effectively destroys hydrophobic interactions, resulting in a clear surface for the following processes. Ethanol was employed at 70% because it is a disinfectant that can kill microbial contaminants; bacteria and fungi that could interfere with the biodegradation process.
The PS-MP specimens were finally dried at room temperature, and the IR spectra of all specimens were recorded [28]. The appeared morphological changes in PS particles were evaluated using a field emission scanning electron microscope (JSM-76104, Jeol, Japan) with the EDX analysis. The samples were coated with a thin layer of platinum using a sputter coater (JEOL Auto Fine Coater [JEC-3000FC]); the accelerating voltage was 15 kV, which is optimal for both surface imaging and EDX analysis. The images were captured at magnifications ranging from 2,000× to 10,000×, depending on the features of interest conducted under high-vacuum conditions. Degradation effects included surface roughening, the creation of holes or fissures, and de-fragmentation. In addition, UV–visible (UV–Vis) spectroscopy (1800 UV spectrophotometer, Shimadzu) was used to gather absorbance spectra between 200 and 600 nm using 1-cm-path quartz cuvettes to test PS-MP degradation. Nonetheless, UV–Vis is limited to plastics that include chromophores (e.g., a conjugated double bond). These analyses can be employed to track the released monomers or alterations in the molecular structure [29].
3 Results
3.1 Bacterial growth
Bacterial growth was monitored through 4 weeks of the experimental period in all B. subtilis inoculated samples of PS-MPs with and without abiotic pretreatment by determining the CFU. Figure 2 shows the pattern of bacterial growth in all samples which was uniform and steady through the experimental period.
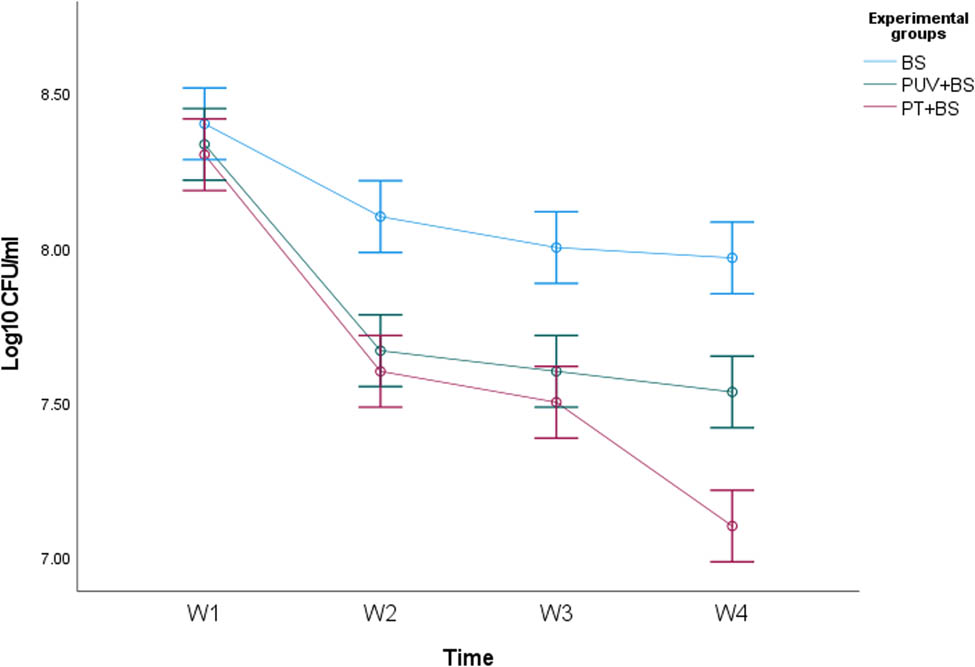
Bacterial growth curve showing log CFU·ml−1 of the bacterium Bacillus subtilis in the optimized medium. Each value of the log (CFU·ml−1) of the curve represents the mean ± SE of at least three replicates.
3.2 UV–Vis spectroscopy
Figure 3(a) and (b) shows the UV–Vis absorption spectra corresponding to the control (the pristine PS-MPs) and the groups with bacterial culture with and without pretreatment after 4 weeks that represent the beginning and end of the biodegradation experiment. A marked variation in the absorbance ratio of different inoculated samples (pretreated and untreated) is observed after 4 weeks in comparison to the control. The observable trends are as follows: Control (pristine PS-MPs, green curve) shows minimal absorbance changes owing to the intrinsic resistance of the pristine PS. Limited peaks indicate little or no structural change during the testing period. For the untreated inoculated group, PS + BS (orange curve): bacterial action induces chemical modifications, including oxidation and the introduction of chromophoric groups (e.g., carbonyls and hydroxyls), resulting in a moderate increase in absorbance in the shorter wavelength range (<300 nm). For the UV pretreatment group, PUV + BS (blue curve) UV pretreatment improves degradation by introducing oxidized functional groups before bacterial exposure, resulting in a higher initial absorbance. These groups absorb heavily in the UV range, indicating partial photo-oxidation. Bacterial growth increases deterioration following UV exposure, resulting in variations in absorbance across wavelengths. The temperature pretreated inoculated group, PT + BS (red curve) shows physical fragmentation increases surface area, facilitating microbial colonization and activity. The red curve shows distinct peaks and elevated absorbance compared to PS and PS + BS, suggesting intensified biodegradation.
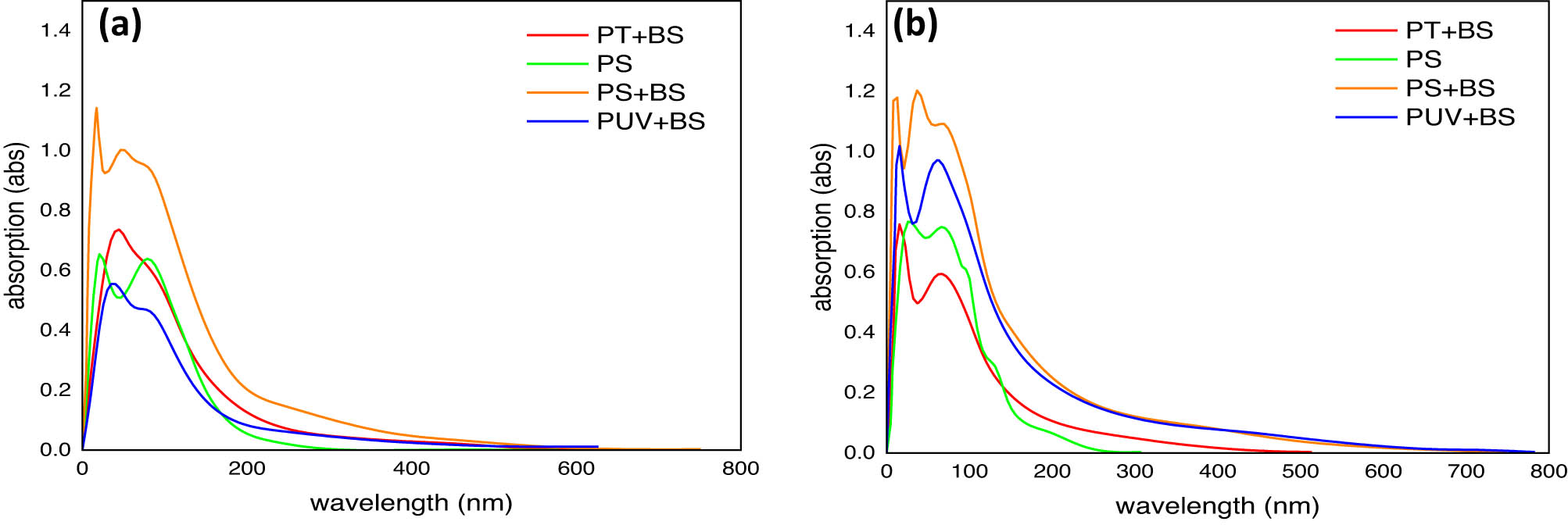
UV–Vis spectra of PS-MP samples: (a) first week and (b) fourth week. PS (control, pristine), PS + BS (untreated inoculated with B. subtilis), PT + BS (temperature-treated PS and inoculated with B. subtilis), and PUV + BS (UV-treated PS and inoculated with B. subtilis).
In addition, a quantitative assessment of the degradation rate of inoculated PS-MPs (PS + BS, PT + BS, PUV + BS) was done on the basis of the reduction rate that was calculated by applying the first-order kinetic model shown under
where K indicates the first-order rate constant for samples uptake per day, t indicates the time in days, C indicates the final concentration of samples, and C 0 indicates the initial concentration of samples. Thus, the formula shown below was employed to compute the half-life of samples by integrating the value generated from the removal rate constant:
Notably, UV pretreated PS-MPs (PUV + BS) exhibited a higher reduction rate (0.008037 day−1) and a shorter half-life (86.2 days) followed by (PT + BS) and (PS + BS) (Table 1). However, the occurrence of biodegradation was further confirmed after analyzing the FTIR spectra.
