Abstract
Effects of the type, chemical structure and NCO/OH of resins on wet adhesion and salt spray resistance of two component waterborne polyurethane coating were studied in this paper. The content of functional groups in resins and films were analysed by areas of their FT-IR absorption peak to study the relationship with anticorrosive performance of coatings. Coatings based on resins with more -OH tend to have stronger wet adhesion and salt spray resistance because resins with more -OH have more possibilities to react with -NCO to form a compact film with a higher crosslinking degree. The wet adhesion of coatings after 8 h of immersion deteriorated from level 1 to level 5 with the increase of NCO/OH due to the decrease of -OH in coatings to link with substrates. And excess -NCO tend to react with H2O to generate CO2 to form cracked physical bubbles, which would weaken the wet adhesion.
1 Introduction
Metal corrosion is chemical or electrochemical reaction between metals and the environment medium such as H2O, O2 and NaCl. To apply anticorrosive coatings (1), such as epoxy (2,3), alkyd resin (4), or polyurethane coating, on the surface of metals is an efficient and economic method
of reducing corrosion. And PU is widely used as the film former of coating due to its good adhesion and resistance to corrosion.
Traditional solvent based PU coating would bring about volatile organic polymerization compounds (VOCs) during coating manufacture and application, which seriously threatens environmental or public safety. Two component waterborne polyurethane (2K WPU) coating (5) provides a good solution for eliminating VOCs and has been paid progressive attention in coating industry.
There are many reviews in the literature dealing with metal corrosion by WPU coatings based on various resins. Xu et al. (6) prepared a high molecular weight polyacrylate dispersion using the synthesized polyacrylate as macromolecular emulsifier by emulsion polymerization. The WPU coating based on the obtained polyacrylate with low VOCs level showed good wet adhesion and chemical resistance. Huang et al. (7) prepared and characterized electroactive waterborne polyurethane (EWPU) containing conjugated segments of electroactive amino-capped aniline trimer unit. And the protection efficiency (PEF%) of EWPU (93.96%) was higher than that of non-electroactive waterborne polyurethane (NEWPU) coating (18.20%) based on electrochemical measurements in 3.5 wt% NaCl electrolyte, which indicated enhanced corrosion protection effects of EWPU on cold-rolled steel (CRS). The enhanced corrosion protection ability of the EWPU coating could be interpreted as that the redox catalytic capabilities of aniline trimer units existed in EWPU induce the formation of passive metal oxide layers on the CRS electrode. Kiosidou (8) used salt spray to test a newly developed polyurethane based marine antifouling coating, and the experimental formulation exhibited superior anticorrosion performance overall compared with acrylic based system.
In addition, it is vital to establish the relationship between resins and anticorrosive properties of coatings such as salt spray resistance (9) and wet adhesion (10,11). Wu et al. (12) found that the wet adhesion of epoxy coatings was significantly influenced by the epoxide content, and excess epoxide groups were highly likely to favor the formation of stronger adhesive bonding at coating-metal interface. Meis et al. (13) concluded that the novel aliphatic resin coatings show better wet adhesion compared to aromatic resin coatings according to the investigation of different epoxy-amine coatings on the adhesion performance on aluminium substrates. Coatings mainly rely on resins and reinforcement to realize superior anticorrosive properties. And the effect of resins in 2K WPU coating on the wet adhesion and salt spray resistance cannot be ignored. But most of works (14,15,16,17) in recent years have focused on the nano particles, such as nano ZnO and graphene oxide, to improve anticorrosive performance of 2K WPU coating. And the relationship among resins, salt spray resistance, and wet adhesion of 2K WPU coating is still not clear.
In this paper, in order to improve anticorrosive performance by selecting resins and optimization of NCO/OH during preparation of coatings, 2K WPU coating on carbon steel surfaces with TiO2 as the barrier and pigment were fabricated. The crosslinking degree, salt spray resistance, and wet adhesion of 2K WPU coating were investigated. The chemical structure of resins and coatings were characterized by Fourier transform infrared spectroscopy (FT-IR), and the content of functional groups were creatively analyzed according to the area of their absorption peak. The scanning electron microscopy (SEM) was used to investigate the micro morphology of coatings. These works intend to study the effect of resins such as their type, chemical structure, and NCO/OH in 2K WPU coating on corrosion protection and establish the relationship among the chemical structure of resins, salt spray resistance, and wet adhesion of coatings.
2 Experimental
2.1 Materials
Waterborne hydroxyl-functional polyacrylic polyol (AR70, AR42, AR46, AR27 and AR01), polyester polyol (ES 66) and polycarbonate polyol (CA50), and polyisocyanurate based on HDI trimmers (CU55) were donated by Covestro. Hydroxyl content of selected polyacrylic polyols is different, as shown in Table 1. Wetting and dispersing agent BYK190, surface active agent BYK349, flatting agent BYK381, defoaming agent BYK022 and BYK093 were purchased from BYK-Chemie GmbH. Rheological agent RM8W was purchased from Rohm and Haas. Surface active agent AD01 were purchased from Air Products.
