Abstract
It is found that forced (reactive) blending of polystyrene (PS) with polymethylmethacrylate (PMMA) involves the covalent binding of heterogeneous macromolecules to afford the paired polymers. For this purpose, the “anchor” N-H unsubstituted tetrazole or oxirane functional groups are preliminarily introduced in the structure of both polymers in a small amount that leads to a covalent binding of the heterogeneous macromolecules. The reaction between the modified PS and PMMA is carried out in dimethylformamide (DMF), toluene and dichloroethane (DCE) at a high total concentration of polymers (10-20 g dL-1). The process is accompanied by gel-formation to deliver cross-linked paired polymers It is established that the highest rate of the paired polymer is attained in the DCE medium, while the lowest rate is observed in DMF. For paired polymers synthesized in DMF, two glass transition temperatures (Tg) of 92°C and 104°C correspond to the original PS and PMMA, respectively. The products of forced blending of PS and PMMA in toluene have one averaged Tg value (99°C), whereas those obtained in DCE show no pronounced glass transition region at 90 ÷ 115°C. In toluene or DCE, the paired polymers are formed, which represent single-phase systems having one glass transition region.
1 Introduction
The forced or reactive blending of higher molecular compounds is based on the covalent bonding of macromolecules belonging to different, sometimes completely incompatible, polymers (1, 2, 3). Among the forced (reactive) blending materials are block and graft copolymers as well as the paired polymers (4), which differ only in the location of the linkage of heterogeneous macromolecular chains. The widespread block copolymers represent chemically bound (across the terminal fragments) different in nature polymer chains (blocks), which in most cases are thermodynamically incompatible (5). The latter circumstance facilitates the spatial segregation of heterogeneous blocks at the molecular level and promotes microphase separation both in liquid and condensed systems based on the block copolymers to give a variety of spatially-regular nanostructures (6, 7, 8, 9, 10). That is why the block copolymers are found wide application as construction and functional materials, matrices for storing information, and in nanotechnologies (7,10, 11, 12). The uniqueness of structure and properties of the block copolymers is due to the fact that they combine in a one macromolecule the long chain fragments (blocks), which inherent properties of the corresponding homopolymers. There are various approaches to the formation of such block structures, for example, via anionic or pseudo-living radical polymerization, and by the interaction of reactive terminal functional groups belonging to heterogeneous macromolecules (7,9,13).
Another approach to the forced blending of higher molecular compounds is based on the formation of the paired polymers via the covalent binding of heterogeneous macromolecules in the course of the reactions involving their “anchor” functional groups, distributed randomly in chains (4,14). Often, such binding of heterogeneous macromolecules is accompanied by the formation of spatial network structures, so called “conetworks”, which are actually a cross-linked block-copolymer (11,15). Due to thermodynamically unfavorable interactions between chemically different segments, the overlapping of macromolecular coils and their chemical interaction occur in a very restricted space, occupied by a coil. The spatial structure of a paired polymer, similar to a diblock copolymer, can be represented as a segregated conformation, in which chemically different, but covalently bound macromolecular coils occupy separate regions in space (Scheme 1). Thus, the paired polymers, like block copolymers, can combine chain fragments with structures inherent in the initial polymer precursors and, therefore, possess properties characteristic of the block copolymers.
In the present paper, we report a method for the forced (reactive) blending of PS with PMMA via the formation of the paired polymers. Also, some of properties of these polymers are discussed. The N-H unsubstituted tetrazole cycles, contained in the structure of the first polymer, and oxirane moiety, presented in the structure of the second polymer, have been chosen as the “anchor” functional groups facilitating the covalent binding of heterogeneous macromolecules for a short time and under relatively mild conditions. The alkylation of the tetrazole ring with an oxirane-containing reagent (Scheme 2) occurs at temperatures below 100°C and does not require additional initiation, leading to the binding of macromolecules (11).
Another reason for choosing these “anchor” groups is a relatively simple procedure for introduction of small amounts of heterocyclic fragments into the structure of PS and PMMA macromolecules via copolymerization
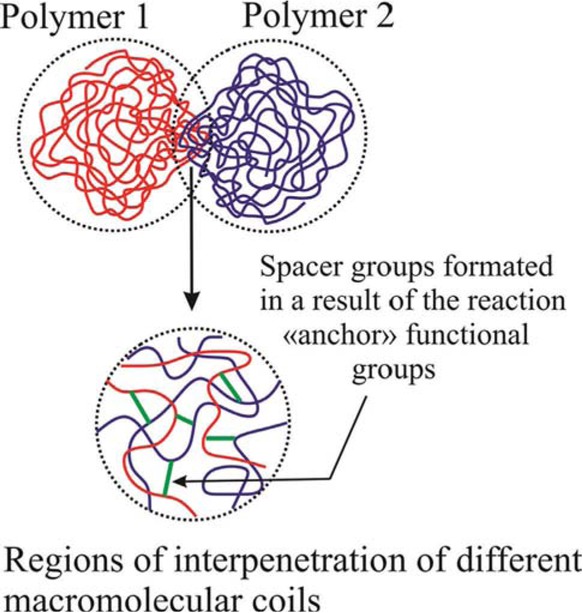
Schematic illustration of paired polymer structure.
