Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
-
Maha Daghestani
Abstract
Green synthesized nanoparticles from plant extracts are being used in various biomedical applications, particularly because of their bactericidal and cytotoxic activities. In this study, silver nanoparticles (AgNPs) were successfully synthesized from the Rosmarinus officinalis aqueous leaf extract. Different spectroscopic and microscopic analyses such as ultraviolet-visible (UV-vis) spectroscopy, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy, and energy-dispersive X-ray spectroscopy were performed to verify the biosynthesized AgNPs in our sample. The formation of nanosilver particles was preliminarily confirmed by UV-vis spectroscopy at 400 nm. The presence of carboxylic or amide groups was confirmed by FTIR, for the reduction of the silver ion. Transmission electron microscopy confirmed a particle size of 12–22 nm. The prepared AgNPs showed good antibacterial activity against human pathogens and good cytotoxic activity against the human breast cancer cell line (MDA MB 231). The nanoparticles prepared from R. officinalis can be used for various biomedical applications.
List of abbreviations
- AgNPs
Silver nanoparticles
- DLS
Dynamic light scattering
- EDX
energy-dispersive X-ray spectrum
- FTIR
Fourier transform infrared spectroscopy
- PDI
Polydispersity index
- SPR
Surface plasmon resonance
- TEM
Transmission electron microscopy
- UV-vis
Ultraviolet-visible absorption
1 Introduction
Silver has been used by mankind for a long time because of its low toxicity and good biocidal properties. It is frequently used in water-purifying systems in hospitals, for healing injuries, and in the field of nanoparticle synthesis [1]. Some bacteria are resistant to silver and are able to accumulate this metal up to 25% of their dry weight biomass in their cells [2], which makes them suitable for extracting silver from ores [3]. Silver nanoparticles (AgNPs) are used as an effective nano-drug against many diseases [4]. Also, they are more efficient in killing silver-resistant bacteria due to their highly developed surface area [5], whereas highly reactive silver ions (Ag+ ions) form insoluble precipitates so are less efficient in killing bacteria [6].
Nanobiotechnology is constantly exploring the synthesis of metal nanoparticles in a nontoxic and ecofriendly manner [7]. The main requirements for the synthesis of a metal nanoparticle are the solvent medium, a reducing agent, and a nontoxic stabilizer of nanoparticles [8]. Plants are rich in active compounds and, thus, can be used as a good source of reducing agents for synthesizing nanoparticles [9]; this has prompted a new field of green synthesis [10]. Almost all parts of the plants are rich in active biomolecules such as amino acids, alkaloids, proteins, polysaccharides, phenolics, terpenoids, and flavones, which can exhibit good reducing and capping properties useful in synthesizing nanoparticles [11]. Additionally, hydroxyl and carboxyl groups present in phenols and flavonoids wrap the nanoparticles and act as capping agents [12].
Rosmarinus officinalis, commonly known as rosemary, contains many different bioactive compounds, and it is used as a flavoring spice in cooking for many years [13]. It is one of the most important commercial crops in Saudi Arabia [14]. Its essential oil has been reported to have analgesic, antispasmodic, antibacterial, and hypnotic effects [15]. It is also used in the treatment of depression and stress-related disorders [16]. Same plants grown under different climatic conditions show different active components [17,18]. Some environmental factors like type of soil and temperature can alter the plant structure and its phytochemical activity, especially the bioactive compounds present in the leaves [19]. Some studies even reported a direct relationship between vitamins and phenolic compounds of plant and soil mineral content of the area where the plant is grown [20]. Natural compounds of R. officinalis leaves change significantly if grown under differential environmental conditions [21]. It was of great interest to synthesize AgNPs by the reduction of aqueous Ag+ ions with the R. officinalis leaf extract collected from Riyadh, Saudi Arabia, and synthesis of AgNPs from R. officinalis collected from deserts has not been reported. The green synthesized AgNPs were characterized using various microscopic and analytical techniques, including ultraviolet-visible absorption (UV-vis) spectroscopy, Fourier transform infrared spectroscopy, and transmission electron microscopy (TEM). The bactericidal and cytotoxic properties of the synthesized nanoparticles were also analyzed.
2 Materials and methods
2.1 Leaf extract
Fresh green leaves of rosemary cultivated in the Riyadh region of Saudi Arabia were used for this study. Ten grams of healthy leaves were washed, chopped, and boiled in 0.1 L of double-distilled water for 30 min. After cooling to room temperature, the broth was filtered and stored at 4°C.