Details about -OH content of resins and -NCO content of hardener.
| Component | No. | Main functional group | Content of -OH or -NCO (wt%) |
|---|---|---|---|
| Polyacrylic polyol | AR01 | -OH | 4.0 |
| Polyacrylic polyol | AR70 | -OH | 3.7 |
| Polyacrylic polyol | AR46 | -OH | 3.5 |
| Polyacrylic polyol | AR42 | -OH | 3.0 |
| Polyacrylic polyol | AR27 | -OH | 2.0 |
| Polyester polyol | ES66 | -OH | 4.1 |
| Polycarbonate polyol | CA50 | -OH | 4.0 |
| Polyisocyanurate | CU55 | -NCO | 20.0 |
Auxiliary solvent PGDA were purchased from DOW. TiO2 (R706) was purchased from DuPont. Acetone and alcohol were supplied by Shanghai Ling Feng Chemical Reagent. All the materials above mentioned were used without further purification.
2.2 Instruments and measurements
FTIR-ATR spectrum of the samples was measured using FTIR spectrophotometer (EQUINOX 55, German). The micro morphologies inside WPU coating was characterized via field-emission SEM (Quanta200, USA).
2.3 Preparation of 2K WPU coating
Firstly, the pigment paste of coatings was prepared by mixing 250.0 g R706, 10.0 g AD01, 25.0 g BYK190, 2.0 g BYK349, 3.0 g BYK022, and 2.0 g RM8W with some deionized water at 2500 rpm in a plastic container at 25°C for 15 min. After grinding, the 36.9 g reaction mixture, 0.5 g BYK381 and 1.0 g BYK093 were added into 61.6 g waterborne hydroxyl-functional resin to mix at 2500 rpm for 20 min and then placed for 12 h to let out air bubbles. In the next step, 2K WPU coating was prepared by mixing the reaction solution with the hardener CU55 in the presence of PGDA by hand for 3 min. Its viscosity can be adjusted by adding deionized water. The calculation of dosage of resins or hardener is based on the content of -OH or -NCO provided by suppliers. And MW of selected resins are nearly equal.
2.4 Application of 2K WPU coating on steel substrates
The carbon steel panels with dimensions of 15 cm × 7.5 cm × 0.1 cm were used as metal substrates. Prior to coating application, panels were polished with SiC papers up to 400 grit, and cleaned by ethanol, followed by drying at 25°C for use. And the obtained 2K WPU coating was applied on the cleaned panels with film applicator, followed by drying in a vacuum oven at 60°C for 16 h to remove possible residual solvents. These samples were allowed to cure at room temperature for 7 days. After curing, these samples with dry film thickness of 55 ± 5 μm were chosen for test.
2.5 Crosslinking degree test
Films encased by a sieve were soaked in acetone for 24 h, and they were dried at 60°C for 1 h. Their crosslinking degree can be calculated based on the decrement of weight using following equations:
Equation 1 describes the ratio of pigment in the film where mp is weight of pigment, mr is weight of resins, nr is solid content of resins, mh is weight of hardener, and nh is solid content of hardener.
Equation 2 describes the crosslinking degree of films where mf is weight of films and md is decrement of weight of films.
2.6 Wet adhesion test
Edges of all the samples were sealed by water-proof tape before immersion. These panels were immersed in the water at 40°C for several hours and then tested immediately after being picked up and wiping water off the coating surface. Cross cut test for films was used to classify the wet adhesion of different coatings on substrates according to ISO 2409:2013. The wet adhesion was characterized by evaluating the intact coating area. Stronger wet adhesion can be marked lower level.
2.7 Salt spray test
The salt spray test was carried out in a DE2006-7 cyclic corrosion tester cabinet. The corrosive nature of the chamber specified by ASTMB 117 is a continuous spraying with a 3.5 wt% solution of NaCl at 35 ± 1°C. The back side and edges of steel panels with x-cut paints (according to EN ISO 17872:2007) were protected with a special pressure adhesive tape (TESA Tape, USA) and epoxy. And the coated samples were placed inside the salt spray chamber angled at 45°. Specimen surfaces and the rusting degree were monitored and rated by visual examination of these panels after 600 h.
3 Results and discussion
3.1 Analysis of functional groups content via FT-IR spectra
Figure 1a shows the FT-IR spectrum of different resins including waterborne polyacrylic polyols (AR70, AR42, AR46, AR27 and AR01) and waterborne hydroxyl-functional polyester polyol (ES66) and polycarbonate polyol (CA50). These spectrums show the absorption
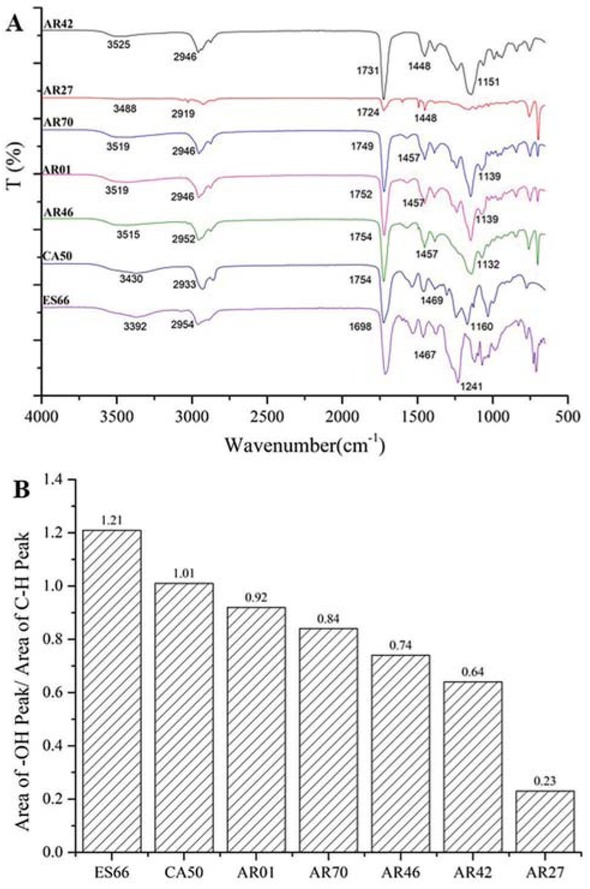
FTIR spectrum (a) of different resins and area of -OH absorption peak (b) in FT-IR spectrum of different resins.