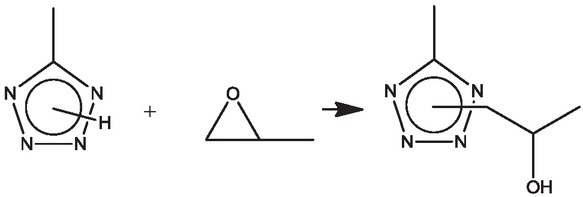
The reaction between tetrazole and oxirane cycles.
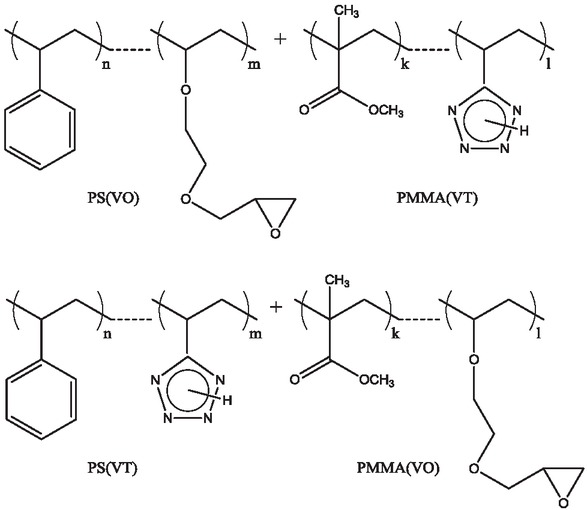
The combination of the polymer reagents.
of styrene or MMA with 5-vinyltetrazole (VT) or 2-(vinyloxyethoxy)methyloxirane (VO), respectively. Moreover, for the forced blending, a double combination of the polymer reagents is used: oxirane-containing PS(VO) - tetrazole-containing PMMA(VT) and, vice versa, tetrazole-containing PS(VT) – oxirane-containing PMMA(VO) (Scheme 3).
2 Experimental
2.1 Materials and physico-chemical characterization
Commercial monomers styrene and MMA (Merck) as well as VO (bp 57°C / 1 mm Hg) and VT (mp 126°C) (manufactured in Russia) were used. Before application, these monomers were rectified or dry distilled in vacuum (VT). The tetrazole or oxirane fragments were introduced into the macromolecular chain of PS and PMMA via co-polymerization of the corresponding pairs of comonomers. The polymerization was carried out using an ampoule method in argon atmosphere under the action of AIBN (0.5 mass% of the weight of monomers) in DMF at 60°C for 48 h. The resulting polymers were precipitated and washed from unreacted monomer residues in the Soxhlet apparatus with diethyl ether (in the case of modified PMMA) or ethanol (in the case of styrene-containing samples), then dried under vacuum to constant weight.
The ratio of the monomer units in the tetrazole-containing polymers was determined by chemical analysis of the N-H acidic tetrazole cycles and elemental analysis, which was performed using the FLASH BA 1112 Series CHN analyzer. The composition of oxirane-containing polymers was established by chemical analysis of the epoxy groups as well as by 1H NMR spectroscopy (for modified PMMA). 1H NMR spectra of copolymer samples dissolved in DMSO d6 were recorded on a Varian NMR Spectrophotometer (500 MHz). The content of monomer units was determined from a ratio of the integrated intensity of the proton signals of the methyl methacrylate units ≥CHCH3 (2.55 ppm) and the oxirane ring ˗O˗CH <(3.75 ppm). All analytical methods gave satisfactory results in evaluation of the copolymers composition. The molecular weights of the copolymers were measured by the GPC method according to polystyrene standards. Chromatographic studies were performed on a Waters GPCV 2000 chromatograph equipped with a refractometer, using a Plgel 5 μm MIXED-C column. DMF was used as an eluent, which was fed with a rate of 1 ml min-1, the chromatography temperature being 70°C. The FTIR spectra of tetrazole-containing PMMA(VT) films were run on an FTIR Fourier spectrometer “Infralum FT-801”. The characteristics of the modified PS and PMMA samples are shown in Table 1.
Characteristics of products of the copolymerization of styrene and methyl methacrylate with 2-(vinyloxyethoxy)methyloxirane (VO) or 5-vinyltetrazole (VT).