2.2 Biosynthesis of AgNPs
The freshly prepared leaf extract was mixed with a silver nitrate solution at a final concentration of 5 mM at room temperature. The reduction of silver ions to AgNPs was indicated by a color change from yellow to brown and recorded by scanning through a UV-vis spectrophotometer at different intervals for 3 h.
2.3 Characterization of the prepared nanoparticles
The as-prepared nanoparticles were analyzed by observing the color change of the reaction mixture. The absorbance of the reaction mixture was measured over the range of 200–700 nm every 30 min. The average size of the as-prepared nanoparticles was determined by carrying out the zeta potential analysis. The hydrodynamic size and zeta potential of the prepared AgNPs were measured at the scattering angle of 173° with a red laser at the wavelength of 633 nm. The average size of the synthesized particles was measured with the help of a TEM. Infrared spectroscopy was used to determine the nature of bio-reducing functional groups.
2.4 Antimicrobial activity
Bacterial strains, namely Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, Salmonella typhi, Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus pneumoniae, were provided by King Khalid Hospital, Riyadh, Saudi Arabia. The antibacterial activity of the synthesized AgNPs against all the mentioned bacteria was determined by the disk diffusion method.
2.5 Screening of cytotoxic activity of the prepared nanoparticles
R. officinalis nanoparticles were screened for cytotoxicity on the breast cancer cell line MDA-231 by the MTT assay. Different concentrations of the synthesized nanoparticles (3.125, 6.25, 12.5, 25, 50, and 100 µL) were used to treat the cancerous cells. The inhibition percentage was calculated.
3 Results
UV-vis spectroscopy was used as a primary method to confirm the synthesis of nanoparticles due to their interaction with specific wavelengths. The synthesis of nanoparticles from silver ions in the presence of the rosemary plant extract was initially confirmed by a color change from yellow to brown. The surface plasmon resonance (SPR) peak at 400 nm confirmed the presence of nanoparticles in the extract (Figure 1). Free electrons in the nanoparticles resonate at certain wavelengths, resulting in SPR. A size reduction of the particles can lower the SPR peak, thus relating the absorption to the particle size [22].
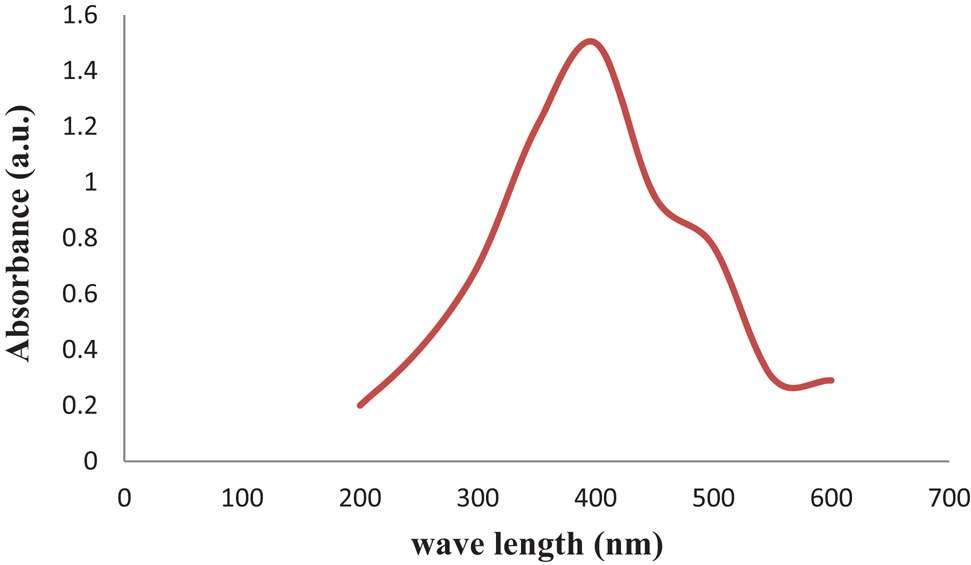
UV-vis spectral analysis of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract.
Recorded infrared (IR) spectra point toward the active compounds of the R. officinalis extract, which facilitate the green synthesis of nanoparticles. Nanoparticles are synthesized in three phases, starting with the activation phase, where the plant metabolites with reducing capacities help to split the metal ion from the salt. Next is the growth phase, where metal ions combine to form nanoparticles, and finally, the termination phase, where capping around the synthesized particle is done with the help of the metabolites present in the plant extract [23]. The IR spectrum of Figure 2 shows an intense peak at 1636.75 cm−1, which is recognized as C═O and C═C bond stretches and identified as carboxylic or amide groups, and a peak at 3304.40 cm−1, which represents the hydroxyl group and suggests the presence of phenols, flavonoids, and alcohols in the plant extract.