peaks at 3500 cm-1 (O-H stretching vibration), 2950 cm-1 (-CH2 and -CH3 stretching vibration), 1750 cm-1 (C=O stretching vibration) and 1140 cm-1 (C-O-C stretching vibration), respectively.
The area of absorption peaks for some functional groups was calculated to reflect their content in resins or coatings based on the internal standard CH absorption peak. And the hydroxyl content in resins reflected by the area of -OH absorption peak was presented in Figure 1b The resins can be sorted according to their hydroxyl content based on the analysis of area of absorption peak: ES66 > CA50 > AR01 > AR70 > AR46 > AR42 > AR27. As waterborne hydroxyl-functional polyester polyol, ES66 has the highest hydroxyl content among these resins. For same waterborne polyacrylic polyol, the content of -OH in AR61 is the highest, while the content of -OH in AR27 is the lowest. The different hydroxyl content in polyacrylic polyols indicates that they are ideal materials to study the effect of hydroxyl content in resins on the salt spray resistance and wet adhesion of coatings.
As shown in Figure 2, the hydroxyl content in 2K WPU coating decreased with the increase of NCO/OH when the ratio is smaller than 1.6, and then kept stable according to the analysis of area of -OH absorption peak. This result demonstrated that the proper increase of -NCO can bring about the consumption of -OH, but excess -NCO is not available for the reaction with -OH. The absorption peak of -OH in the case of excess -NCO means that -OH in coatings cannot be completely consumed by -NCO. The result can be explained by that chain segments are more difficult to move in 2K WPU coating with the chemical reaction going on, and it is difficult for the rest of -OH to react with -NCO.
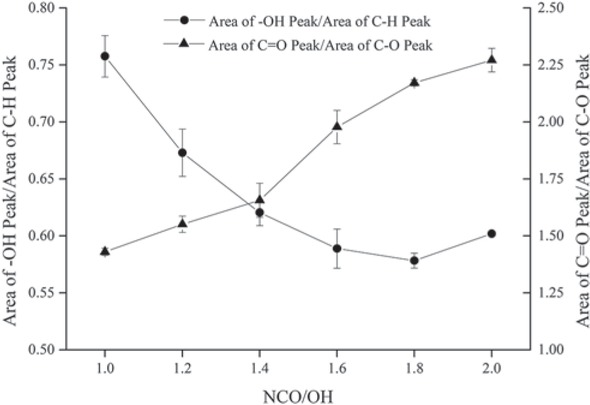
Area of -OH absorption peak and C=O/C-O ratio in 2K WPU coating with increase of NCO/OH.
No absorption peak of -NCO in coatings detected by FTIR indicates that the excess -NCO was completely used up. In general, 2K WPU coating mainly needs the reaction between -NCO and -OH at the molar ratio of 1:1 to form a compact PU film, as expressed in Scheme 1. According to the integration for absorption peaks of C=O and C-O in FTIR spectra of 2K WPU coating with different NCO/OH, the result was listed in Figure 2. The C=O/C-O ratio in coatings raises with the increase of NCO/OH, which means excess -NCO reacted with H2O to generate CO2 and urea without C-O, as shown in Scheme 1. When NCO/OH is smaller than 1.6, the reaction with -NCO prevailed because -OH is easier to react with -NCO than H2O. When NCO/OH is larger than 1.6, the reaction to generate CO2 and urea dominated due to less -OH in coatings. The generated CO2 would gather to form physical bubbles in coatings. And these bubbles would remain or break up inside the film while the motion of PU macromolecule is restricted, which is regarded as a micro defect in 2K WPU film.
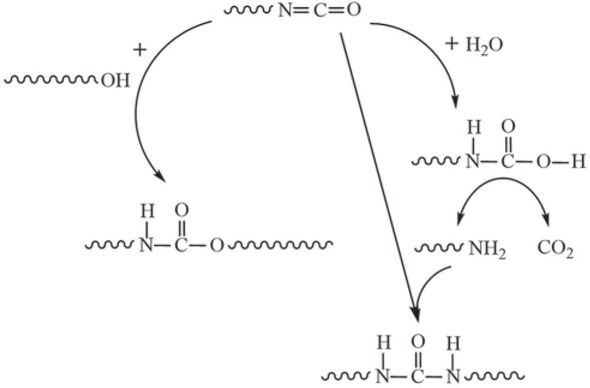
Reaction mechanism between excess -NCO and -OH or H2O.