Biodegradation of inoculated PS-MP samples (treated and untreated) based on the reduction rate
| Samples | Rate of constant (day−1) | Half-life (day) |
|---|---|---|
| PS + BS | 0.00096 | 722.9 |
| PT + BS | 0.00118 | 587.3 |
| PUV + BS | 0.008037 | 86.2 |
3.3 FTIR analysis
The FTIR spectra were evaluated corresponding to the first week (Figure 4(a)–(d)) and the fourth week of the biodegradation experiment. It is in the region ranging between 4,000 and 250 cm−1 of the spectrum that illustrates the most significant changes in different experimental setups. At the initial stage, all the inoculated groups share the same constituent functional groups as the control group (pristine PS-MPs). The prominent peaks between 3,800 and 3,200 cm−1 correspond to the O–H stretching attributed to the alcohol group. The peaks at 2,422.59 and 2,376.30 cm−1 correspond to the O═C═O stretching characterized by carbon dioxide. The bands at 1,635.64 and 840.96 cm−1 correspond to C═C stretching followed by a band at 1,388.75 cm−1 attributed to C–H bending. The bands between 1,111.00 and 925.83 cm−1 correspond to C–O stretching. The band at 2,090.84 was observed only for the pristine PS-MPs that is attributed to the N═C═S group of isothiocyanates. Between the pristine PS-MPs and the inoculated groups (with and without thermal and UV pretreatment), a marked difference in the absorbance intensity was observed in the 3,100–3,441 cm−1 and in the 1,388.75 cm−1 region, which explains the alterations induced by the pretreatments. Figure 5(a)–(c) shows the FTIR spectra after 4 weeks of inoculation in both pretreated and untreated samples of PS-MPs. Inoculation without pretreatment (Figure 5a) showed marked changes in the peak intensities as well as the appearance of new bands 3,032.10 and 2,924.09 cm−1 attributed to C–H stretching. Furthermore, additional peaks corresponding to C═O stretching at 1,728.22 cm−1 followed by the appearance of a peak at 956.69 cm−1 attributed to C–O stretching were observed for all three inoculated groups (Figure 4(a)–(c)) after 4 weeks. A closer comparison of the FTIR spectra of week 1 and week 4 for the inoculated sample that received thermal pretreatment (Figures 4(c) and 5(b)) illustrates that the most discernable changes that were observed after 4 weeks were the change in the intensity of peaks at 3,147.83 and 3,433.29 cm−1 corresponding to O–H stretching and change in the intensity of the peaks at 1,643.35, 1,388.75, and 1,118.71 cm−1. In addition, the FTIR spectra for week 1 and week 4 for inoculated UV-pretreated samples showed a conspicuous change in the peak intensity of bands at 3,402.43 and 3,147.83 cm−1 corresponding to O–H stretching. Furthermore, observable change in the peak intensity was noted for the prominent peaks at 1,627.92, 1,388.75, and 1,111.00 cm−1 corresponding to C═C stretching, C–H bending, and C–O stretching, respectively (Figures 4(d) and 5(c)). Furthermore, the extent of organic material degradation can be assessed based on the change in the FTIR absorption peaks, the evolution of oxygen-containing (e.g., hydroxyl, carbonyl, ester, etc.), or the disappearance of distinct functional groups (Figure 6). The oxygen evolution due to oxidative degradation can be monitored using one of the corresponding oxygen-containing functional groups like hydroxyl (OH), carbonyl (C═O), or ether (C–O–C), those typically observed at frequencies close to the ranges of 3,700–3,100 cm−1 (OH stretching), 1,850–1,650 cm−1 (C═O stretching), and 1,200–900 cm−1 (C–O stretching). The OH and C–O bands are vulnerable to alteration by, e.g., moisture and overlapping with various other structural bands (e.g., C–C, C–N stretching, and C–H bending), respectively, and thus are less useful for reaction monitoring. The carbonyl band, on the other hand, is one typical product of oxidative degradation that has high molar absorptivity (more detectable) and thus being more acceptable for quantitative calculations. The CI can be calculated from the ratio between the area (or sometimes the intensity) under the C═O stretching band (1,650–1,850 cm−1) and that of the methylene δ C–H scissoring peak (1,380–1,340 cm−1) as given in the equation below:
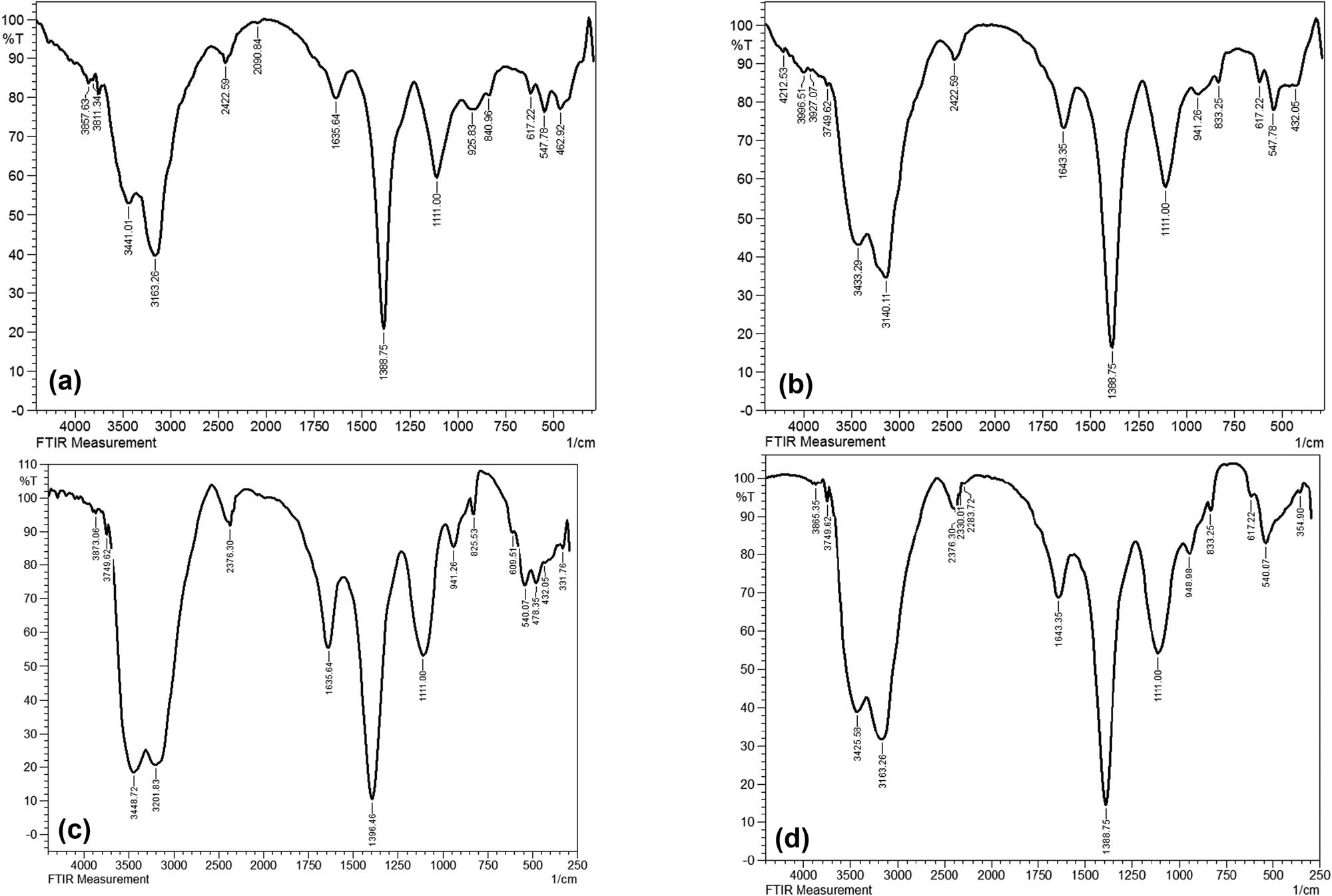
FTIR spectra of PS-MP samples in the first week (a) Control; pristine PS-MPs, (b) PS + BS (untreated inoculated with B. subtilis), (c) PT + BS (temperature treated PS and inoculated with B. subtilis), and (d) PUV + BS (UV-treated PS and inoculated with B. subtilis).
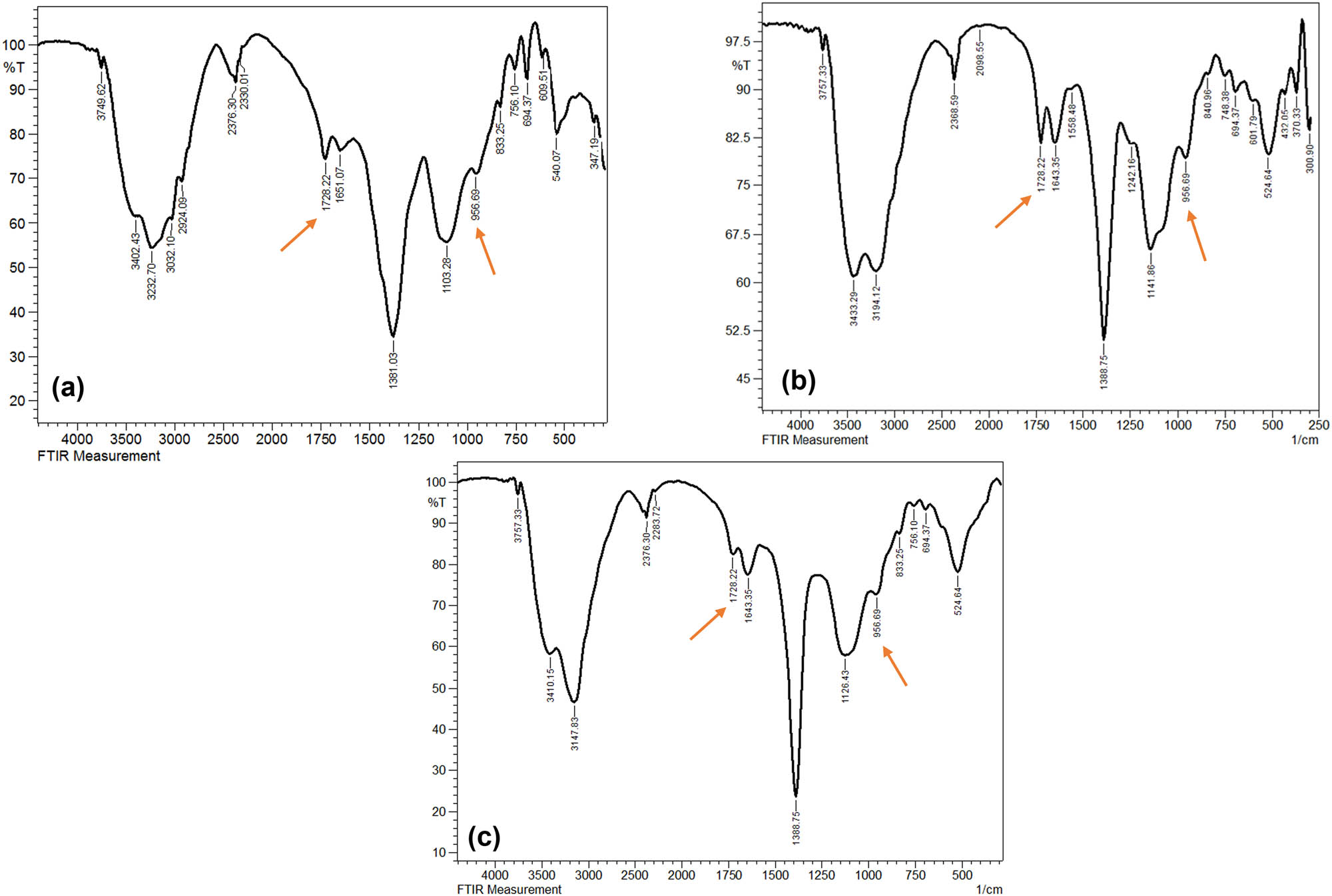
FTIR spectra of PS-MP samples in the fourth week (a) PS + BS (untreated inoculated with B. subtilis), (b) PT + BS (temperature-treated PS and inoculated with B. subtilis), and (c) PUV + BS (UV-treated PS and inoculated with B. subtilis).
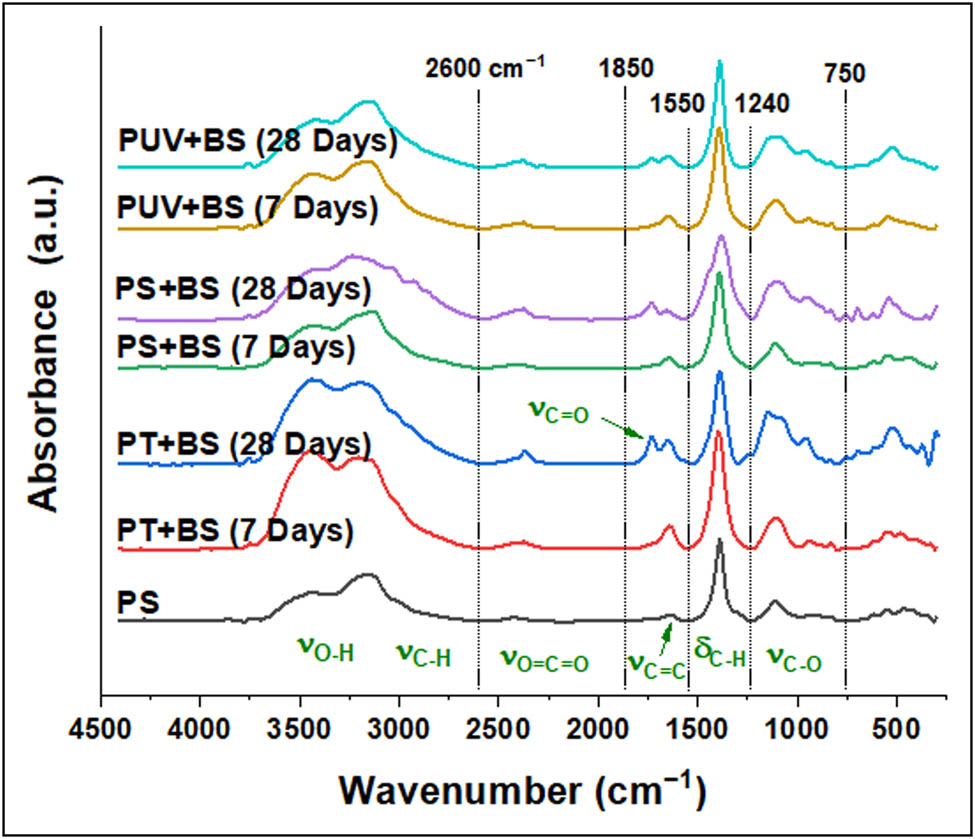
FTIR spectra of plastic waste: PS pristine; plastic treated with temperature for a week (PT + BS-w1) and for 4 weeks (PT + BS-w4); treated with microorganism for a week (PS + BS-w1) and 4 weeks (PS + BS-w4); treated with light irradiation and microorganism for a week (PUV + BS-w1) and for 4 weeks (PUV + BS-w4). Spectral peaks were roughly categorized into six regions for simplicity.