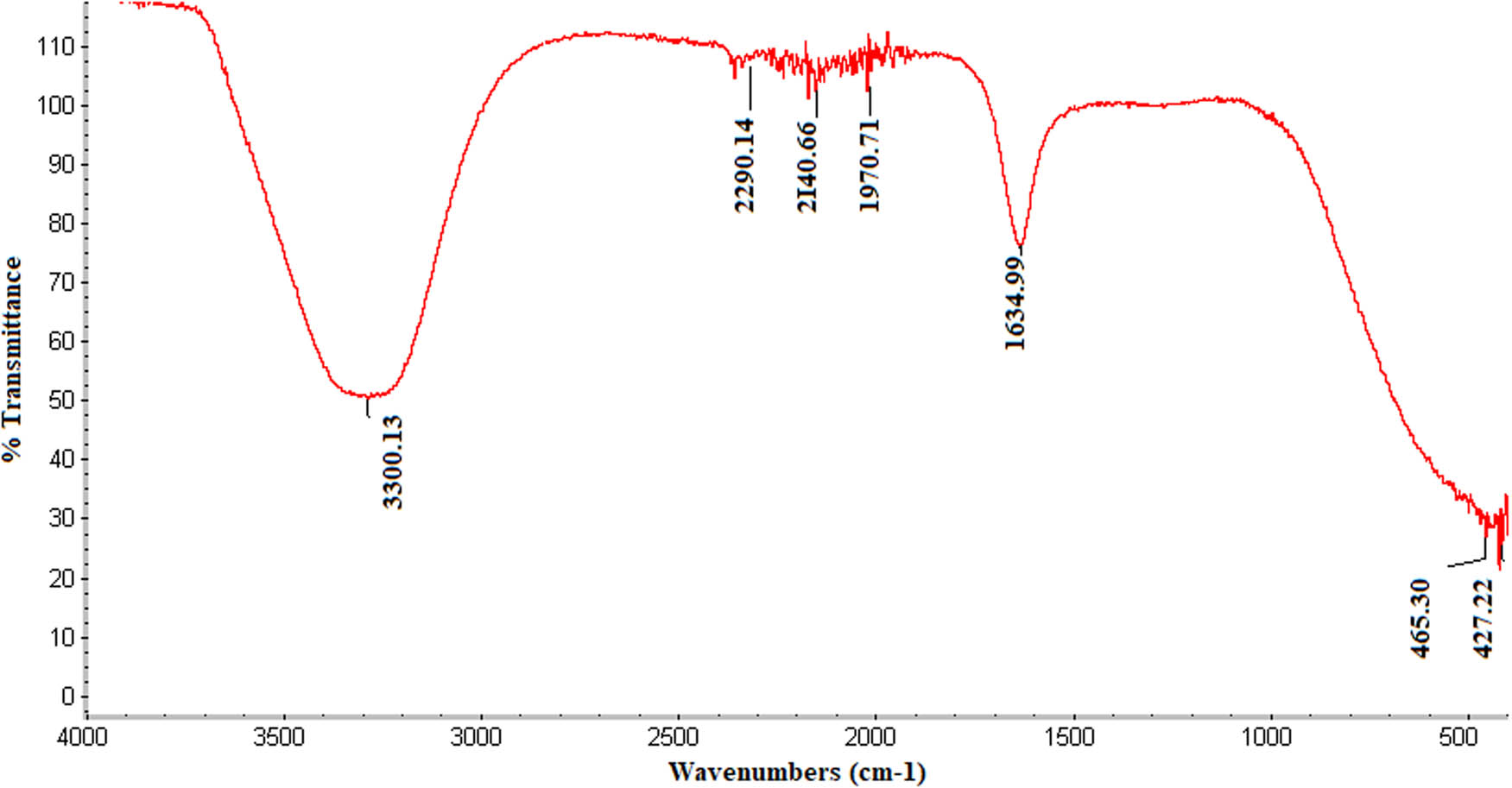
IR spectra of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract.