3.2 Effect of resins on the crosslinking degree of 2K WPU coating
The crosslinking degree of 2K WPU coating based on different resins was presented in Figure 3a The coating based on polyester polyol ES66 and polycarbonate polyol CA50 showed a higher crosslinking degree than polyacrylic polyol AR01. And resins can be sorted according to the crosslinking degree of 2K WPU coating: ES66 > CA50 > AR01 > AR70 > AR46 > AR42 > AR27. The performance in crosslinking degree of coatings is consistent with the hydroxyl content in resins. Resins with more hydroxyl groups reacted and crosslinked with polyisocyanurate as
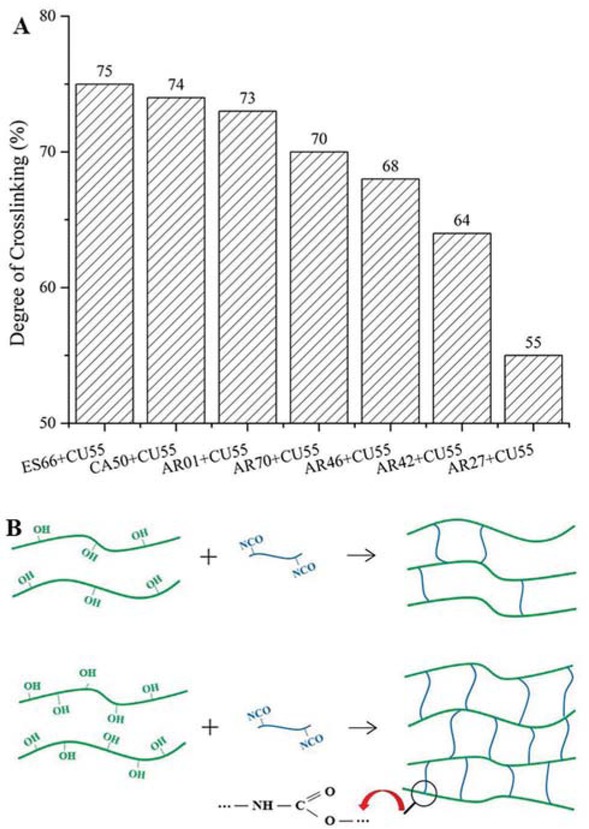
The crosslinking degree (a) of 2K WPU coating based on different resins and main reaction mechanism (b) between resins with different hydroxyl content and polyisocyanurate.
shown in Figure 3b, which contributed to the formation of a compact and tight PU film.
3.3 Morphology analysis of 2K WPU coating via SEM
SEM image of micro morphology inside 2K WPU coating based on different resins was presented in Figure 4. The micro structure of 2K WPU coating based on AR01, ES66, and CA50 seem compact and tight, and enough PU resin wrapped around some pigment particles. The micro structure of 2K WPU coating based on AR27 seem loose by comparison, and many pigment particles are gathering and exposed in coating for lack of enough PU resin. This difference in micro structure is related to the hydroxyl content of resins and crosslinking degree of coatings. The coating based on resin with more -OH tends to have a high crosslinking degree, and then to form enough PU to wrap around