To calculate the peak area, the FTIR spectra were set in the absorption mode. The data were drawn using Origin software; the baseline correction, derivatization, peak integration, and deconvolution were performed using the integrated functions.
Due to the overlapping of C═O peaks with C═C, a deconvolution was performed; an example of deconvolution results is demonstrated in Figure 7 for all groups (control and inoculated). Furthermore, as oxidative degradation typically results in various types of C═O containing compounds (e.g., aldehyde, ketone, acid, amid, etc.), multi-peaks were detected in this region; however, the complete region was treated as one during calculation while the peak center was selected at the highest intensity. As can be seen in Table 2, the CI values for the thermally pretreated inoculated group (PT + BS) were the highest compared to other samples, PS + BS and PUV + BS. The CI value of PT + BS samples was doubled after a week of conditioning compared to the pristine PS-MPs (i.e., increased from 0.053 to 0.106). After 4 weeks (PT + BS -w4), the CI has reached the value of 0.245 which is the highest detected CI value in the study. On the other hand, CI values of PS + BS did change moderately, while for PUV + BS there was no noticeable change observed, suggesting negligible oxidation. However, the extent of degradation has become considerable after 4 weeks of conditioning. Overall, the degree of degradation was in the order of PT + BS > PS + BS > PUV + BS.
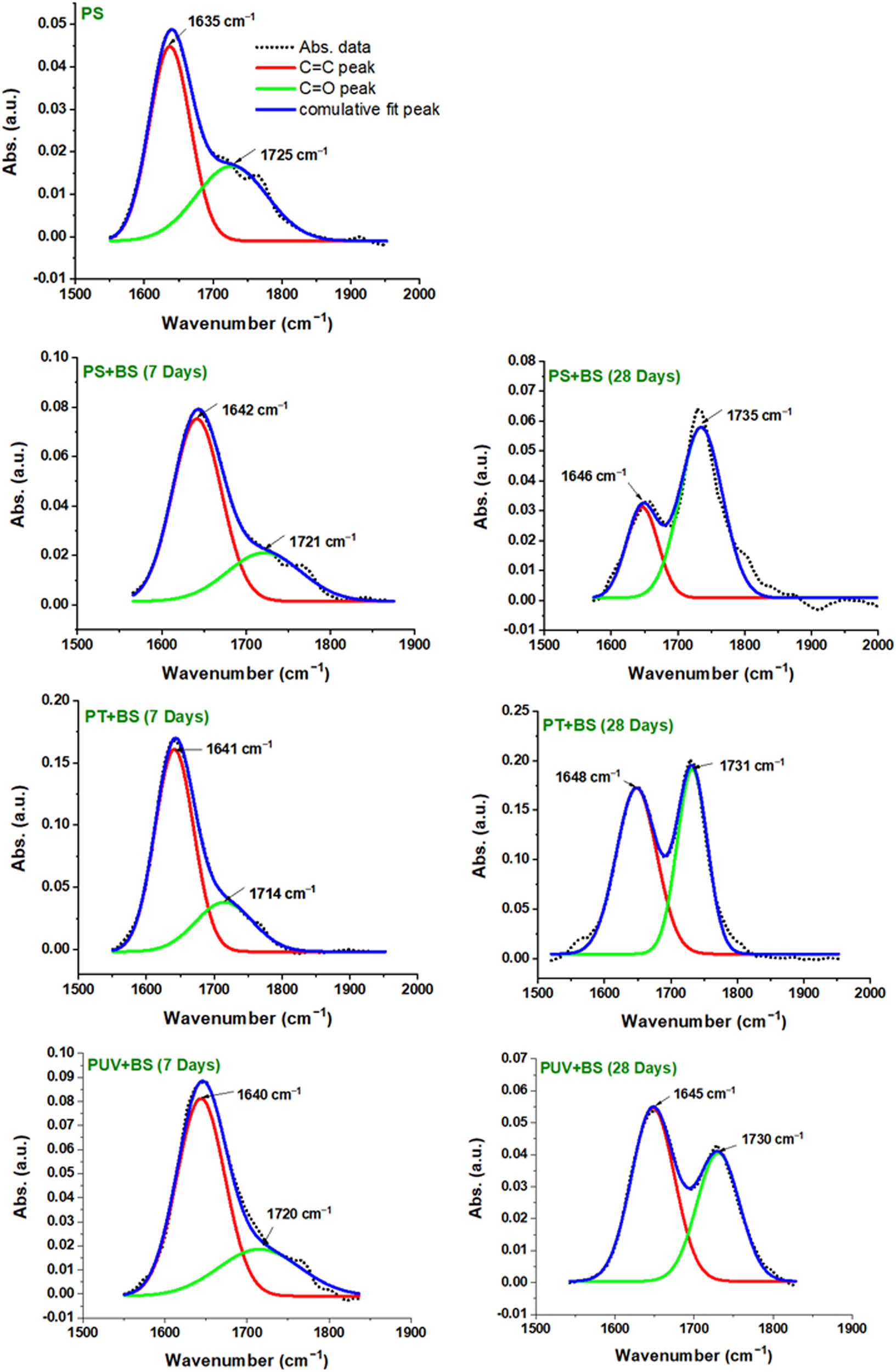
Illustration of C═C and C═O peaks after deconvolution of the absorption band between, e.g., 1,550 and 1,850 cm−1. PS-MPs, control pristine; PS + BS, plastic inoculated with B. subtilis; PT + BS, plastic pretreated with temperature and with B. subtilis and PUV + BS, plastic pretreated with light and with B. subtilis for 7 and 28 days.
Carbonyl index for pristine plastic (PS-MPs) and its corresponding inoculated samples with and without abiotic pretreatment for week 1 and week 4
| Peak center (cm−1) | Carbonyl index | ||||||
|---|---|---|---|---|---|---|---|
| PS | PT + BS | PS + BS | PUV + BS | ||||
| Week 1 | Week 4 | Week 1 | Week 4 | Week 1 | Week 4 | ||
| 1,725 | 0.053 | 0.106 | 0.245 | 0.049 | 0.144 | 0.042 | 0.093 |
3.4 Surface morphological and elemental analysis
Scanning electron microscopy (SEM) coupled with EDX was used to observe the surface morphological changes with the elemental analysis to further validate the degree of PS-MP biodeterioration. The pristine PS-MP control exhibited a smooth surface, as shown in Figure 8(a). However, thermal-pretreated and UV-pretreated PS-MP samples demonstrated various irregularities on their surface (Figure 8(b) and (d)). The SEM micrographs of the inoculated PS samples with prior UV and heat treatment showed the appearance of mechanical fatigue in week 4 characterized by the onset of the fracture/cracks occurring close to the surface (Figure 8(c) and (e). In addition, the micrographs after 4 weeks of bacterial inoculation without abiotic pretreatment also showed marked morphological changes, demonstrating the biodeterioration of the brittle PS-MP samples (Figure 8(f) and (g)).
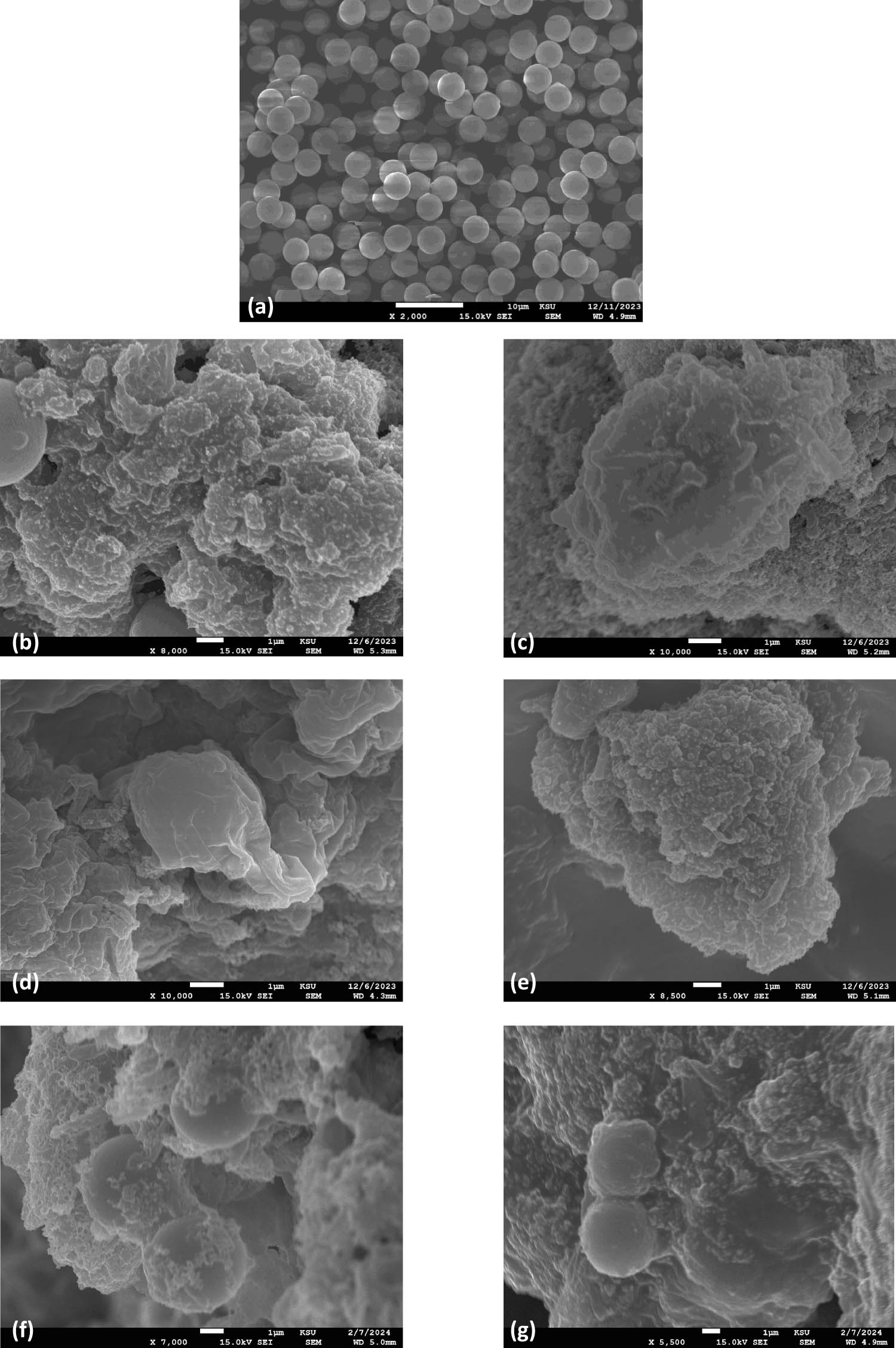
SEM micrographs of PS-MP samples (a) PS (control, pristine), (b) PS + BS (untreated inoculated with B. subtilis) in the first week and (c) fourth week, (d) PT + BS (temperature treated PS and inoculated with B. subtilis) in the first week and (e) fourth week, (f) PUV + BS (UV treated PS and inoculated with B. subtilis) in the first week and (g) fourth week.