In this study, we used dynamic light scattering (DLS) to determine the particle size distribution of the synthesized AgNPs (Figure 3). The Z-average mean of the synthesized AgNPs was 66.79 nm, with a polydispersity index (PDI) of 0.177. The PDI is best measured with DLS. Our results with respect to nanoparticles size are acceptable, as a PDI value of more than 0.7 indicates a broad size distribution [24]. Plant extracts are good reducing agents for the synthesis of metal nanoparticles. The interface of the particles mainly depends on the zeta potential since a larger zeta potential results in a particle abundance, thus providing stability to the particles (Figure 4) [25]. The energy-dispersive X-ray spectrum (EDX) of the synthesized nanoparticles exhibited strong signals in the oxygen, potassium, and chlorine regions (Figure 5). The presence of other elements such as carbon, calcium, sodium, and magnesium was also observed in the spectrum. A TEM image was used to find the average size of the prepared nanoparticles, which was in the range of 12–22 nm (Figure 6). TEM is typically an ultrathin sample image showing the 2D structure with the help of an absorption beam passing through the sample [26]. Nowadays, AgNPs are highly important in detergent and disinfectant manufacturing industries because of the resistance level of microbes to chemical fungicides and disinfectants [27]. We examined the antibacterial efficiency of AgNPs prepared from R. officinalis against some pathogenic bacteria. The varying degree of inhibition by both the Gram-positive and Gram-negative bacteria is shown in Table 1.
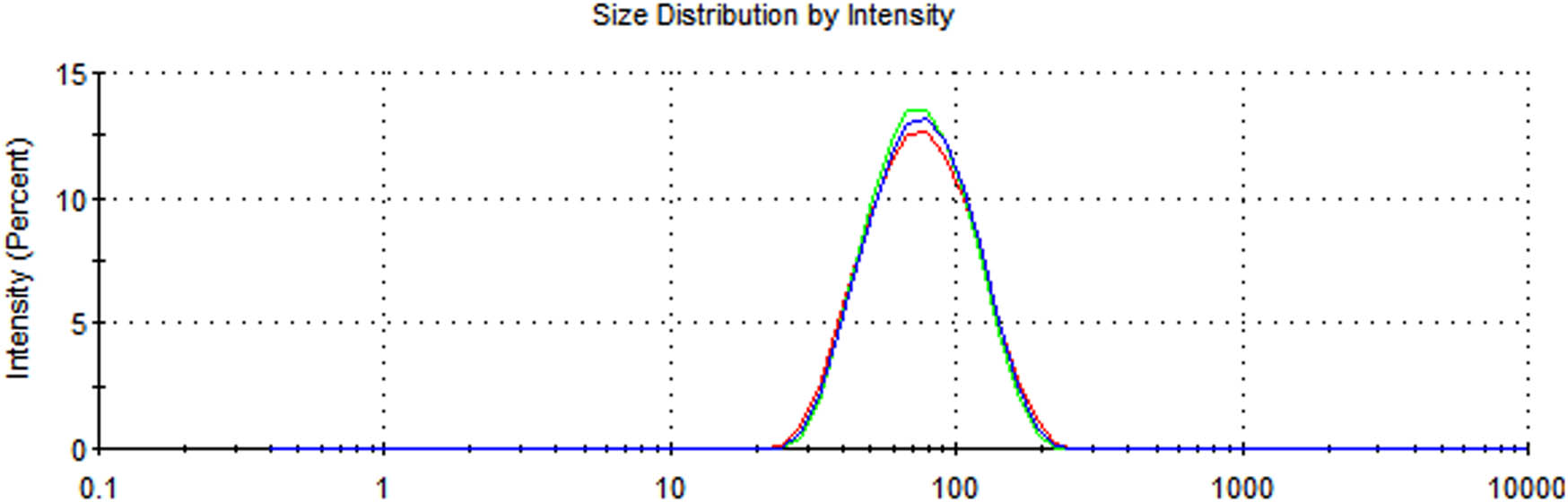
Average size of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract.