pigment particles. And the formation of compact and tight PU film containing pigment particles is beneficial to shield from corrosive medium, such as H2O, O2, Cl-, Na+ and so on. The loose micro structure of WPU coating based on AR27 is caused by low hydroxyl content of resin and low crosslinking degree of coating, which may lead to the decrease of anticorrosive performance.
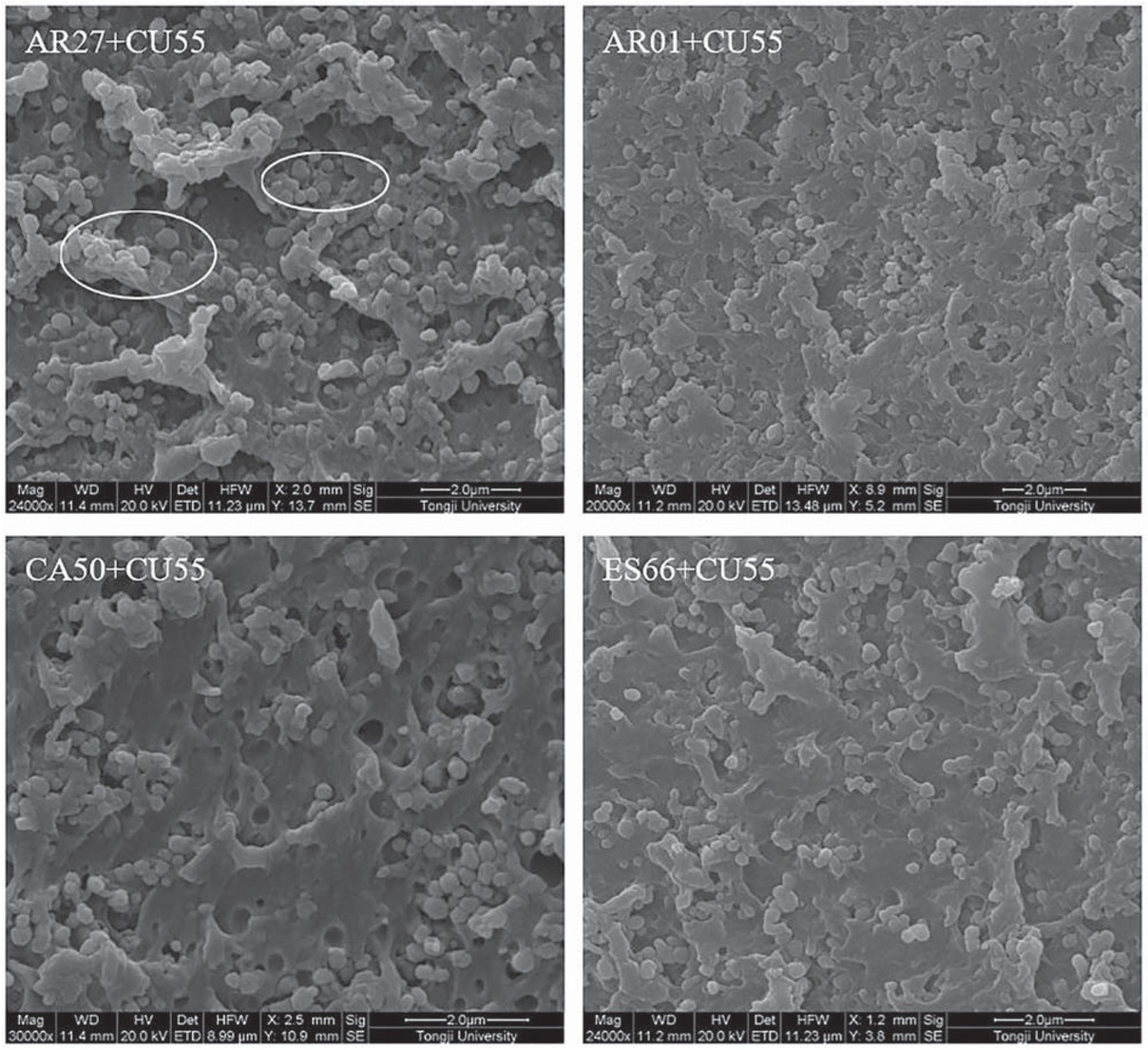
SEM image of micro morphology inside 2K WPU coating based on different resins.
3.4 Effect of resins on the salt spray resistance
3.4.1 Effect of hydroxyl content in resins on the salt spray resistance
The corrosion degree of coatings for polyacrylic polyols with different hydroxyl content can be sorted as follows: AR01 > AR70 > AR46 > AR42 > AR27, as shown in Figure 5a Obviously, the salt spray resistance of 2K WPU coating is related with the hydroxyl content in resins. For polyacrylic polyol, more hydroxyl group in resins means more possibilities to react with -NCO. As a result, PU film will become more compact because of its higher crosslinking degree, and its salt spray resistance will be stronger. Consequently, much rust and blister can be seen from the coating based on AR27 with least hydroxyl group at the OH/CH ratio of 0.23, which exhibited almost no barrier to rust accumulation. And the coating based on AR01 with most hydroxyl group at the OH/CH of 0.92 showed the best appearance among these samples.
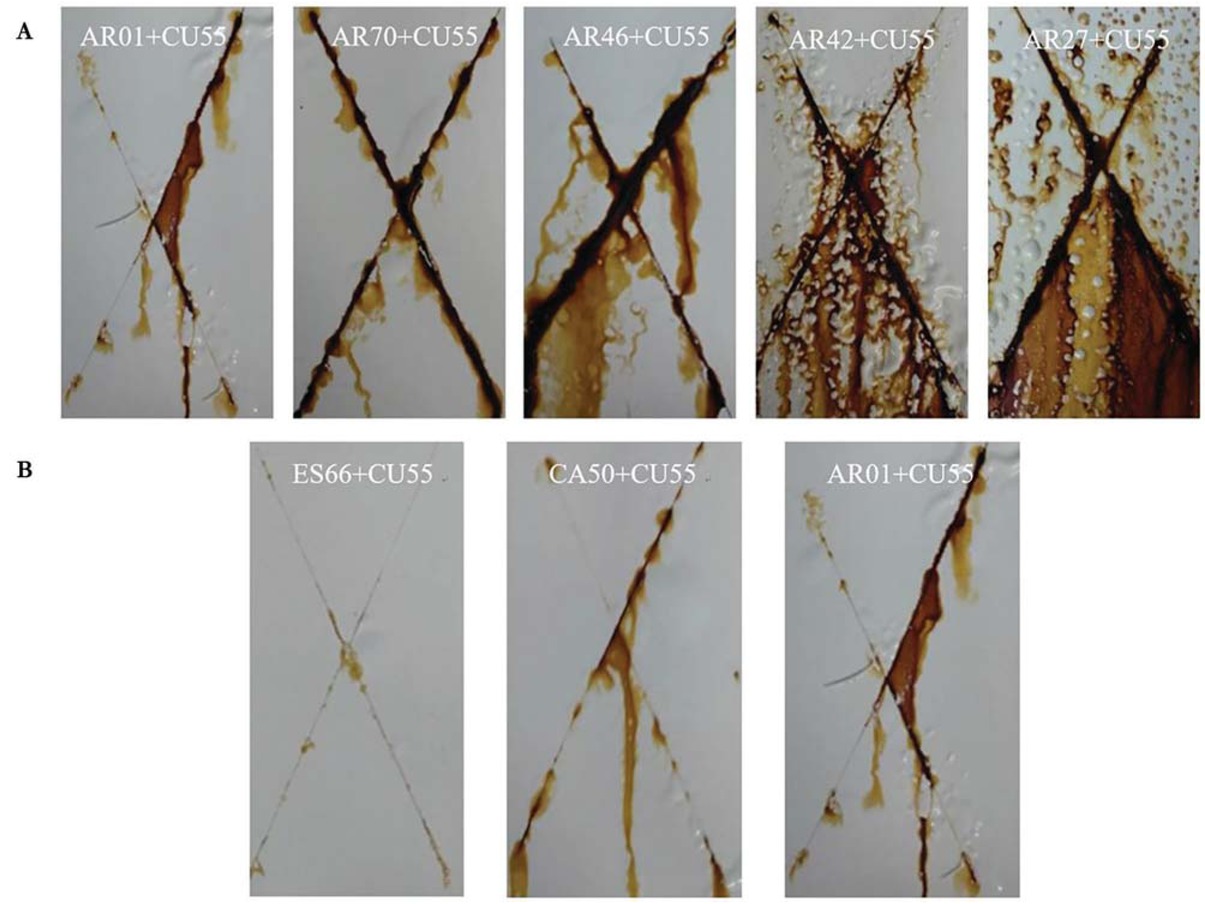
Images of 2K WPU coating based on polyacrylic polyols with different hydroxyl content (a) and different resins (b) after salt spray for 600 h.
The salt spray resistance of 2K WPU coating always depends on the compactness and wet adhesion of the film. In most cases, the compactness of coating relies on the content of groups to react in resins. The more -OH in resins, the more possibilities for resins to react and crosslink with the polyisocyanurate. Therefore, the compactness of PU film will be improved by selecting high hydroxyl content resins. As for the wet adhesion, it is not only the last barrier of coating to prevent the surface of substrates from corrosive medium, but also the crucial factor to realize its salt spray resistance.
3.4.2 Effect of different types of resins on the salt spray resistance
Image of 2K WPU coating on the substrate for different resins including polyacrylic polyol, polyester polyol, and polycarbonate polyol after salt spray for 600 h were presented in Figure 5b Little rust and blister on the surface of coatings based on ES66 and CA50 shows the superiority of polyester and polycarbonate polyol on the
salt spray resistance. Obviously, average rust width along two scribe marks on the coating based on AR01 was larger. Hence, polyacrylic polyol exhibited less barrier to rust accumulation and spreading.
The result was consistent with the prediction according to the hydroxyl content of resins and crosslinking degree of coatings. The OH/CH ratio of polyester polyol ES66, polycarbonate polyol CA50, and polyacrylic polyol AR01 are 1.21, 1.01, and 0.92, respectively. And crosslinking degrees of coatings based on ES66, CA50, and AR01 are 75%, 74%, and 73%, respectively. Accordingly, ES66 and CA50 with high hydroxyl content tended to form a compact PU film with high crosslinking degree, and showed perfect salt spray resistance. Moreover, the strong cohesive energy brought by ester group in ES66 and CA50 is beneficial to the formation of compact and tight PU film and the improvement of salt spray resistance.
3.5 Effect of resins on the wet adhesion
3.5.1 Effect of hydroxyl content in resins on the wet adhesion
The wet adhesion of 2K WPU coating based on polyacrylic polyols with different hydroxyl content were presented in Figure 6a: AR01 > AR70 > AR46 > AR42 > AR27.
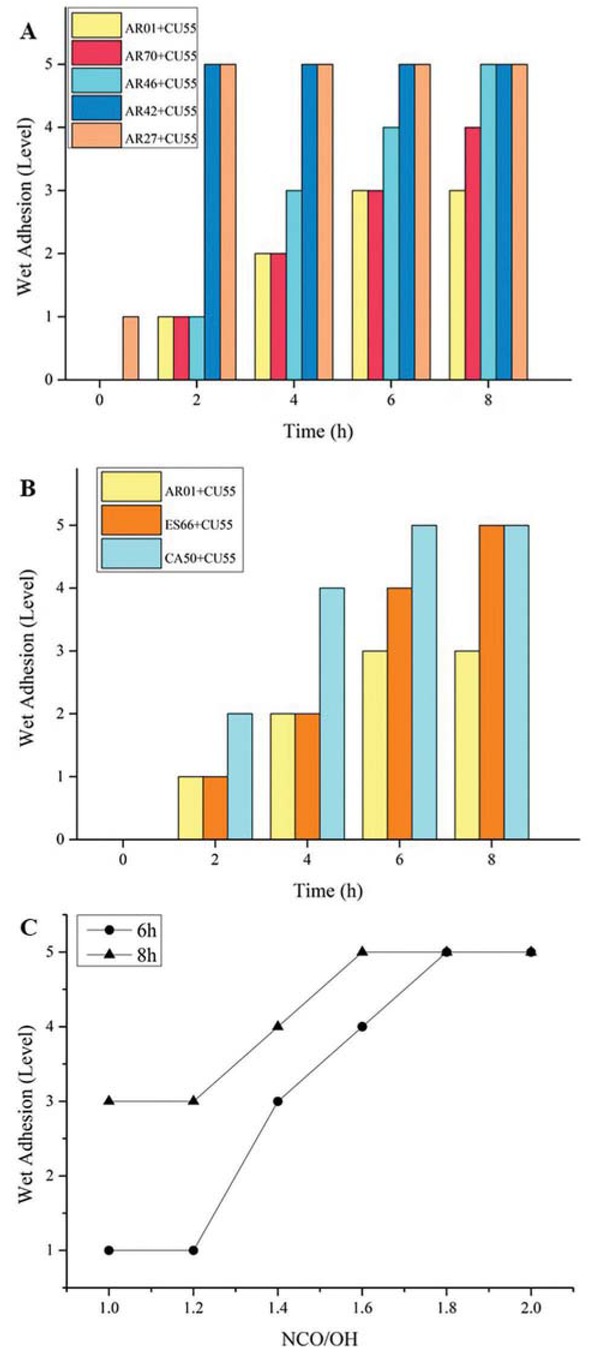
The wet adhesion of 2K WPU coating based on polyacrylic polyols with different hydroxyl content (a), different resins (b), and different NCO/OH (c).