In addition, the samples were examined for PS-MP biodegradation by SEM/EDX analysis.
Based on their element footprints and surface modifications/properties. The detected elements included C, O, Na, and Si, which are frequently observed in samples of environmental water that contain microplastics (MPs). The elements detected were C, O, Na, and Si, which have been typically identified in environmental water samples containing MPs. Carbon and oxygen (C, O) were dominant, visibly illustrating the organic matrix of the PS-MPs (Figures 9–14). Table 3 shows the EDX analysis with the elemental map of each PS-MP sample (control, inoculated groups; pretreated and untreated). Variations in the surface atomic O/C ratio of all samples are visible, indicating that the scission reaction involves an oxidation process. The mean atomic O/C ratio for the control sample was 0.40 ± 0.01667, followed by a significant (p ≤ 0.05) observable increase in the ratio for the inoculated samples; 0.67 ± 0.00577 for untreated PS-MPs, 0.48 ± 0.00882 for UV-pretreated and 0.65 ± 0.00577 for heat/temperature treated (Table 4).
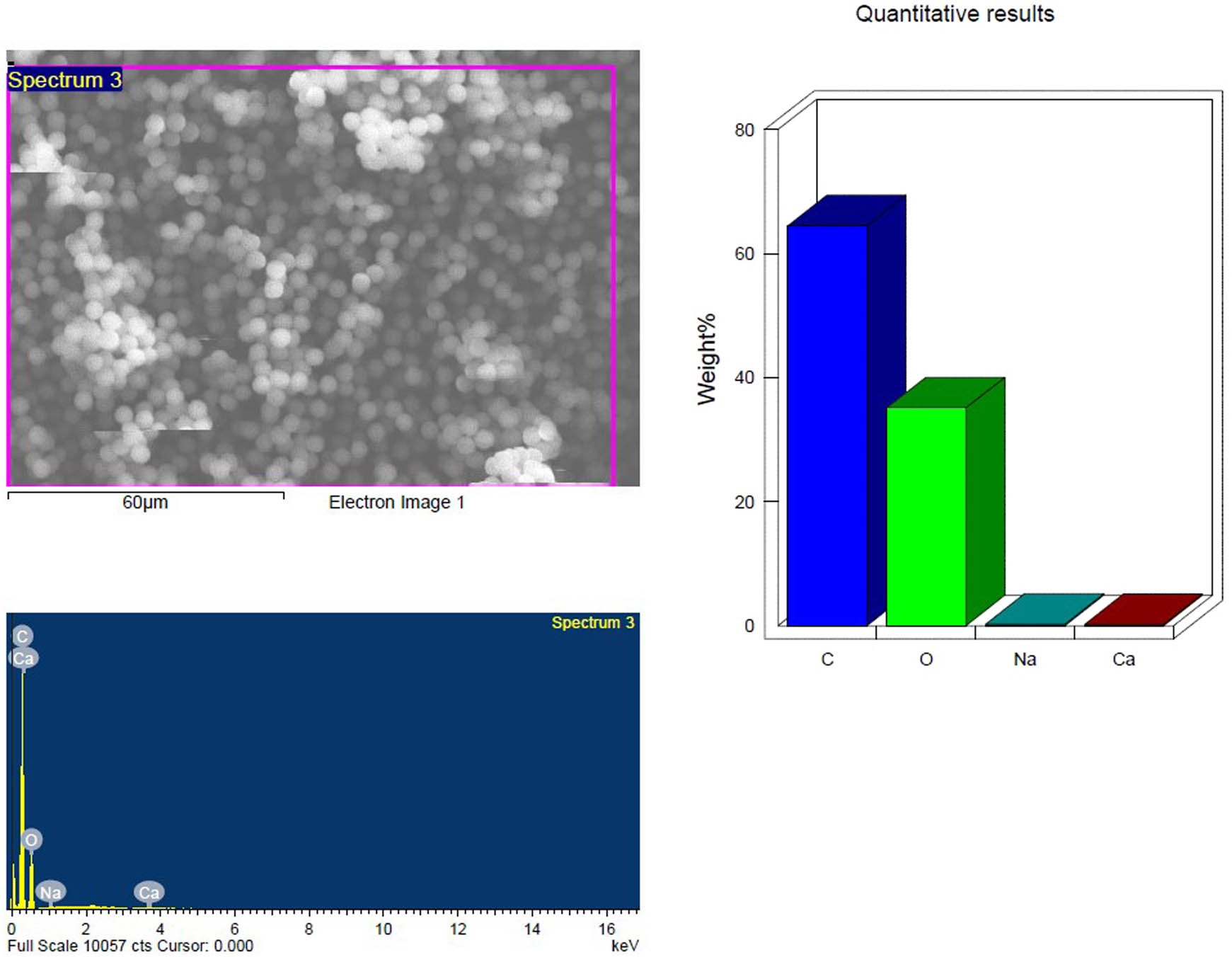
SEM and EDX analysis of the pristine PS-MP samples; control showing the elemental composition.
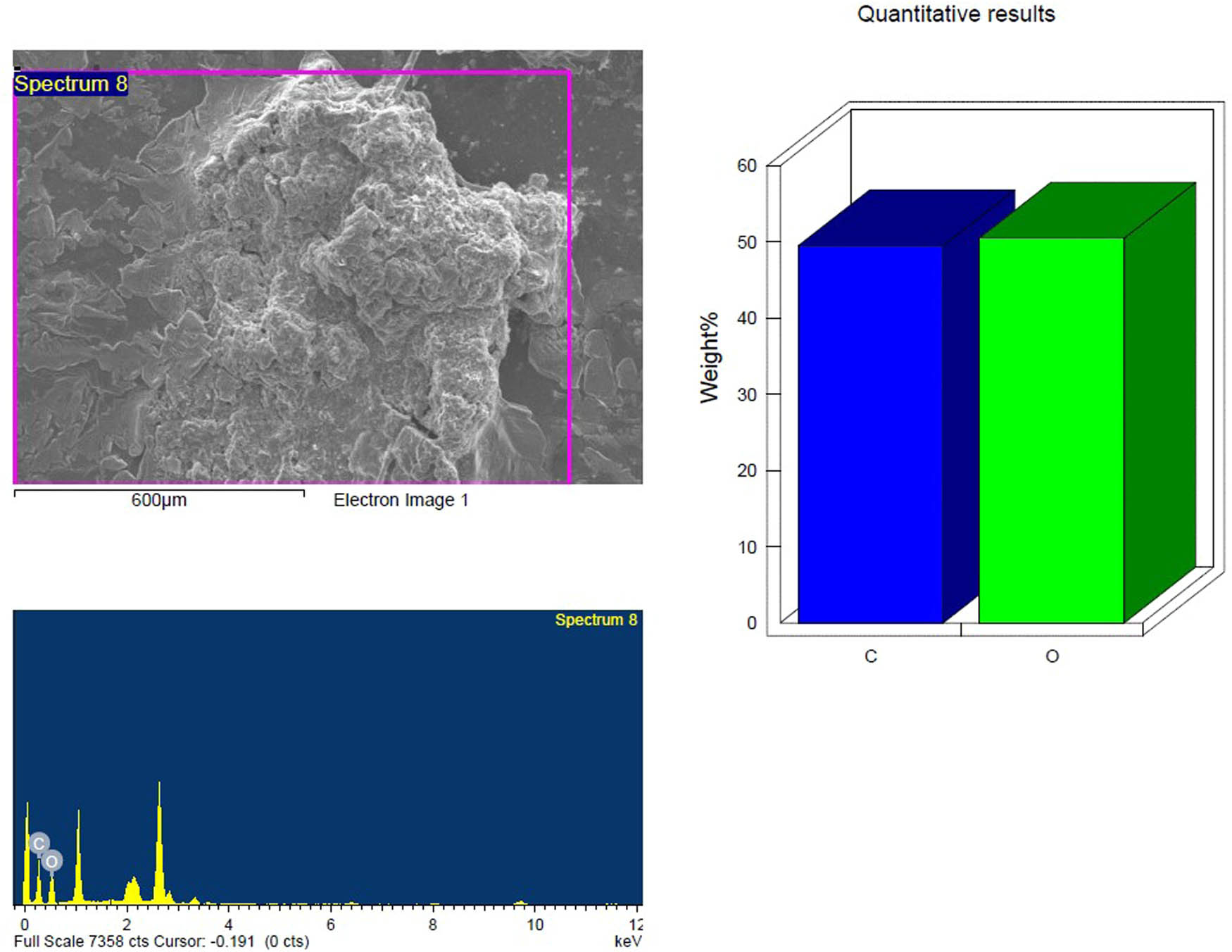
SEM and EDX analysis of the untreated inoculated PS-MP sample after 4 weeks showing the elemental composition.
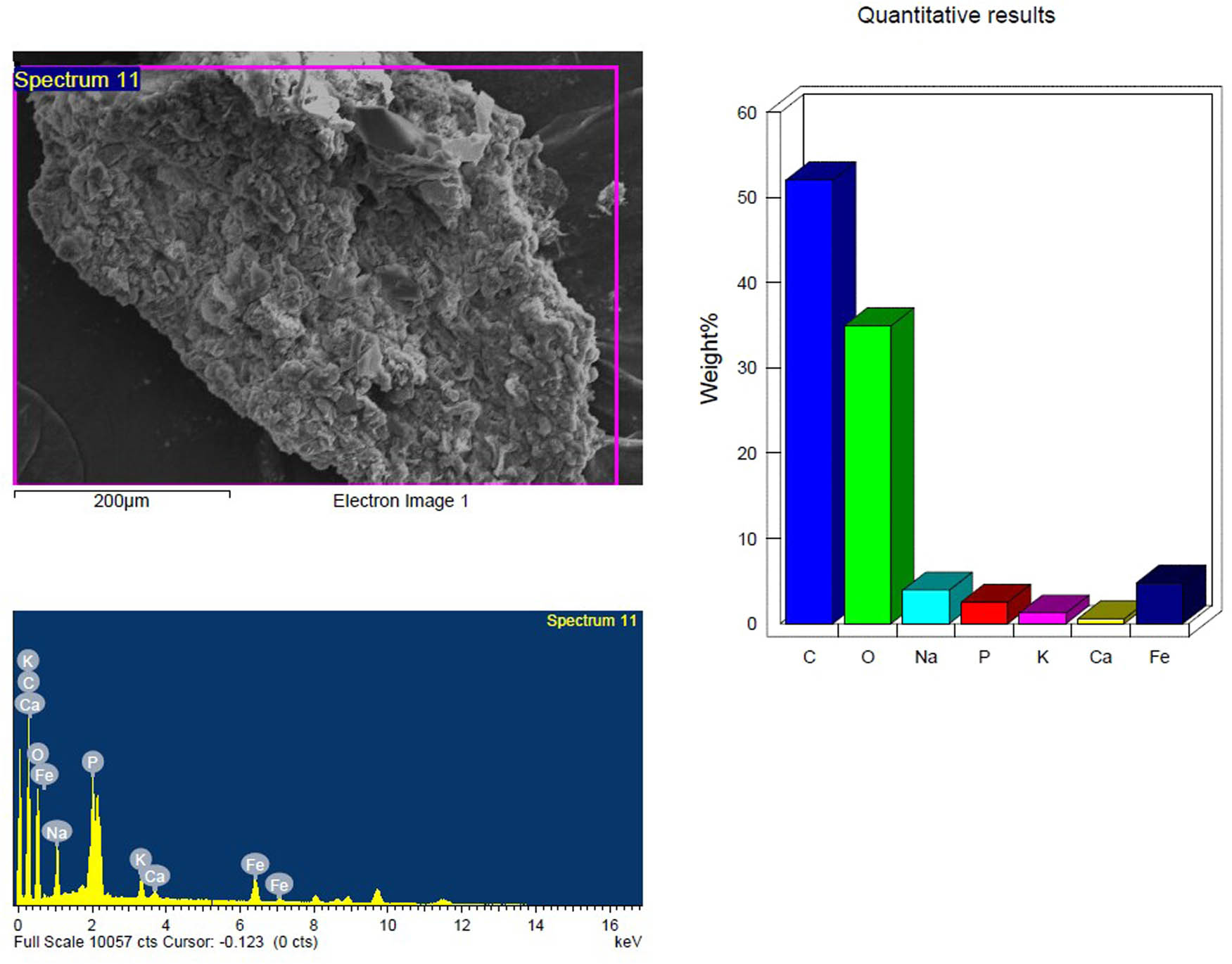
SEM and EDX analysis of the temperature-treated inoculated PS-MP sample in the first week showing the elemental composition.