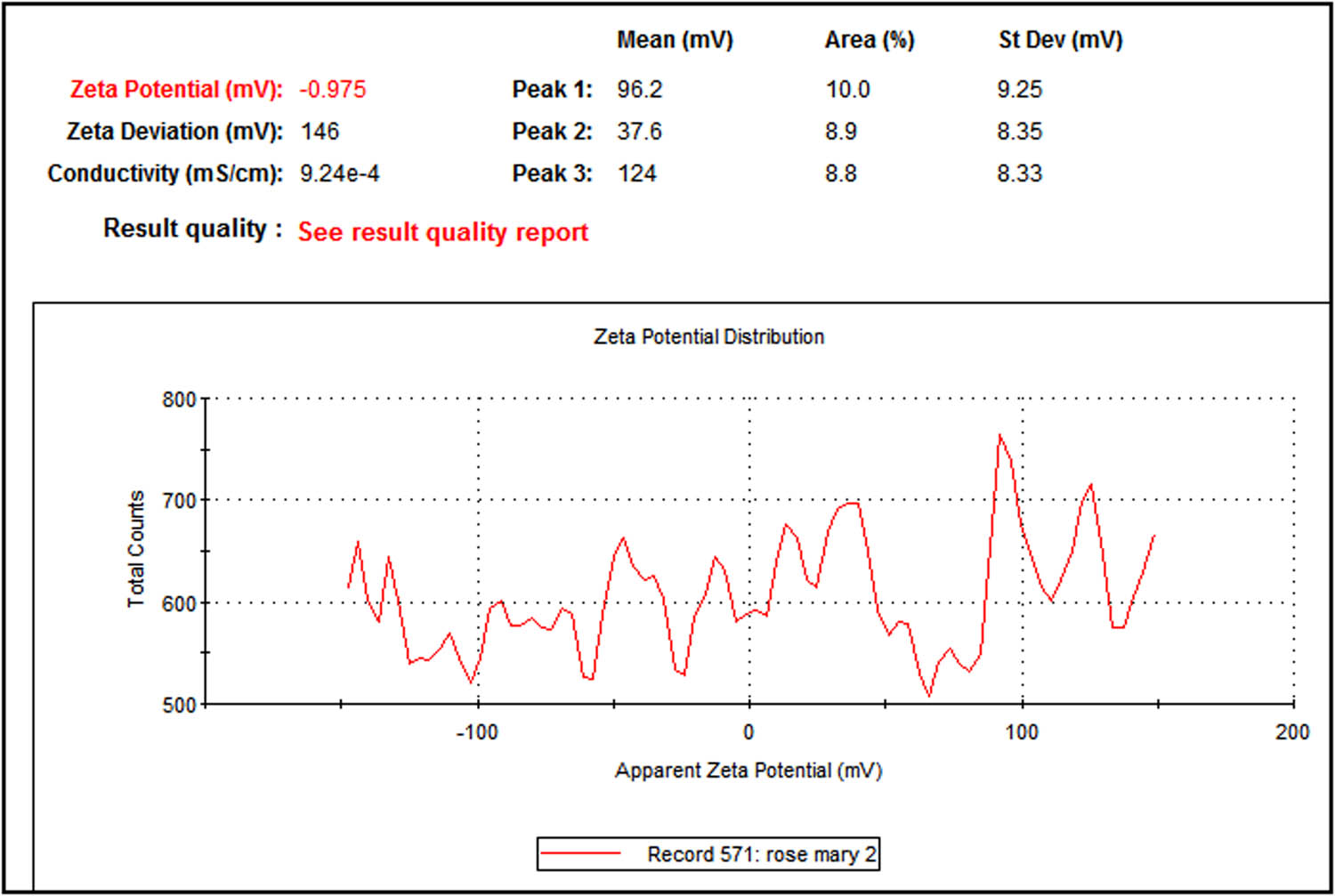
Zeta potential distribution of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract.
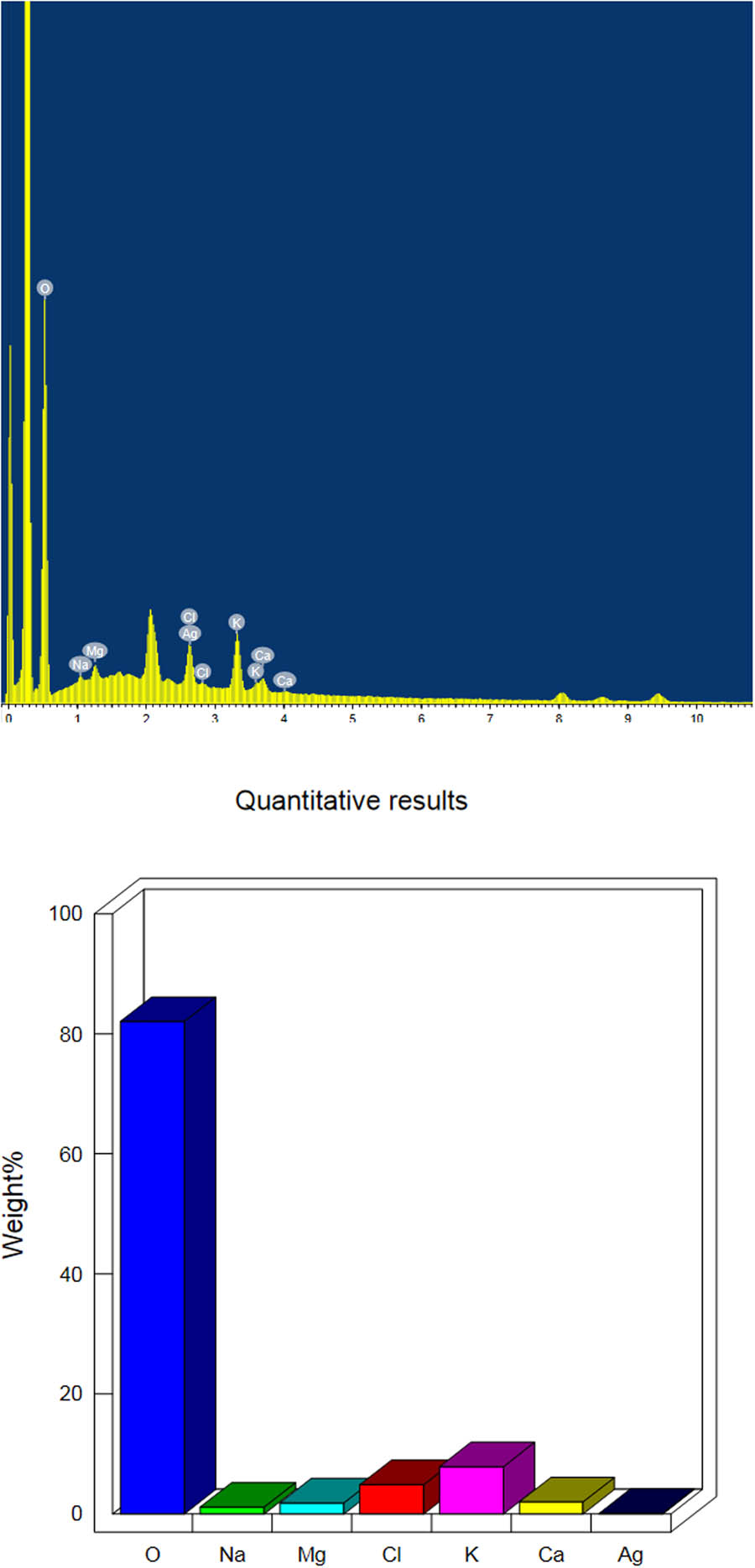
EDX elemental analysis of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract.

TEM micrograph of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract (particle size shown in range from 12 to 22 nm).
Antibacterial activity of the biosynthesized AgNPs prepared with aqueous R. officinalis leaf extract against eight bacterial species tested by disc diffusion assay
| Strains | Zone of inhibition (mm) | |
|---|---|---|
| Gram positive | S. aureus | 11 ± 0.30 |
| S. epidermidis | 8 ± 0.13 | |
| S. pneumonia | 10 ± 0.25 | |
| E. faecalis | 10 ± 0.50 | |
| B. subtilis | 11 ± 0.20 | |
| Gram negative | E. coli | 13 ± 0.10 |
| S. typhi | 14 ± 0.31 | |
| K. pneumoniae | 9 ± 0.11 |
4 Discussion
The antibacterial efficacy of a particle depends on its small size and round shape. Our results agree with many previous studies reporting high antimicrobial activities of round-shaped nanoparticles with a size of less than 10 nm [28]. It has been suggested that the antibacterial properties of the plant-derived AgNPs could be due to either the particles’ ability to permeate the cell and interact with the genetic material deoxyribonucleic acid (DNA) and other important constituents, leading to cell death, or their adherence to the negatively charged cell surface that alters the chemical and physical properties of the cell wall and cell membrane [29]. In this study, the green synthesized AgNPs from R. officinalis were evaluated at various concentrations for the cytotoxic effects on the breast cancer cell lines (MB 231) in vitro. As observed in Figure 7, both plant extracts and nanoparticles decrease the viability of cancer cell lines (MB 231), but the cytotoxicity of AgNPs was higher than the cytotoxicity of the plant extract. The cytotoxic effect of nanoparticles on cell viability has a major role in antitumor activity, thereby reducing disease progression [30]. The cytotoxic effects of silver result from the active physiochemical interaction of silver atoms with functional groups of intracellular proteins, as well as with the nitrogen bases and phosphate groups in DNA [31].