This sequence of coatings based on polyacrylic polyols was consistent with salt spray resistance, which means that wet adhesion is the key property to realize its salt spray resistance for steel panels with x-cut paints. And the wet adhesion of 2K WPU coating always decreases before rusting during salt spray.
Good agreement was found between hydroxyl content in resins, crosslinking degree, and wet adhesion of 2K WPU coating. Resins with more -OH tended to react with -NCO provided by polyisocyanurate to form PU film with higher crosslinking degree. During immersion in water, H2O molecules can diffuse into the interface via some micro defects because some pigment particles cannot be wrapped by enough PU in coatings based on the low hydroxyl content resins such as AR27. And the accumulation of H2O at the interface would form a lateral pressure to weaken the adhesion of coatings. Hence, the compact and tight coatings based on the resin with more hydroxyl groups tend to show more excellent water resistance. AR01 is the best case on the hydroxyl content and wet adhesion of coatings among these polyacrylic polyols. The wet adhesion of 2K WPU coating based on AR01 with the OH/CH of 0.92 was graded as level 3 even if the sample was immersed in water at 40°C for 8 h.
3.5.2 Effect of different types of resins on the wet adhesion
The wet adhesion of 2K WPU coating on the substrate for different resins after immersed in water for several hours was presented in Figure 6b: AR01 > ES66 > CA50. And it is easier for the wet adhesion of coatings based on polyester polyol ES66 and polycarbonate polyol CA50 to deteriorate from level 0 to level 5 than AR01.
Obviously, ES66 and CA50 have a worse wet adhesion than AR01 even though they have more -OH, which proves
the weakness of polyester or polycarbonate polyol on resistance to water even though its salt spray resistance and hydroxyl content was best of them. The wet adhesion is a key property to realize its salt spray resistance of coating, but not the only one factor to decide its performance on salt spray. Because the ester group in these resins is easy to hydrolyze in warm water, PU macromolecular main chains are easy to break accordingly, and then the cohesion and wet adhesion of the PU film wrapping pigment particles will be weakened.
3.5.3 Effect of NCO/OH on the wet adhesion
In practical use, excess polyisocyanurate would be added into coatings so as to consume enough hydroxyl groups in resins because of too much water in 2K WPU coating. According to the reaction mechanism in theory between -OH and -NCO and some engineering practice, the effect of NCO/OH ranging from 1.0 to 2.0 on wet adhesion of coatings was studied in this paper. Figure 6c presents the wet adhesion of 2K WPU coating with different NCO/OH. The wet adhesion of coatings cannot be improved by excess -NCO, and it deteriorated from level 1 or 3 to level 5 with the increase of NCO/OH after immersed in the water at 40°C for 6 h or 8 h.
There are two reasons for effect of NCO/OH on the wet adhesion. On the one hand, the decrease of hydroxyl content in coatings is the main cause for the deterioration of wet adhesion. In general, the adhesion was regarded as a linkage between hydroxyl group of coatings and hydroxyl group of substrates. Addition of -NCO can be mainly used to react with -OH of resin in the first stage, and the rest of -OH has decreased with the increase of NCO/OH accordingly. As a result, there is less and less -OH in 2K WPU coating with the increase of NCO/OH to link with other groups of metal substrates. On the other hand, excess -NCO can react with H2O to generate CO2 and urea without C-O, which was proved by the increase of C=O/C-O in Figure 2. And cracked physical bubbles or other micro defects inside the PU film caused by CO2 in the reaction between -NCO and H2O would form a path for water to permeate into the interface. Furthermore, the accumulation of H2O at the interface would form a lateral pressure to weaken the adhesion of coatings. Therefore, excess -NCO will bring about the deterioration of wet adhesion of 2K WPU coating.
4 Conclusions
In this study, the effect of resins on salt spray resistance and wet adhesion of 2K WPU coating was studied to further provide some theory foundation for selecting resins and optimization of NCO/OH to improve anticorrosive performance of coatings. The results could be concluded as follows.
Resins with more -OH tend to react with polyisocyanurate to form a compact PU film with higher crosslinking degree, which leads to the better wet adhesion and salt spray resistance. The sequence of hydroxyl content in polyacrylic polyols was consistent with their crosslinking degree, salt spray resistance and wet adhesion of coatings.
For commonly used resins, polyester polyol and polycarbonate polyol had better performance than polyacrylic polyol on salt spray resistance due to more hydroxyl and ester groups in resins. The wet adhesion of coatings based on polyester and polycarbonate polyol decreased from level 1 to level 5 with the increase of soak time because of the easily hydrolyzed ester group in resins.
The wet adhesion of 2K WPU coating would decrease from level 1 to level 5 with the increase of NCO/OH. Because there are less -OH in coatings to link with substrates with the increase of NCO/OH. Moreover, cracked physical bubbles inside the film caused by CO2 generated by the reaction between -NCO and H2O would form a path for water to permeate into the interface, which weaken the adhesion of coatings.
Acknowledgments
Thanks to the company Covestro Polymers (China) Co., Ltd. and the employees for supporting this work. This study was finished during the internship in Covestro.
References
1 Sørensen P.A., Kiil S., Dam-Johansen K., Weinell C.E., Anticorrosive coatings: a review. J. Coat. Technol. Res., 2009, 6, 135-176.10.1007/s11998-008-9144-2Suche in Google Scholar
2 Yan J., Shi J.J., Zhang P.G., Tian W.B., Zhang Y.M., Preparation and properties of epoxy/basalt flakes anticorrosive coatings. Mater. Corros., 2018, 69, 1-7.10.1002/maco.201810039Suche in Google Scholar
3 Armelin E., Pla R., Liesa F., Ramis X., Iribarren J.I., Aleman C., Corrosion protection with polyaniline and polypyrrole as anticorrosive additives for epoxy paint. Corros. Sci., 2008, 50, 721-728.10.1016/j.corsci.2007.10.006Suche in Google Scholar
4 Hofland A., Alkyd resins: from down and out to alive and kicking. Prog. Org. Coat., 2012, 73, 274-282.10.1016/j.porgcoat.2011.01.014Suche in Google Scholar
5 Noreen A., Zia K.M., Zuber M., Tabasum S., Saif M.J., Recent trends in environmentally friendly water-borne polyurethane coatings: a review. Korean J. Chem. Eng., 2016, 33, 388-400.10.1007/s11814-015-0241-5Suche in Google Scholar
6 Xu F., Qian B.R., Hu Z., Chen W.D., Zhuang Z.Y., Zhu B.Y., et al., A novel route to emulsifier-free, waterborne hydroxyl functional polyacrylate with low VOC level and its application in 2K-WPU coatings. J. Macromol. Sci. A., 2013, 50, 555-561.10.1080/10601325.2013.781465Suche in Google Scholar
7 Huang H.Y., Huang T.C., Lin J.C., Chang J.H., Lee Y.T., Yeh J.M., Advanced environmentally friendly coatings prepared from amine-capped aniline trimer-based waterborne electroactive polyurethane. Mater. Chem. Phys., 2013, 137, 772-780.10.1016/j.matchemphys.2012.09.063Suche in Google Scholar
8 Kiosidou E.D., Karantonis A., Pantelis D.I., Silva E.R., Bordado J.C.M., Rust morphology characterization of polyurethane and acrylic-based marine antifouling paints after salt spray test on scribed specimens. J. Coat. Technol. Res., 2017, 14, 1381-1395.10.1007/s11998-017-9939-0Suche in Google Scholar
9 Huang M.X., Yang J.L., Salt spray and EIS studies on HDI microcapsule-based self-healing anticorrosive coatings. Prog. Org. Coat., 2014, 77, 168-175.10.1016/j.porgcoat.2013.09.002Suche in Google Scholar
10 Miyauchi K., Takita Y., Yamabe H., Yuasa M., A study of adhesion on stainless steel in an epoxy/dicyandiamide coating system: influence of glass transition temperature on wet adhesion. Prog. Org. Coat., 2016, 99, 302-307.10.1016/j.porgcoat.2016.06.002Suche in Google Scholar
11 Glass P., Chung H., Washburn N.R., Sitti M., Enhanced wet adhesion and shear of elastomeric micro-fiber arrays with mushroom tip geometry and a photopolymerized p(DMA-co-MEA) tip coating. Langmuir, 2010, 26, 17357-17362.10.1021/la1029245Suche in Google Scholar PubMed
12 Wu T.H., Foyet A., Kodentsov A., van der Ven L.G.J., van Benthem R.A.T.M., de With G., Wet adhesion of epoxy-amine coatings on 2024-T3 aluminum alloy. Mater. Chem. Phys., 2014, 145, 342-349.10.1016/j.matchemphys.2014.02.022Suche in Google Scholar
13 Meis N.N.A.H., van der Ven L.G.J., van Benthem R.A.T.M., de With G., Extreme wet adhesion of a novel epoxy-amine coating on aluminum alloy 2024-T3. Prog. Org. Coat., 2014, 77, 176-183.10.1016/j.porgcoat.2013.09.001Suche in Google Scholar
14 Akbarian M., Olya M.E., Mahdavian M., Ataeefard M., Effects of nanoparticulate silver on the corrosion protection performance of polyurethane coatings on mild steel in sodium chloride solution. Prog. Org. Coat., 2014, 77, 1233-1240.10.1016/j.porgcoat.2014.03.023Suche in Google Scholar
15 Cai K.W., Zuo S.X., Luo S.P., Yao C., Liu W.J., Ma J.F., et al., Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv., 2016, 6, 95965-95972.10.1039/C6RA19618GSuche in Google Scholar
16 Rashvand M., Ranjbar Z., Effect of nano-ZnO particles on the corrosion resistance of polyurethane-based waterborne coatings immersed in sodium chloride solution via EIS technique. Prog. Org. Coat., 2017, 76, 1413-1417.10.1016/j.porgcoat.2013.04.013Suche in Google Scholar
17 Li J., Cui J.C., Yang J.Y., Li Y.Y., Qiu H.X., Yang J.H., Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol., 2016, 129, 30-37.10.1016/j.compscitech.2016.04.017Suche in Google Scholar
© 2019 Xu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