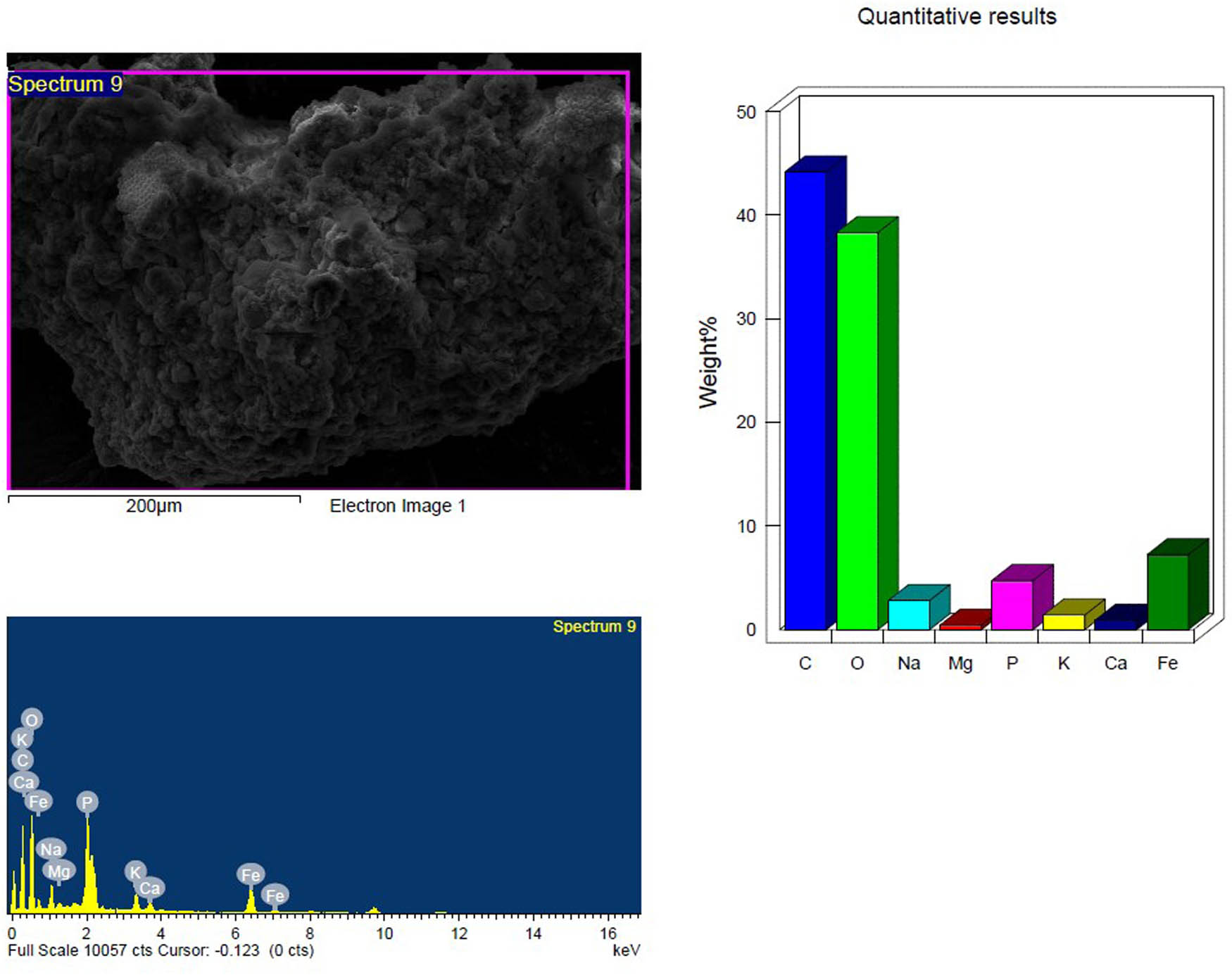
SEM and EDX analysis of the temperature-treated inoculated PS-MP sample after 4 weeks showing the elemental composition.
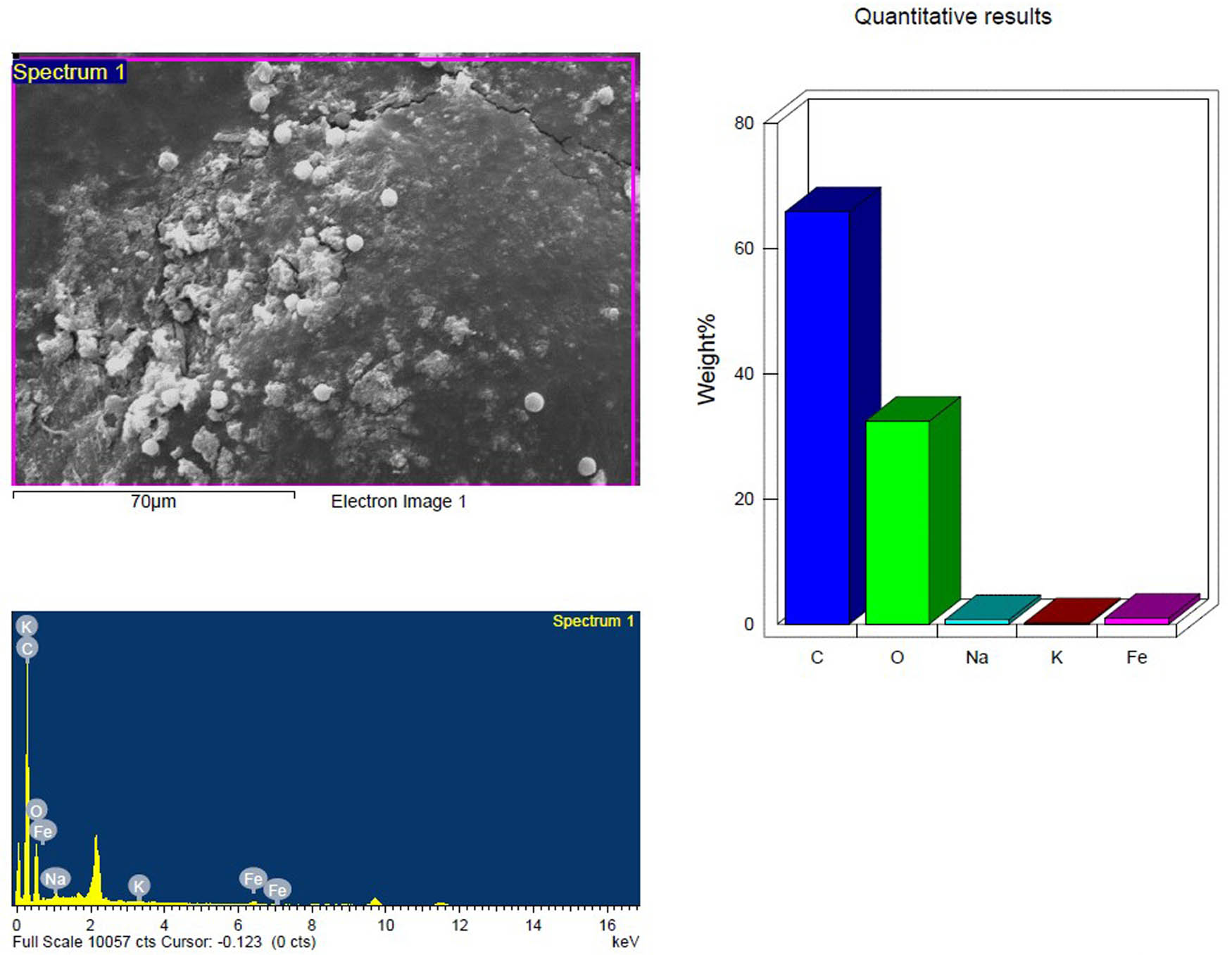
SEM and EDX analysis of the UV treated inoculated PS-MP sample in the first week showing the elemental composition.
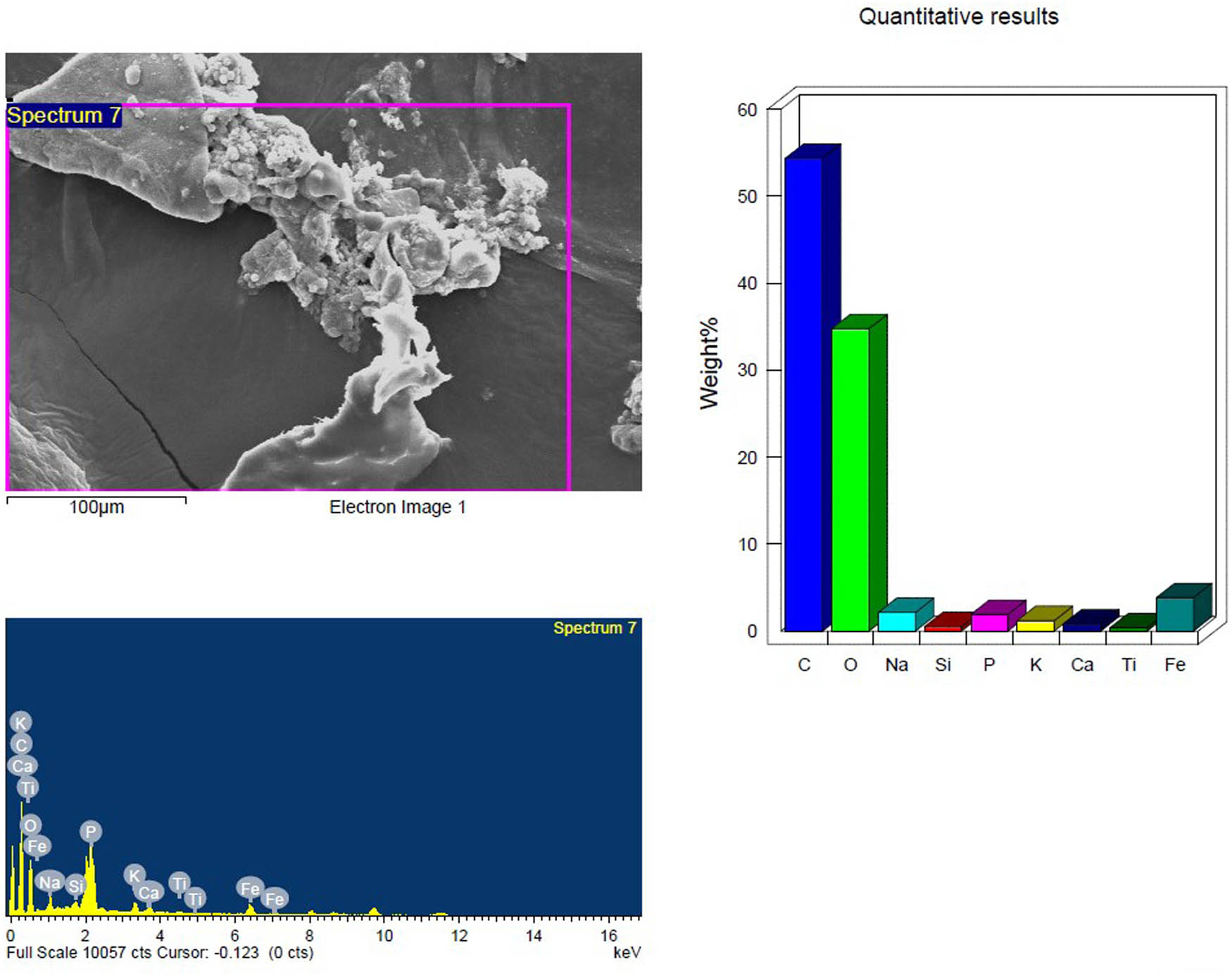
SEM and EDX analysis of the UV treated inoculated PS-MP sample after 4 weeks showing the elemental composition.