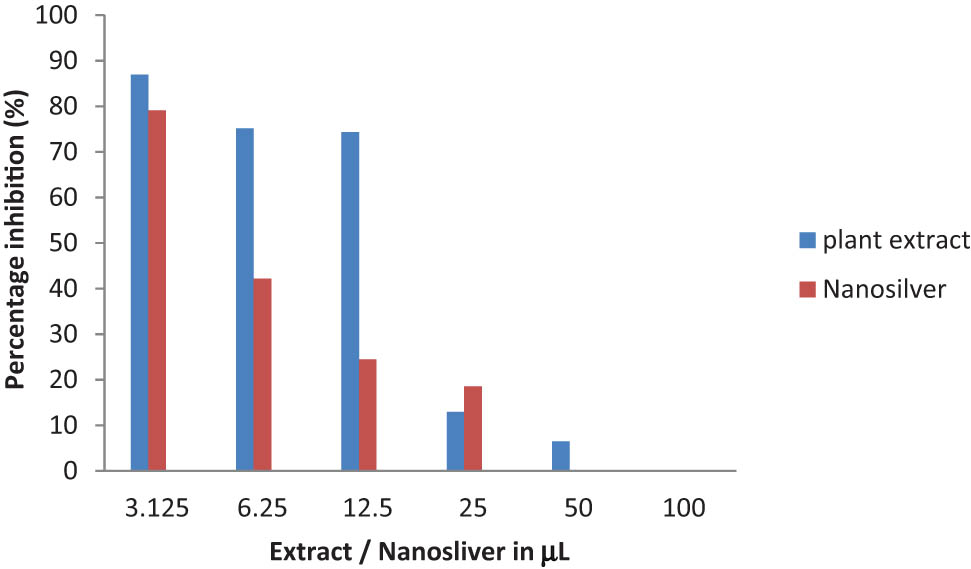
Cytotoxicity of aqueous R. officinalis leaf extract and its AgNPs on breast cancer cell lines (MDA MB 231) following 24 h exposure.
Acknowledgments
This research project was supported by a grant from the Research Centre for Female Scientific and Medical Colleges at King Saud University. The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
References
[1] Graham C. The role of silver in wound healing. Br J Nurs. 2005;14(19):S22.10.12968/bjon.2005.14.Sup5.19954Suche in Google Scholar
[2] Seifelnassr AAS, Abouzeid AZM. Exploitation of bacterial activities in mineral industry and environmental preservation: an overview. J Min. 2013;2013:507168.10.1155/2013/507168Suche in Google Scholar
[3] Pooley FD. Bacteria accumulate silver during leaching of sulphide ore minerals. Nature. 1982;296(5858):642–3.10.1038/296642a0Suche in Google Scholar
[4] Rasheed T, Bilal M, Li C, Iqbal HMN. Biomedical potentialities of taraxacum officinale-based nanoparticles biosynthesized using methanolic leaf extract. Curr Pharm Biotechnol. 2017;18(14):1116–23.10.2174/1389201019666180214145421Suche in Google Scholar
[5] Ying JY. The era of nanotechnology. Nano Today. 2008;3:1.10.1016/S1748-0132(08)70049-5Suche in Google Scholar
[6] Sun Z, Fan C, Tang X, Zhao J, Song Y, Shao Z, et al. Characterization and antibacterial properties of porous fibers containing silver ions. Appl Surf Sci. 2016;387:828–38.10.1016/j.apsusc.2016.07.015Suche in Google Scholar
[7] Rasheed T, Bilal M, Li C, Nabeel F, Khalid M, Iqbal HMN. Catalytic potentialof bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J Photochem Photobiol B. 2018;181:44–52.10.1016/j.jphotobiol.2018.02.024Suche in Google Scholar PubMed
[8] Raveendran P, Fu J, Wallen SL. Completely “Green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–1.10.1021/ja029267jSuche in Google Scholar PubMed
[9] Barkat MA, Harshita, Beg S, Naim MJ, Pottoo FH, Singh SP, et al. Current progress in synthesis, characterization and applications of silver nanoparticles: precepts and prospects. Recent Pat Antiinfect Drug Discov. 2018;13(1):53–69.10.2174/1574891X12666171006102833Suche in Google Scholar PubMed
[10] Rasheed T, Bilal M, Iqbal HMN, Li C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf B. 2017;1(158):408–15.10.1016/j.colsurfb.2017.07.020Suche in Google Scholar PubMed
[11] Ahumada M, Suuronen EJ, Alarcon EI. Biomolecule silver nanoparticle-based materials for biomedical applications. In: Martínez L, Kharissova O, Kharisov B, editors. Handbook of Ecomaterials. Cham: Springer. 2019.10.1007/978-3-319-68255-6_161Suche in Google Scholar
[12] Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta naturae. 2014;6(1):35–44.10.32607/20758251-2014-6-1-35-44Suche in Google Scholar
[13] Borrás-Linares I, Stojanović Z, Quirantes-Piné R, Arráez-Román D, Švarc-Gajić J, Fernández-Gutiérrez A, et al. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int J Mol Sci. 2014;15(11):20585–606. 10.3390/ijms151120585.Suche in Google Scholar PubMed PubMed Central
[14] Bazaid SA, El-Amoudi MS, Ali EF, Abdel-Hameed ES. Volatile oil studies of some aromatic plants in Taif region. J Medicinal Plants Stud. 2013;1(5):119–28.Suche in Google Scholar
[15] de Oliveira JR, Camargo SEA, de Oliveira LD. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J Biomed Sci. 2019;26(1):5.10.1186/s12929-019-0499-8Suche in Google Scholar PubMed PubMed Central