| Copolymer | VO or VT Content | Мw | Copolymer Solubility | ||
|---|---|---|---|---|---|
| in Copolymer, (mol. fraction) | (kDa) | DMF | Toluene | DCE | |
| PS(VO) | 0.04 | 136 | Sol. | Sol. | Sol. |
| PS(VO) | 0.10 | 114 | Sol. | Sol. | Sol. |
| PS(VT) | 0.02 | 445 | Sol. | Sol. | Sol. |
| PS(VT) | 0.06 | Sol. | Insol. | Sol. | |
| PS(VT) | 0.11 | Sol. | Insol. | Insol. | |
| PMMA(VO) | 0.05 | 112 | Sol. | Sol. | Sol. |
| PMMA(VO) | 0.07 | 83 | Sol. | Sol. | Sol. |
| PMMA(VO) | 0.09 | 65 | Sol. | Sol. | Sol. |
| PMMA(VT) | 0.02 | 519 | Sol. | Insol. | Insol. |
| PMMA(VT) | 0. 09 | - | Sol. | Insol. | Insol. |
2.2 Synthesis and characterization of the paired polymers
The interaction between PS and PMMA, containing “anchor” tetrazole and oxirane groups, was carried out in DMF, toluene, and DCE at varied temperatures (25-80°C), total polymer concentrations (5-20 g dL-1) and time. To synthesize the paired polymers, preprepared solutions of PS and PMMA were mixed at room temperature in an appropriate solvent. Then the mixture was loaded into ampoules, blown with argon, the ampules were sealed and kept in a thermostat at the given temperature and time. The reaction between the polymers was accompanied by gel formation. The time of the system fluidity loss was fixed visually in two parallel experiments. After completion of the reaction, the resulting gel-like mass was treated with diethyl ether or the solvent was removed in vacuum. In the former case, samples of the paired polymers were obtained as powdered products, while in the latter case, glass-like blocks were formed. Films of polymer mixtures were prepared for different reaction time by casting the reaction mixture up to gel formation on a glass substrate followed by removal of the solvent. The swelling degree of cross-linked paired polymers in various solvents was determined by gravimetric method and was calculated from formula Кsw = (mh − mp) / mp, where mh and mp are the mass of the swollen hydrogel and the dry polymer, respectively. The temperature of the reaction involving polymers and that of the swelling processes was maintained using Memmert air thermostat (thermostat accuracy was ± 0.5°C). Viscosimetric study of the reaction was carried out by stopping the process at different times until the beginning of gel formation, diluting the reaction mass with DMF to a total polymer concentration of 1.0 g dL-1, and measuring the viscosity of the solution. All viscosimetric measurements, including viscosimetric titration of PS solutions with PMMA solutions in DMF, toluene and DCE (polymer concentration 1 g dL-1) were performed using a capillary viscosimeter with a hanging level at 25°C. The additive values of the reduced viscosities of polymer mixture solutions at different ratios were calculated in accordance with the procedure (16). Glass transition temperatures (Tg) of the initial modified PS and PMMA, their mixtures, and the resulting paired polymers were determined using differential scanning calorimetry (DSC) with a STA 449 F3 Jupiter (Netzsch) under nitrogen flow (gas flow rate 5 ml min-1) at a heating rate of 5 deg min-1. For the analysis, carefully dried samples in an amount of 5-10 mg were used.
3 Results and discussion
The abovementioned presence of “anchor” functional groups in the structure of reacting heterogeneous macromolecules is not the only prerequisite for the formation of a pair polymer. The interacting polymers should have a common solvent and should be compatible with it, allowing the preparation of reaction mixtures without phase separation at operating concentrations. In the present paper, for a pair of PS and PMMA from liquids satisfying the indicated condition, DMF, toluene and DCE, which are thermodynamically “good” solvents with respect to both polymers, were chosen (17,18). Introduction of monomer units in amount of 0.04 ÷ 0.10 mol. fraction into the polymer structure does not affect the solubility of PS(VO) and PMMA(VO) as compared to homopolymers (Table 1). The presence of the same amount of “anchor” tetrazole cycles in the structure of the modified polymers restricts a set of the appropriate solvents, especially in the case of PMMA(VT).
The nature of the solvent dramatically influences the compatibility of polymers, and at the molecular level determines the presence of interpenetration regions of heterogeneous macromolecular coils. Figure 1 shows dependencies of the reduced viscosity of the polymer mixture solutions simulating the reaction system, PS(VT) and PMMA(VO) on the ratio of polymer components in DMF, toluene and DCE. In all cases, visual phase separation in the entire range of the component ratios is not detected. However, the results of viscosimetric titration of PMMA solutions with PS solutions indicate that the worst compatibility of polymers is observed in DMF. The experimentally determined reduced viscosities of PS/PMMA mixtures are lower than the calculated additive values obtained for any polymer ratios (Figure 1). This behavior is probably due to the compactification of heterogeneous macromolecular coils upon their simultaneous presence in solution, which should negatively affect the formation of coil interpenetration regions. In toluene and DCE, the experimental and calculated values of the reduced viscosity of the solutions, regardless of the polymer component ratio, coincide, which evidences the absence of conformational “perturbations” in macromolecular coils upon mixing. Consequently, in these solvents the interpenetration of heterogeneous macromolecular coils is more likely to occur.