Spearman’s correlation (rho) between half-life (days) and CI within all experimental groups
| Half-life (days) | Carbonyl index | |||
|---|---|---|---|---|
| Spearman’s rho | Half-life (days) | Correlation coefficient | 1.000 | 0.500 |
| Sig. (2-tailed) | 0.667 | |||
| N | 3 | 3 | ||
| Carbonyl index | Correlation coefficient | 0.500 | 1.000 | |
| Sig. (2-tailed) | 0.667 | |||
| N | 3 | 3 |
There is no statistically significant correlation at the significance level of (0.05) between half-life and CI.
EDX analysis with the elemental composition of control and inoculated PS-MP samples with Bacillus subtilis showing the O/C ratio after 4 weeks
| Elements | Control (pristine PS-MPs) | Untreated inoculated PS-MPs | UV-pretreated inoculated PS-MPs | Temperature-pretreated inoculated PS-MPs | ||||
|---|---|---|---|---|---|---|---|---|
| Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% | |
| C K | 64.48 | 70.85 | 52.84 | 59.88 | 54.38 | 64.65 | 44.16 | 56.11 |
| O K | 35.11 | 28.96 | 47.16 | 40.12 | 34.78 | 31.04 | 38.31 | 36.54 |
| Other elements (Na, K, Ca) | ||||||||
| Mean atomic O/C ratio | 0.40 ± 0.01667a | 0.67 ± 0.00577b | 0.48 ± 0.00882c | 0.65 ± 0.00577b | ||||
Values of the O/C ratio represent mean ± SE. Different superscripts are statistically significant (p ≤ 0.05).
4 Discussion
Research on the biodegradation of PS has demonstrated that this polymer may be broken down by specific microbes, including fungi and bacteria. According to Ho et al. [22] and Wen et al. [30], these organisms release enzymes that break down PS into smaller particles that can be further metabolized. The genera Bacillus, Pseudomonas, in addition to Paenibacillus, and Rhodococcus, are the most frequently utilized bacteria for PS breakdown [31,32,33].
The bacterial growth in all inoculated samples was stable through the experimental period of 4 weeks. The UV–Vis spectroscopy results showed that the absorbance ratios of the inoculated samples exhibited variation in comparison to the control (pristine PS-MPs) from week 1 to week 4. It has been suggested that biodegradation would happen if the sample absorbance ratio values do not remain constant [34]. The variations in absorbance ratios highlight the interplay of bacterial growth and pretreatment methods in facilitating PS degradation. Higher absorbance indicates the presence of chromophoric degradation intermediates (e.g., carbonyl-rich compounds) and reflects increased microbial metabolic activity, breaking down PS into oxidized by-products. Consequently, this variation implies that the polymer’s molecular structure was altered and that degradation might have occurred. UV-Vis spectroscopy is intrinsically confined to analyzing compounds containing chromophores, which are chemical structures that absorb ultraviolet or visible light. Thus, this limitation implies that the data acquired may not represent global changes in the overall plastic structure but rather specialized/localized changes in specific functional groups or additives. However, PS contains a benzene ring that absorbs significantly in the ultraviolet (200–280 nm) area. Thus, UV-Vis data will mostly reflect changes to these aromatic groups or any additions containing chromophores. Considering this, complementary analyses such as FTIR can detect changes in the entire molecular structure, including non-chromophoric functional groups and the aliphatic backbone [35].
Characteristic PS-MP peaks have been reported at 3,024, 2,847, 1,601, 1,027, and 694 cm−1 in previous studies [33,36], which are close to the range of peaks corresponding to the results of the present study. Most of the characteristic peaks showed a decrease in their intensities after biodegradation by B. subtilis (with and without pretreatment). A similar decrement in intensities of characteristic FTIR peaks was reported after treatment by Pseudomonas alcaligenes (M2-23), as well. New peaks at 3,032.10 and 2,924.09 cm−1, representing C−H stretching corresponding to aldehydes, and at 1,728.22 cm−1 corresponding to C═O stretching attributed to the carbonyl group appeared in the spectra of inoculated PS_MP samples without pretreatment. The functional groups observed via FTIR spectra (–OH, C═O) are intermediates that can be metabolized by microorganisms and funneled into the TCA cycle. It has been postulated that during the process of oxidation, which is involved in biodegradation, functional groups such as hydroxyl or carbonyl groups could be formed via β-oxidation, which are known to be used in the citric acid (TCA) cycle or by the bacteria for energy metabolism, which subsequently enhances the hydrophilicity [33,37]. Thus, the integration of FTIR and metabolic pathways emphasizes the interplay between chemical analysis and biological processes in biodegradation research. In addition, the pretreated (thermal and UV) inoculated samples also showed the appearance of 1,728.22 cm−1 corresponding to C═O stretching attributed to the carbonyl group validating the biodegradative process. The presence of the characteristic carbonyl band and its intensity directs the occurrence of oxidation reactions with chain scission [38]. These results are in line with the study by Miloloža et al. [33], where a similar characteristic weak peak at 1,715 cm−1 was detected in the FTIR spectra of biodegraded PS by Bacillus cereus and P. alcaligenes, indicating the formation of carbonyl groups (−C═O). Furthermore, all inoculated groups showed the appearance of a band at 956 cm−1 after 4 weeks. It has been previously reported that the band at 956 cm−1 suggests its association with the C–O stretching mode of primary alcohols [39]. Consequently, the presence of more oxygen atoms on the plastic surface in regions where bacteria are growing is clear evidence of PS degradation [40]. Furthermore, based on previous literature, the carbonyl index (CI) has been applied for monitoring the physicochemical change and is one of the most acceptable among other methods used to probe the extent of oxidation [41]. Using the area under the band in FTIR spectroscopy is widely regarded as the most accurate and effective method for determining the CI [42]. The CI values calculated showed a notable change in the extent of degradation in all inoculated samples of PS-MPs after 4 weeks. Overall, the degree of biodegradation was in the order of PT + BS > PS + BS > PUV + BS. This increase in the CI value reflects the increase in carbonyl groups due to oxidative degradation, with the temperature pretreated sample being most pronounced. The CI values of degraded plastics have been reported in the range of 0–2 [41] but mostly less than 1 [43], which is in line with the obtained range in the present investigation (0–0.245).
These findings further corroborate the results of the SEM/EDX investigation, which showed a discernible increase in the atomic O/C ratio and was associated with the appearance of peaks in the FTIR spectra that were assigned to oxygen-containing functional groups (alcohol, aldehyde, and carbonyl). The SEM micrographs also demonstrated marked surface modifications of PS-MPs attributed to the abiotic pretreatment followed by the bacterial inoculation as well as the bacterial inoculation alone. Thus, the results clearly illustrated that bacterial inoculation of PS-MP samples with and without pretreatment modified the surface morphology after 4 weeks, as the presence of cracks/ridges indicates that the PS samples did become fragile [23].
It has been documented that PS biodegradation commences when microorganisms grow on its surface and release their enzymes, which break down the polymer into smaller molecular fragments such as oligomers and possibly monomeric units. Based on existing reports, proposed PS-biodegradation pathways and mechanisms involve the oxidation of styrene followed by its metabolic breakdown via the TCA cycle. Degradation might begin with either the cleavage of the main chain or the side chain, resulting in various degradation routes [44]. The breakage of the main chain can produce several aromatic monomers of PS, such as styrene and toluene, followed by two catabolic processes that use aromatic molecules as carbon sources. The first suggested process converts styrene into intermediate chemicals, which are then transformed into acetyl-CoA via the β-oxidation pathway. The citric acid (TCA) cycle leads to the core biosynthetic pathways. Another hypothesized mechanism begins with the cleavage of PS polymers’ side chains; monooxygenases or aromatic ring hydroxylases are viable candidates for breaking the aromatic ring of PS; however, the specific degradation pathway and enzymes involved have yet to be revealed [45]. Thus, certain microbes can utilize styrene as a carbon source for growth [22]. The results of the present study evidently support this explanation of PS biodegradation. The endpoints based on the biodegradability tests displayed an effective degradation of the PS-MPs in all inoculated groups with and without the abiotic pretreatment. The Bacillus strain alone was adequate to direct the biodeterioration process, which was observed in the larger magnitude of the modifications observed in the FTIR spectra, a moderately high CI, and the atomic O/C ratio of the untreated inoculated PS-MP sample. This is contrary to some previous studies that have reported that abiotic pretreatment (UV and thermal) enhances the biodegradative efficacy of microbial strains on polymers [23,38]. On the other hand, a study by Yao et al. [3] supported the notion that B. subtilis and B. licheniformis can effectively degrade PE without any abiotic pretreatment and could potentially be used as plastic-biodegrading microorganisms. Miloloža et al. [33] also investigated the biodegradation of MP-PS particles by the bacteria B. cereus and P. alcaligenes and reported that these bacterial strains offer a promising biodegradative potential for MPs in the environment.
When assessing the biodegradation of plastics, both half-life and carbonyl index are commonly used metrics, but they reflect different aspects of the degradation process and do not always correlate directly. This is in congruence with the quantitative data on half-life and CI in all inoculated groups in the current study. Spearman correlation coefficient, r s , did not show a statistically significant (p ≤ 0.05) correlation between half-life and CI in the study. Half-life represents the overall rate of disintegration in terms of plastic mass or integrity, while the CI reveals chemical changes in the polymer; it mostly signifies oxidation and disintegration of the plastic’s molecular structure. UV-pretreated inoculated PSMPs exhibited the highest reduction rate of 0.008037 day−1 and half-life of 86.2 days among all the inoculated groups, though the CI index was the least for this group. It has been reported that UV exposure causes the generation of free radicals in the polymer matrix, resulting in rapid chain scission without the formation of numerous carbonyl groups. This causes faster deterioration (shorter half-life) but lower CI. In addition, UV radiation might produce alternative degradation products (e.g., hydrocarbons, volatile organic compounds) instead of carbonyl-containing intermediates, lowering the measured CI [46,47]. On the other hand, the untreated inoculated group (PS + BS) showed a low reduction rate of 0.00096 day−1 and half-life of 722.9 days even though the CI was moderately high along with the perceptible shift observed in the FTIR spectra. This explains that PS undergoes slow oxidative degradation, producing carbonyl-rich intermediates that accumulate because of the resistance of these materials to microbial degradation, extending the half-life [48].