[16] Fernández LF, Palomino OM, Frutos G. Effectiveness of Rosmarinus officinalis essential oil as antihypotensive agent in primary hypotensive patients and its influence on health-related quality of life. J Ethnopharmacol. 2014;151:509–16.10.1016/j.jep.2013.11.006Suche in Google Scholar PubMed
[17] Guo H, Chamberlain SA, Elhaik E, Jalli I, Lynes AR, Marczak L, et al. Geographic variation in plant community structure of salt marshes: species, functional and phylogenetic perspectives. PLoS One. 2015;10(5):e0127781.10.1371/journal.pone.0127781Suche in Google Scholar PubMed PubMed Central
[18] Liu W, Liu J, Yin D, Zhao X. Influence of ecological factors on the production of active substances in the anti-cancer plant sinopodophyllum hexandrum (Royle) T. S. Ying. PLoS One. 2015;10(4):e0122981.10.1371/journal.pone.0122981Suche in Google Scholar PubMed PubMed Central
[19] Liu W, Yin D, Li N, Hou X, Wang D, Li D, et al. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci Rep. 2016;6:28591.10.1038/srep28591Suche in Google Scholar PubMed PubMed Central
[20] Tsao R, Khanizadeh S, Dale A. Designer fruits and vegetables with enriched phytochemicals for human health. Can J Plant Sci. 2006;86:773–86.10.4141/P05-138Suche in Google Scholar
[21] Peñuelas J, Llusià J. Effects of carbon dioxide, water supply, and seasonality on terpene content and emission by Rosmarinus officinalis. J Chem Ecol. 1997;23:979.10.1023/B:JOEC.0000006383.29650.d7Suche in Google Scholar
[22] Peng S, McMahon JM, Schatz GC, Gray SK, Sun Y. Reversing the size-dependence of surface plasmon resonances. Proc Natl Acad Sci U S A. 2010;107(33):14530–4.10.1073/pnas.1007524107Suche in Google Scholar PubMed PubMed Central
[23] Malik P, Shankar R, Malik V, Sharma N, Mukherjee T. Green chemistry based benign routes for nanoparticle synthesis. J Nanopart. 2014;2014:302429.10.1155/2014/302429Suche in Google Scholar
[24] Clayton KN, Salameh JW, Wereley ST, Kinzer-Ursem TL. Physical characterization of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics. 2016;10(5):054107. 10.1063/1.4962992.Suche in Google Scholar PubMed PubMed Central
[25] Meléndrez MF, Cárdenas G, Arbiol J. Synthesis and characterization of gallium colloidal nanoparticles. J Colloid Interface Sci. 2010;346(2):279–28.10.1016/j.jcis.2009.11.069Suche in Google Scholar PubMed
[26] Rauwel P, Küünal S, Ferdov S, Rauwel E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv Mater Sci Eng. 2015;2015:682749.10.1155/2015/682749Suche in Google Scholar
[27] Hedberg J, Skoglund S, Karlsson ME, Wold S, Odnevall Wallinder I, Hedberg Y. Sequential studies of silver released from silver nanoparticles in aqueous media simulating sweat, laundry detergent solutions and surface water. Env Sci Technol. 2014;48(13):7314–22. 10.1021/es500234y. Epub 2014 Jun 19.Suche in Google Scholar PubMed
[28] Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol. 2012;112:841–52.10.1111/j.1365-2672.2012.05253.xSuche in Google Scholar PubMed
[29] Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57.10.1038/nmat2442Suche in Google Scholar PubMed
[30] Bilal M, Rasheed T, Iqbal HMN, Li C, Hu H, Zhang X. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int J Biol Macromol. 2017;105(1):393–400.10.1016/j.ijbiomac.2017.07.047Suche in Google Scholar PubMed
[31] Blagoi YuP, Galkin VL, Gladchenko GO, Kornilova SV, Sorokin VA, Shkorbatov AG. Metal complexes of nucleic acids in solutions. Naukova Dumka. 1991; p. 272.Suche in Google Scholar
© 2020 Maha Daghestani et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Artikel in diesem Heft
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis