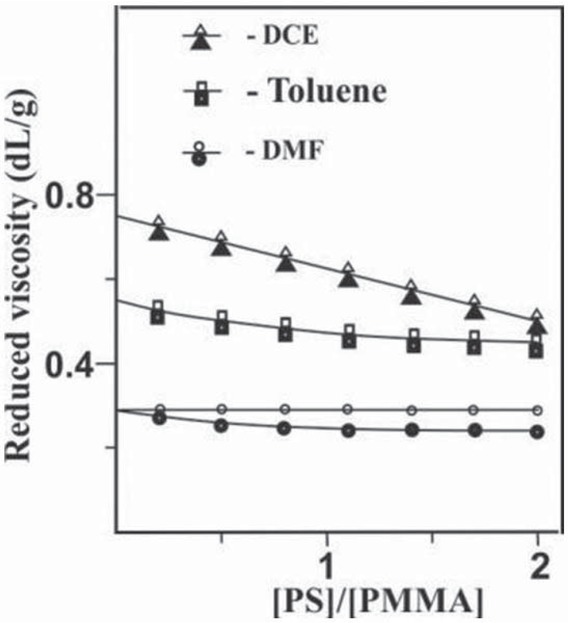
Reduced viscosity of solutions of PS(VT) blends with PMMA (VO) in DMF, Toluene and DCE at 25°C as a function the weight ratio components. (●, ■, ▴) – Experimental dependences and (○, □, Δ) corresponding calculated additivity dependences.
In the systems under study, the structural factor also influences the compatibility of polymers. One of the selected “anchor” functional groups in the polymer structure is the proton-donor tetrazole ring, prone to the formation of hydrogen bonds, including those with the carbonyl group of the ester fragment of the PMMA monomer unit (Scheme 4). The FTIR spectra of PMMA, modified by the introduction of monomer units of VT, contain two bands of stretching vibrations of the C=O group assigned to the free ester carbonyl group at 1729 cm-1 and participating in the formation of hydrogen bonds with a tetrazole ring at 1680 cm-1 (Figure 2). In addition, a broad absorption band with a maximum at 3160 cm-1 corresponds to the stretching vibrations of the N–H bond of the tetrazole
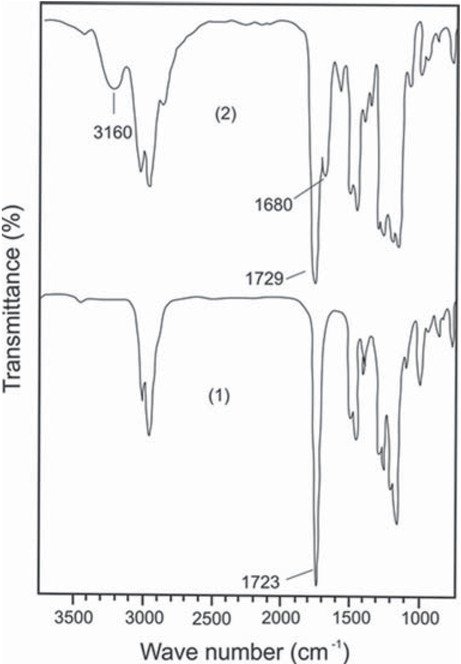
FTIR spectra of PMMA (1) and PMMA, modified by the introduction of monomer units (0.15 mol. fract.) of VT (2).
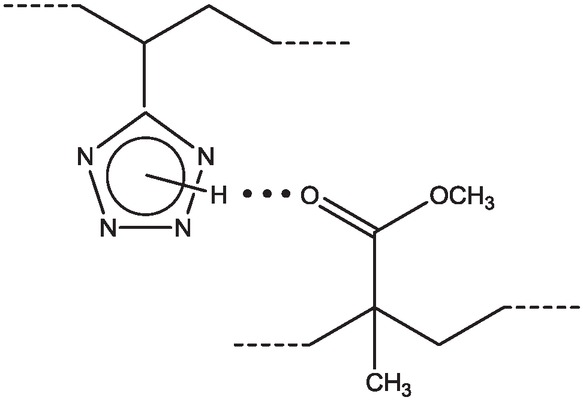
The formation of hydrogen bonds in PMMA(VT) macromolecules.
ring involved in the formation of a hydrogen bond (19). The possibility of hydrogen bonding between structural fragments indicates that the compatibility of PS and PMMA depends on a macromolecule containing the VT “anchor” links. In the case of tetrazole-containing PMMA(VT), internal macromolecular formation of hydrogen bonds takes place, which facilitates the compacting of PMMA coils and the deteriorates its compatibility with PS(VO). In contrast, the interaction between tetrazole-containing PS(VT) and PMMA(VO) leads to formation of intermacromolecular hydrogen bonds, which favor the contact of heterogeneous polymer coils and improve the compatibility of the polymers. Thus, phase separation is observed for the system PS(VO)–PMMA(VT) in DMF with a total polymer concentration of more than 15 g dL-1, while the system PS(VT)–PMMA(VO) remain homogeneous at concentrations above 20 g dL-1.