Although several microbes with PS biodegradation potential have been identified, the mechanism and enzymes responsible for degradation have not been thoroughly investigated. However, researchers have proposed potential PS-degrading enzymes that include lipases, esterase, and oxidative enzymes [44]. Alkane hydroxylases can break PS main-chain C–C bonds under acidic or alkaline environments, as the stability of C–C bonds reduces [24]. In addition, depolymerizing enzymes that cleave the polymer backbone are required for reducing PS to monomers/oligomers such as styrene. Further degradation of styrene occurs via aromatic ring cleavage enzyme pathways, resulting in simpler molecules such as acetyl-CoA and succinyl-CoA that eventually enter the TCA cycle, which has been described above [44].
However, some study limitations should be acknowledged. The study could be planned over a longer period, elucidating the ability of B. subtilis to maintain its biodegradation efficiency over prolonged periods. There could be more analytical resolution and quantitative data. In addition, the environmental impact of by-products was not explored. These limitations could direct future research on investigating the ability of B. subtilis to degrade other types of plastics across different particle sizes (nano to larger fragments) and varied morphologies. In addition, using the bacterial strain in a natural consortium, optimization of abiotic treatments, and studies on understanding the enzymatic strategies involved in microbial degradation are imperative.
5 Conclusion
Taken together, the study highlights that B. subtilis, a commonly found bacteria in the soil, was effective in biodegrading the PS-MPs, which was evident from an integrated approach of using complementary techniques: UV–Vis, FTIR, SEM/EDX to provide a holistic view of both local and global degradation. On the basis of all the metrics assessed, pretreatment with temperature and untreated inoculated PS-MPs showed a perceptible oxidative chain scission. Thus, the bacterial strain was efficacious in biodegrading the PS-MPs with and without the abiotic pretreatment, which offers an alternative to abate energy-intensive processes, ensuring the credentials of green chemistry. Hence, the findings propose sustainable resolutions in waste management strategies, which are pivotal in the recent global scenario of plastic pollution.
Acknowledgements
The authors acknowledge the Research Institute/Centre supporting program (RICSP-25-1), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: The authors acknowledge the Research Institute/Centre supporting program (RICSP-25-1), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Norah Raqi Al-Otaibi: methodology, formal analysis, writing – original draft; Promy Virk: conceptualization, project administration, formal analysis, writing –original draft, writing – review and editing; Rasha Elsayim: methodology, formal analysis, writing – original draft; Mohammed Elbagir Amin: resources, formal analysis; Asma Mudhahi Alshammari: resources, formal analysis; Alanoud Tariq Al Sudairi: methodology, resources; Nada Ali Almohawis: methodology, resources; Dalia Fouad Ibrahim: resources; Manal A. Awad: Analysis, writing and reviewing; Abdel-Basit Al-Odayni: Analysis, writing and reviewing; Gadah Albasher: project administration, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] da Costa JP. Micro- and nanoplastics in the environment: Research and policymaking. Curr Opin Environ Sci Health. 2018;1:12–6. 10.1016/j.coesh.2017.11.002.Search in Google Scholar
[2] Chalmin P. The history of plastics: From the Capitol to the Tarpeian Rock. Field actions science reports. J Field Actions. 2019(Special Issue 19):6–11. https://journals.openedition.org/factsreports/5071.Search in Google Scholar
[3] Yao Z, Seong HJ, Jang YS. Degradation of low-density polyethylene by Bacillus species. Appl Biol Chem. 2022;65:84. 10.1186/s13765-022-00753-3.Search in Google Scholar
[4] Enyoh CE, Shafea L, Verla AW, Verla EN, Qingyue W, Chowdhury T, et al. Microplastics exposure routes and toxicity studies to ecosystems: An overview. Environ Anal Health Toxicol. 2020;35(1):e2020004. 10.5620/eaht.e2020004.Search in Google Scholar PubMed PubMed Central
[5] Zarus GM, Muianga C, Hunter CM, Pappas RS. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci Total Environ. 2021;756:144010. 10.1016/j.scitotenv.2020.144010.Search in Google Scholar PubMed PubMed Central
[6] Prata JC. Airborne microplastics: Consequences to human health? Environ Pollut. 2018;234:115–26. 10.1016/j.envpol.2017.11.043.Search in Google Scholar PubMed
[7] Pironti C, Ricciardi M, Motta O, Miele Y, Proto A, Montano L. Microplastics in the environment: Intake through the food web, human exposure and toxicological effects. Toxics. 2021;9(9):224. 10.3390/toxics9090224.Search in Google Scholar PubMed PubMed Central
[8] Chang X, Xue Y, Li J, Zou L, Tang M. Potential health impact of environmental micro- and nanoplastics pollution. J Appl Toxicol. 2020;40(1):4–15. 10.1002/jat.3915.Search in Google Scholar PubMed
[9] Khuyen VTK, Le DV, Fischer AR, Dornack C. Comparison of microplastic pollution in beach sediment and seawater at UNESCO can gio mangrove biosphere reserve. Glob Chall. 2021;5(11):2100044. 10.1002/gch2.202100044.Search in Google Scholar PubMed PubMed Central
[10] Hwang J, Choi D, Han S, Choi J, Hong J. Potential toxicity of polystyrene microplastic particles. Sci Rep. 2020;10(1):7391. 10.1038/s41598-020-64464-9.Search in Google Scholar PubMed PubMed Central
[11] Rani M, Shanker U. Plastic degradation and its environmental implications. Degrad Plast. 2021;99:290–324. 10.21741/9781644901335-12.Search in Google Scholar
[12] Elahi A, Bukhari DA, Shamim S, Rehman A. Plastics degradation by microbes: A sustainable approach. J King Saud Univ – Sci. 2021;33(6):101538. 10.1016/j.jksus.2021.101538.Search in Google Scholar
[13] Moharir RV, Kumar S. Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review. J Clean Prod. 2019;208:65–76. 10.1016/j.jclepro.2018.10.059.Search in Google Scholar
[14] Jachimowicz P, Nosek D, Cydzik-Kwiatkowska A. Chemical and microbiological changes on the surface of microplastic after long term exposition to different concentrations of ammonium in the environment. Sci Total Environ. 2022;830:154784. 10.1016/j.scitotenv.2022.154784.Search in Google Scholar PubMed
[15] Qi X, Ren Y, Wang X. New advances in the biodegradation of Poly(lactic) acid. Int Biodeterior Biodegrad. 2017;117:215–23. 10.1016/j.ibiod.2017.01.010.Search in Google Scholar
[16] Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci Total Environ. 2020;715:136968. 10.1016/j.scitotenv.2020.136968.Search in Google Scholar PubMed
[17] Amobonye A, Bhagwat P, Singh S, Pillai S. Plastic biodegradation: Frontline microbes and their enzymes. Sci Total Environ. 2021;759:143536. 10.1016/j.scitotenv.2020.143536.Search in Google Scholar PubMed
[18] Vimala PP, Mathew L. Biodegradation of polyethylene using Bacillus subtilis. Procedia Technol. 2016;24:232–9. 10.1016/j.protcy.2016.05.031.Search in Google Scholar
[19] Li X, Guo Q, Wang Y, Xu J, Wei Q, Chen L, et al. Enhancing nitrogen and phosphorus removal by applying effective microorganisms to constructed wetlands. Water. 2020;12(9):2443. 10.3390/w12092443.Search in Google Scholar
[20] Olmos J, Acosta M, Mendoza G, Pitones V. Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch Microbiol. 2020;202:427–35. 10.1007/s00203-019-01757-2.Search in Google Scholar PubMed
[21] Cai Z, Li M, Zhu Z, Wang X, Huang Y, Li T, et al. Biological degradation of plastics and microplastics: A recent perspective on associated mechanisms and influencing factors. Microorganisms. 2023;11(7):1661. 10.3390/microorganisms11071661.Search in Google Scholar PubMed PubMed Central
[22] Ho BT, Roberts TK, Lucas S. An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach. Crit Rev Biotechnol. 2018;38(2):308–20. 10.1080/07388551.2017.1355293.Search in Google Scholar PubMed
[23] Samat AF, Carter D, Abbas A. Biodeterioration of pre-treated polypropylene by Aspergillus terreus and Engyodontium album. NPJ Mater Degrad. 2023;7:28. 10.1038/s41529-023-00342-9.Search in Google Scholar
[24] Tarafdar A, Sinha A, Masto RE. Biodegradation of anthracene by a newly isolated bacterial strain, Bacillus thuringiensis AT.ISM.1, isolated from a fly ash deposition site. Lett Appl Microbiol. 2017;65(4):327–34. 10.1111/lam.12785.Search in Google Scholar PubMed
[25] Jeyakumar D, Chirsteen J, Doble M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour Technol. 2013;148:78–85. 10.1016/j.biortech.2013.08.074.Search in Google Scholar PubMed
[26] Arkatkar A, Juwarkar AA, Bhaduri S, Uppara PV, Doble M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int Biodeterior Biodegrad. 2010;64(6):530–6. 10.1016/j.ibiod.2010.06.002.Search in Google Scholar
[27] Prabhakar S, Kandeepan DC, Charulatha R. Isolation, Optimization and Characterization of Cellulose Enzyme Production by Bacillus subtilis. Int J Res Anal Rev. 2019;6(2):103–12, doi: Isolation__Optimization_and_Characterization_of20190625-95196-1ogb8y9-libre.pdf (d1wqtxts1xzle7.cloudfront.net).Search in Google Scholar
[28] Mohamed AE, Elgammal WE, Dawaba AM, Ibrahim AG, Fouda A, Hassan SM. A novel 1,3,4-thiadiazole modified chitosan: synthesis, characterization, antimicrobial activity, and release study from film dressings. Appl Biol Chem. 2022;65(1):54. 10.1186/s13765-022-00725-7.Search in Google Scholar
[29] Su WF. Characterization of polymer. Principles of polymer design and synthesis. 2013;82:89–110. 10.1007/978-3-642-38730-2_5.Search in Google Scholar
[30] Wen YCH, Tang DYYT, Khoo KS, Lup ANK, Chew KW. Nature’s fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Environ Sci Ecotechnol. 2020;4:100065. 10.1016/j.ese.2020.100065.Search in Google Scholar PubMed PubMed Central
[31] Mohan AJ, Sekhar VC, Bhaskar T, Nampoothiri KM. Microbial assisted high impact polystyrene (HIPS) degradation. Bioresour Technol. 2016;213:204–7. 10.1016/j.biortech.2016.03.021.Search in Google Scholar PubMed
[32] Kučić Grgić D, Miloloža M, Lovrinčić E, Kovačević A, Cvetnić M, Ocelić Bulatović V, et al. Bioremediation of MP-polluted waters using bacteria Bacillus licheniformis, Lysinibacillus massiliensis, and mixed culture of Bacillus sp. and Delftia acidovorans. Chem Biochem Eng Q. 2021;35(2):205–24. 10.15255/CABEQ.2021.1915.Search in Google Scholar
[33] Miloloža M, Ukić Š, Cvetnić M, Bolanča T, Grgić DK. Optimization of polystyrene biodegradation by Bacillus cereus and Pseudomonas alcaligenes using full factorial design. Polymers. 2022;14(20):4299. 10.3390/polym14204299.Search in Google Scholar PubMed PubMed Central
[34] Graziely PS, Erica A, Carlos C. Evaluation of biodegradation process of textile azo dye in solution by Aspergillus oryzae by UV-VIS and FTIR analysis. In: Industrial, medical and environmental applications of microorganisms. Wageningen Academic Publishers; 2014. p. 109–14. 10.3920/9789086867950_018.Search in Google Scholar