The reaction between modified PS and PMMA was carried out at a total concentration of polymers of 10-20 g dL-1, i.e. in the concentration range at which there are multiple contacts between polymeric coils forming a spatial network. Therefore, under the selected conditions, the process was accompanied by gel formation to afford the cross-linked products of forced blending of PS with PMMA. In general, the generation of fluctuation networks in the initial solution of polymers mixture is mandatory for the gel formation upon covalent binding of heterogeneous macromolecules containing “anchor” groups. Theoretically, this should be observed in the transition to the moderately concentrated solution (crossover region), which is characterized by a crossover concentration c* = 1.08/[η] (Debye criterion) (20). However, for the system PS(VT)–PMMA(VO) in all three solvents, the value of the minimum total polymer concentration, cgel, at which the interaction of polymers is accompanied by the gel formation, is approximately 2 times higher than the values of c* (Table 2). This fact may indicate that, due to a lower compatibility of PS with PMMA, the formation of a spatial fluctuation network from heterogeneous macromolecules is realized at much higher concentration of polymers.
Critical total polymer concentrations at gelation in the system PS(VT)–PMMA(VO) (1 : 1) at 80°C.
| Polymer characteristic | Solvent | ||
|---|---|---|---|
| DMF | Toluene | DCE | |
| [η]PS (dL g-1) | 0.22 | 0.33 | 0.28 |
| [η]PMMA (dL g-1) | 0.24 | 0.48 | 0.70 |
| [η]ada (dL g-1) | 0.23 | 0.40 | 0.49 |
| с* (g dL-1) | 4.7 | 2.7 | 2.2 |
| сgel (g dL-1) | 10.0 | 7.0 | 5.0 |
a Additivity value for polymer mixture 1 : 1.
Gel formation during the interaction of the modified PS and PMMA is preceded by an induction period, which can last minutes or days, depending on the structure of the selected polymers and the reaction conditions. However, the formation of a paired polymer begins long before the gel point. The increase in viscosity of the reaction system up to the moment of gel formation (Figure 3) indicates the associative processes that can be caused by covalent binding of heterogeneous macromolecular coils. Moreover, for the system PS(VT)–PMMA(VO)–DCE, this process proceeds for abnormally short time. The formation of paired polymers before gel formation is supported also by augmentation of the homogeneity (transparency) of films, poured from the reaction mixture in DMF as duration of the process increases (with an increase in the degree of completion of the reaction between the polymers) (Figure 4). The paired polymer formed in situ in the reaction system, like diblock copolymers (21,22), can act as a compatibilizer, which improves the compatibility of polymer mixtures.
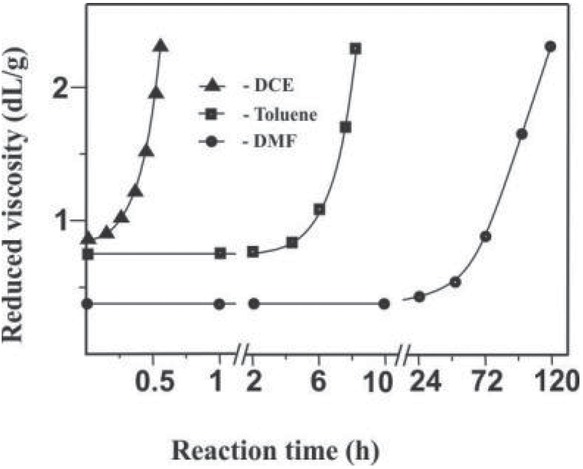
Reduced viscosity of solutions of PS(VT) blends with PMMA (VO) in DMF, Toluene and DCE at 25°C as a function the time of reaction between polymers. Reaction conditions: PS(VT) : PMMA (VO) = 1:1; molar fractions of VT units in PS 0,02 and VO units in PMMA 0,05; T = 80°C.
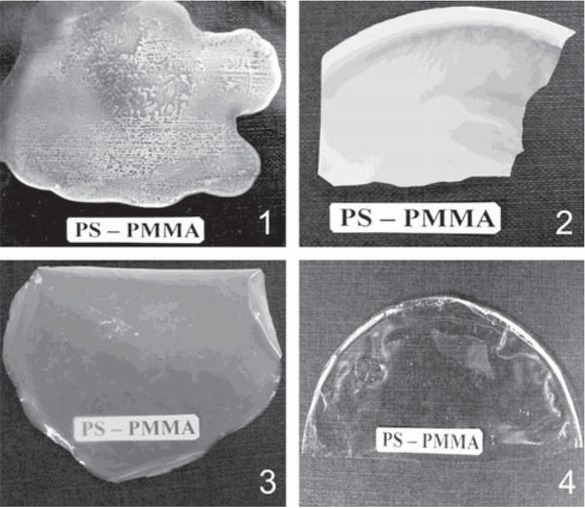
Films based on the PS(VT)–PMMA(VO) mixture (1) and the PS–PMMA paired polymer obtained over 24 (2), 72 (3) and 120 h (4). Reaction conditions: PS(VT) : PMMA (VO) = 1:1; molar fractions of VT units in PS 0,02 and VO units in PMMA 0,05; T = 80°C.
The gel formation in the reaction system is caused by generation of a single spatial network. Time of fluidity loss, τ, allows estimating the effects of various internal (structural) and external (concentration, nature of the solvent) factors on the formation of network paired polymers PS–PMMA. Different positions of “anchor”
tetrazole and oxirane fragments in PS and PMMA macromolecules affect the compatibility of polymers, and, consequently, the regularities of the reaction between polymers. Thus, the fluidity loss times for systems PC(VT, 0.11 mol. fraction)–PMMA(VO, 0.09 mol. fraction) and PS(VO, 0.1 mol. fraction)–PMMA(VT, 0.09 mol. fraction) in DMF at a total polymer concentration of 15 g dL-1 at 80°C are 50 and 96 h, respectively. Therefore, better compatibility of the polymers in the former system
promotes the formation of the network structure of the paired polymer over a shorter period of time. The faster formation of a spatial network is facilitated by an increase in the content of “anchor” groups in PS and PMMA macromolecules (Figure 5), as well as augmentation of total concentration of the polymers and the reaction temperature (Figure 6).
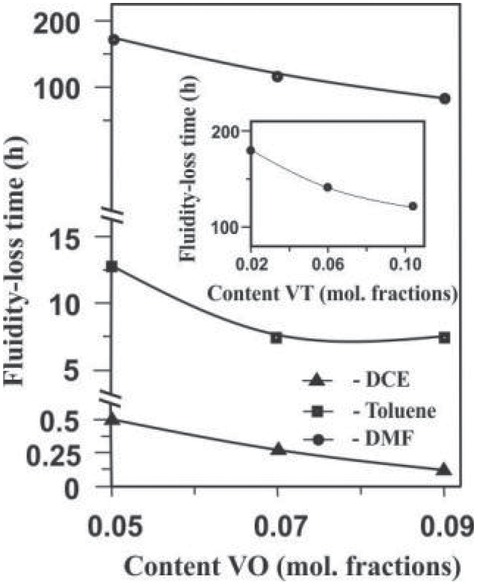
Fluidity-loss time of the PS(VT)–PMMA(VO) reaction system in DMF, Toluene and DCE as a function of molar fractions of VO units in PMMA (VT units in PS 0,02) and VT units in PS (VO units in PMMA 0,05). Reaction conditions: PS(VT) : PMMA (VO) = 1:1; T = 80°C.
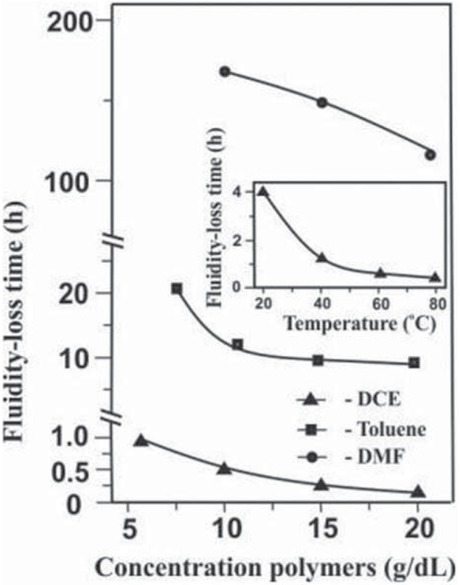
Fluidity-loss time of the PS(VT)–PMMA(VO) reaction system in DMF, Toluene and DCE as a function of total polymer concentration and reaction temperature. Reaction conditions: PS(VT) : PMMA (VO) = 1:1; molar fractions of VT units in PS 0,02 and VO units in PMMA 0,05; T = 80°C.
It is noteworthy that under the same reaction conditions gel formation in DCE occurs for a much shorter time (tens of minutes) than in toluene (hours) or DMF (days), even at lower concentrations of the polymers, process temperature (Figure 6) and different ratios of the reagents (Figure 7). In all systems, the covalent bonding of heterogeneous macromolecules is the result of the same reaction between “anchor” tetrazole and oxirane rings, for which such a dramatic influence of the solvent on the kinetic regularities is not observed (23). Consequently, the most probable cause of higher rates of network paired polymers formation in DCE is the better compatibility of PS and PMMA, which leads to a more pronounced fluctuation network of heterogeneous macromolecular coils in the initial solution.
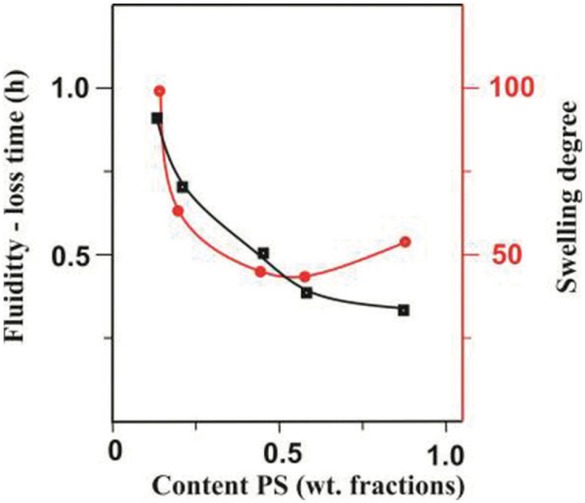
Fluidity-loss time of the PS(VT)–PMMA(VO) reaction system in DCE and degree of swelling of PS–PMMA paired polymers in DCE at 25°C as a function of the weight fraction of PS(VT) in the initial polymer blend. Reaction conditions: molar fractions of VT units in PS 0,02 and VO units in PMMA 0,05; T = 80°C.