[35] Tillman ES, Roof AC, Palmer SM, Zarko BA, Goodman CC, Roland AM. Synthesis of chromophore-labeled polymers and their molecular weight determination using UV–Vis spectroscopy. J Chem Educ. 2006;83(8):1215. 10.1021/ed083p1215.Search in Google Scholar
[36] Jung MR, Horgen FD, Orski SV, Rodriguez V, Beers KL, Balazs GH, et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull. 2018;127:704–16. 10.1016/j.marpolbul.2017.12.061.Search in Google Scholar PubMed
[37] Mooney A, Ward PG, O’Connor KE. Microbial degradation of styrene: biochemistry, molecular genetics, and perspectives for biotechnological applications. Appl Microbiol Biotechnol. 2006;72:1–10. 10.1007/s00253-006-0443-1.Search in Google Scholar PubMed
[38] de Castro Monsores KG, da Silva AO, Oliveira SA, Weber RP, Filho PF, Monteiro SN. Influence of ultraviolet radiation on polystyrene. J Mater Res Technol. 2021;13:359–65. 10.1016/j.jmrt.2021.04.035.Search in Google Scholar
[39] Liu Y. Recent progress in fourier transform infrared (FTIR) spectroscopy study of compositional, structural and physical attributes of developmental cotton fibers. Materials. 2013;6(1):299–313. 10.3390/ma6010299.Search in Google Scholar PubMed PubMed Central
[40] Kim H, Jo JH, Kim Y, Le T, Cho C, Yun C, et al. Biodegradation of polystyrene by bacteria from the soil in common environments. J Hazard Mater. 2021;416:126239. 10.1016/j.jhazmat.2021.126239.Search in Google Scholar PubMed
[41] Almond J, Sugumaar P, Wenzel MN, Hill G, Wallis C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers. 2020;20:369–81.10.1515/epoly-2020-0041Search in Google Scholar
[42] Zeghal E, Vaksmaa A, Vielfaure H, Boekhout T, Niemann H. The potential role of marine fungi in plastic degradation—a review. Front Mar Sci. 2021;8:738877.10.3389/fmars.2021.738877Search in Google Scholar
[43] Ángeles-López Y, Gutiérrez-Mayen A, Velasco-Pérez M, Beltrán-Villavicencio M, Vázquez-Morillas A, Cano-Blanco M. Abiotic degradation of plastic films. In Proceedings of the Journal of Physics: Conference Series; 2017. p. 012027.10.1088/1742-6596/792/1/012027Search in Google Scholar
[44] Hou L, Majumder EL. Potential for and distribution of enzymatic biodegradation of polystyrene by environmental microorganisms. Materials (Basel). 2021;14(3):503. 10.3390/ma14030503.Search in Google Scholar PubMed PubMed Central
[45] Zhang Y, Pedersen JN, Eser BE, Guo Z. Biodegradation of polyethylene and polystyrene: From microbial deterioration to enzyme discovery. Biotechnol Adv. 2022;60:107991. 10.1016/j.biotechadv.2022.107991.Search in Google Scholar PubMed
[46] Urbanek AK, Rymowicz W, Mirończuk AM. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl Microbiol Biotechnol. 2018;102(18):7669–78. 10.1007/s00253-018-9195-y.Search in Google Scholar PubMed PubMed Central
[47] Ojeda TFM, Dalmolin E, Forte MMC, Jacques RJS, Bento FM, Camargo FAO. Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Polym Degrad Stab. 2009;94(6):965–70. 10.1016/j.polymdegradstab.2009.03.011.Search in Google Scholar
[48] Vollmer I, Jenks MJ, Roelands MC, White RJ, Van Harmelen T, De Wild P, et al. Beyond mechanical recycling: Giving new life to plastic waste. Angew Chem Int Ed. 2020;59:15402. 10.1002/anie.201915651.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”
Articles in the same Issue
- Research Articles
- Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential
- Green adsorbents for water remediation: Removal of Cr(vi) and Ni(ii) using Prosopis glandulosa sawdust and biochar
- Green approach for the synthesis of zinc oxide nanoparticles from methanolic stem extract of Andrographis paniculata and evaluation of antidiabetic activity: In silico GSK-3β analysis
- Development of a green and rapid ethanol-based HPLC assay for aspirin tablets and feasibility evaluation of domestically produced bioethanol in Thailand as a sustainable mobile phase
- A facile biodegradation of polystyrene microplastic by Bacillus subtilis
- Enhanced synthesis of fly ash-derived hydrated sodium silicate adsorbents via low-temperature alkaline hydrothermal treatment for advanced environmental applications
- Impact of metal nanoparticles biosynthesized using camel milk on bacterial growth and copper removal from wastewater
- Preparation of Co/Cr-MOFs for efficient removal of fleroxacin and Rhodamine B
- Applying nanocarbon prepared from coal as an anode in lithium-ion batteries
- Improved electrochemical synthesis of Cu–Fe/brass foil alloy followed by combustion for high-efficiency photoelectrodes and hydrogen production in alkaline solutions
- Precipitation of terephthalic acid from post-consumer polyethylene terephthalate waste fractions
- Biosynthesized zinc oxide nanoparticles: Multifunctional potential applications in anticancer, antibacterial, and B. subtilis DNA gyrase docking
- Anticancer and antimicrobial effects of green-synthesized silver nanoparticles using Teucrium polium leaves extract
- Green synthesis of eco-friendly bioplastics from Chlorella and Lithothamnion algae for safe and sustainable solutions for food packaging
- Optimizing coal water slurry concentration via synergistic coal blending and particle size distribution
- Green synthesis of Ag@Cu and silver nanowire using Pterospermum heterophyllum extracts for surface-enhanced Raman scattering
- Green synthesis of copper oxide nanoparticles from Algerian propolis: Exploring biochemical, structural, antimicrobial, and anti-diabetic properties
- Simultaneous quantification of mefenamic acid and paracetamol in fixed-dose combination tablet dosage forms using the green HPTLC method
- Green synthesis of titanium dioxide nanoparticles using green tea (Camellia sinensis) extract: Characteristics and applications
- Pharmaceutical properties for green fabricated ZnO and Ag nanoparticle-mediated Borago officinalis: In silico predications study
- Synthesis and optimization of gemcitabine-loaded nanoparticles by using Box–Behnken design for treating prostate cancer: In vitro characterization and in vivo pharmacokinetic study
- A comparative analysis of single-step and multi-step methods for producing magnetic activated carbon from palm kernel shells: Adsorption of methyl orange dye
- Sustainable green synthesis of silver nanoparticles using walnut septum waste: Characterization and antibacterial properties
- Efficient electrocatalytic reduction of CO2 to CO over Ni/Y diatomic catalysts
- Greener and magnetic Fe3O4 nanoparticles as a recyclable catalyst for Knoevenagel condensation and degradation of industrial Congo red dye
- Recycling of HDPE-giant reed composites: Processability and performance
- Fabrication of antibacterial chitosan/PVA nanofibers co-loaded with curcumin and cefadroxil for wound healing
- Cost-effective one-pot fabrication of iron(iii) oxychloride–iron(iii) oxide nanomaterials for supercapacitor charge storage
- Novel trimetallic (TiO2–MgO–Au) nanoparticles: Biosynthesis, characterization, antimicrobial, and anticancer activities
- Green-synthesized chromium oxide nanoparticles using pomegranate husk extract: Multifunctional bioactivity in antioxidant potential, lipase and amylase inhibition, and cytotoxicity
- Therapeutic potential of sustainable zinc oxide nanoparticles biosynthesized using Tradescantia spathacea aqueous leaf extract
- Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: Evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells
- Antioxidant potential of peptide fractions from tuna dark muscle protein isolate: A green enzymatic approach
- Clerodendron phlomoides leaf extract-mediated synthesis of selenium nanoparticles for multi-applications
- Optimization of cellulose yield from oil palm trunks with deep eutectic solvents using response surface methodology
- Nitrogen-doped carbon dots from Brahmi (Bacopa monnieri): Metal-free probe for efficient detection of metal pollutants and methylene blue dye degradation
- High energy density pseudocapacitor based on a nanoporous tungsten(VI) oxide iodide/poly(2-amino-1-mercaptobenzene) composite
- Green synthesized Ag–Cu nanocomposites as an improved strategy to fight multidrug-resistant bacteria by inhibition of biofilm formation: In vitro and in silico assessment study
- In vitro evaluation of antibacterial activity and associated cytotoxicity of biogenic silver nanoparticles using various extracts of Tabernaemontana ventricosa
- Fabrication of novel composite materials by impregnating ZnO particles into bacterial cellulose nanofibers for antimicrobial applications
- Solidification floating organic drop for dispersive liquid–liquid microextraction estimation of copper in different water samples
- Kinetics and synthesis of formation of phosphate composites from low-grade phosphorites in the presence of phosphate–siliceous shales and oil sludge
- Removal of minocycline and terramycin by graphene oxide and Cr/Mn base metal–organic framework composites
- Microfluidic preparation of ceramide E liposomes and properties
- Therapeutic potential of Anamirta cocculus (L.) Wight & Arn. leaf aqueous extract-mediated biogenic gold nanoparticles
- Antioxidant-rich Micromeria imbricata leaf extract as a medium for the eco-friendly preparation of silver-doped zinc oxide nanoparticles with antibacterial properties
- Influence of different colors with light regime on Chlorella sp., biomass, pigments, and lipids quantity and quality
- Experimental vibrational analysis of natural fiber composite reinforced with waste materials for energy absorbing applications
- Green synthesis of sea buckthorn-mediated ZnO nanoparticles: Biological applications and acute nanotoxicity studies
- Production of liquid smoke by consecutive electroporation and microwave-assisted pyrolysis of empty fruit bunches
- Synthesis of MPAA based on polyacrylamide and gossypol resin and applications in the encapsulation of ammophos
- Application of iron-based catalysts in the microwave treatment of environmental pollutants
- Enhanced adsorption of Cu(ii) from wastewater using potassium humate-modified coconut husk biochar
- Adsorption of heavy metal ions from water by Fe3O4 nano-particles
- Green synthesis of parsley-derived silver nanoparticles and their enhanced antimicrobial and antioxidant effects against foodborne resistant bacteria
- Unwrapping the phytofabrication of bimetallic silver–selenium nanoparticles: Antibacterial, Anti-virulence (Targeting magA and toxA genes), anti-diabetic, antioxidant, anti-ovarian, and anti-prostate cancer activities
- Review Article
- Sustainable innovations in garlic extraction: A comprehensive review and bibliometric analysis of green extraction methods
- Rapid Communication
- In situ supported rhodium catalyst on mesoporous silica for chemoselective hydrogenation of nitriles to primary amines
- Special Issue: Valorisation of Biowaste to Nanomaterials for Environmental Applications
- Valorization of coconut husk into biochar for lead (Pb2+) adsorption
- Corrigendum
- Corrigendum to “An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity”