Thus, the composition and structure of the polymer reagents as well as the reaction conditions have a significant effect on the forced blending of PS with PMMA. In addition, these factors also affect the properties of the forced blending products including the sorption ability of
network paired polymers towards various liquids to form gels. The quantitative characteristic of the sorption ability (swelling coefficient Ksw) is determined by a degree of heterogeneous polymers binding, which, in turn, depends on the conditions of the paired polymer synthesis. Thus, the better compatibility of polymer reagents in the system PS(VT, 0.11 mol. fraction)–PMMA(VO, 0.09 mol. fraction) as compared to the system PS(VO, 0.1 mol. fraction)– PMMA(VT, 0.09 mol. fraction) promotes to the formation of a more rigid network structure, which is confirmed by the values of Ksw in DMF of the paired polymers obtained under the same conditions (28 and 135, respectively). A solvent, which is employed in reaction between PS and PMMA, should expectedly have a strong effect on sorption properties of the products obtained. For example, for the products synthesized in the system PS(VT, 0.02 mol. fraction)–PMMA(VO, 0.05 mol. fraction) in the presence of different solvents and under similar other conditions, the highest degree of swelling in DMF is observed for a sample obtained in DMF (Ksw 90), while the lowest values is detected in toluene (Ksw 9). Ksw of a paired polymer synthesized in DCE is equal to 45. A similar trend is observed for the sorption properties, when these paired polymers are swelled in toluene or DCE. The presented results are in good agreement with the data obtained by the viscosimetric studies. The worst compatibility of PS and PMMA in DMF hinders the formation of interpenetrating regions of heterogeneous macromolecular coils, which eventually leads to a looser network structure of the paired polymers. In toluene, which ensures better compatibility of PS and PMMA, the reaction between polymers involves the formation of a significantly more rigid network and, correspondingly, the values of Ksw become lower. According to the results of viscosimetric study, the sorption properties of the paired polymers synthesized in DCE and toluene should be comparable. However, a much faster formation of a network structure in DCE is likely accompanied by the formation of a large number of the network defects that improves sorption characteristics of the pair polymers. The effect of such factors as the content of “anchor” tetrazole and oxirane fragments in the structure of PS and PMMA macromolecules, the value of the total concentration and the ratio of polymer reagents (Figure 8) on the sorption properties of the paired polymers is generally symbate to their effects on the time characteristics of the gel formation process occurring in the reaction systems. An increase of the anchor groups in polymers and augmentation of the reagents concentration accelerate the formation of a more rigid network structure, which is expressed in a decrease of the Ksw values of the paired polymers.
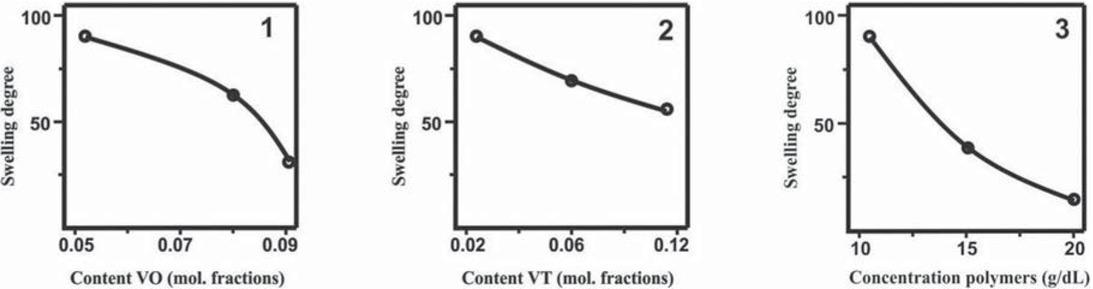
Degree of swelling of PS–PMMA paired polymers in DMF at 25°C as a function of molar fractions of VO units in PMMA (VT units in PS 0,02) (1), VT units in PS (VO units in PMMA 0,05) (2) and total polymer concentration (3). Reaction conditions: PS(VT) : PMMA (VO) = 1:1; T = 80°C.
During the forced blending of PS with PMMA in DMF, toluene or DCE, the reaction systems are converted into transparent gels without visible signs of phase separation. The composition of the isolated network paired polymers is similar to that of the initial polymer reagents; hence,
quantitative binding of heterogeneous polymers occurs. However, the nature of the reaction medium influences the microphase state of the forced blending products, which affects the glass transition temperatures of the paired polymers (Table 3). Model mixtures of the forced blending components, PS(VT, 0.02 mol. fraction)–PMMA(VO, 0.05 mol. fraction) were prepared as films from solutions in DMF, toluene or DCE with a total polymer concentration of 10 g/dL. Unlike the heterogeneous mixture formed after removal of DMF, the polymer film obtained from solutions in toluene or DCE are transparent without signs of phase separation. Nevertheless, in all three cases, two glass transition temperatures (Tg) corresponding to the initial PS(VT) and PMMA (VO) are observed. A similar result is obtained for a paired polymer synthesized in DMF, while the forced blending products of PS with PMMA in toluene are characterized by a single average value of Tg, and those prepared in DCE show no pronounced glass transition region at 90-115°C.
Glass transition temperature for mixture and PS–PMMA paired polymers.
| Sample | Solvent | Tg (°C) |
|---|---|---|
| PS(VT, 0.02 mol. fract.) | 96 | |
| PMMA(VO, 0.05 mol. fract.) | 115 | |
| Mixture PS(VT)–PMMA(VO) | DMF | 95 and 110 |
| Mixture PS(VT)–PMMA(VO) | Toluene | 96 and 115 |
| Mixture PS(VT)–PMMA(VO) | DCE | 94 and 112 |
| Paired polymer PS(VT)–PMMA(VO) | DMF | 92 and 104 |
| Paired polymer PS(VT)–PMMA(VO) | Toluene | 99 |
| Paired polymer PS(VT)–PMMA(VO) | DCE | - |
This result is in agreement with the conclusions on the effect of the medium nature on the forced blending of polymers made on the basis of viscosimetry of initial polymer solutions and studying the regularities of the paired polymers formation in the PS–PMMA system. The worst compatibility of PS and PMMA in DMF leads to the formation of a supramolecular structure of the paired polymers with a pronounced microphase separation. The presence of two glass transition temperatures (inherent in the initial components) in the paired polymer is explained by relatively large structural regions of similar polymers. In toluene or DCE promoting the compatibility of PS and PMMA, the paired polymers with a higher degree of heterogeneous macromolecules binding are formed. These polymers, therefore, represent already single-phase systems with only one glass transition temperature. An increase in the degree of macromolecules binding in DMF using a PS with a large content of “anchor” tetrazole fragments (0.11 mol. fraction) or an increase in the total concentration of polymer reagents (up to 20 g dL-1) also results in the formation of more structurally homogeneous paired polymers, characterized by a single glass transition region at 96-98°C.
4 Conclusions
Block copolymers of PS with PMMA provoke research interest not only as model systems for the study of self-organization processes in polymer systems, but also as building blocks for the synthesis of a variety of multifaceted polymeric materials (24, 25, 26, 27, 28, 29, 30, 31). In the present paper, we have reported another variant of combination in a one polymeric structure of two thermodynamically unblended macrochain fragments via a process called forced blending of polymers to afford the paired polymers. The obtained results show that the formation of paired polymers as well as the structure and properties of the forced blending products strongly depend on the nature of the reaction medium. Reacting polymers should have appropriate compatibility, which should facilitate the formation of interpenetration regions of heterogeneous macromolecular coils, where their covalent binding occurs. It is the nature of the chosen solvent that determines the compatibility of polymers in the initial reaction mixture and, as a consequence, the degree of binding of heterogeneous macromolecules. Thus, the forced blending of PS with PMMA in solutions of DMF, toluene or DCE (other conditions being the same) occurs most rapidly in the presence of the latter solvent. It should be noted that the paired polymers represent the most structurally homogeneous, single-phase systems. On the other hand, the paired polymers of PS with PMMA obtained in DMF probably give supramolecular structures with more pronounced segregated regions formed via self-organization of similar macromolecules. Consequently, the products of forced blending should possess individual properties of the initial polymers.
Acknowledgments
This work was supported by the Russian Fond Basic Researches and Government of Irkutsk Region (17-43-380005).
References
1 Utracki L.A., Polymer Alloys and Blends: Thermodynamics and Rheology. Hanser Publication, Munich, 1989.Suche in Google Scholar
2 Xanthos M., Dagli S.S., Compatibilization of polymer blends by reactive processing. Polym. Eng. Sci., 1991, 31(13), 929-935.10.1002/pen.760311302Suche in Google Scholar
3 Brown S.B., In: Xanthos M. (Ed.), Reactive Extrusion: Principles and Practice, Ch. 4. Hanser Publication, Munich, 1992.Suche in Google Scholar
4 Askadskii A.A., The effect of strong intermolecular and chemical interaction on the miscibility of polymers. Russ. Chem. Rev., 1999, 68(4), 317-331.10.1070/RC1999v068n04ABEH000503Suche in Google Scholar
5 Bates F.S., Fredrickson G.H., Block copolymers-designer soft materials. Phys. Today, 1999, 52(2), 32-38.10.1063/1.882522Suche in Google Scholar
6 Riess G., Micellization of block copolymers. Prog. Polym. Sci., 2003, 28(7), 1107-1170.10.1016/S0079-6700(03)00015-7Suche in Google Scholar
7 Hadjichristidis N., Pispas S., Floudas G., Block Copolymers: Synthesis Strategies, Physical Properties, and Applications. Wiley, Hoboken, 2003.10.1002/0471269808Suche in Google Scholar
8 Hamley W., Developments in Block Copolymer Science and Technology. Wiley, Hoboken, 2004.10.1002/0470093943Suche in Google Scholar
9 Bussels R., (Multi)block Copolymer Synthesis via Controlled Radical Polymerization in Aqueous Dispersions. Technishe Universiteit Eindhoven, Eindhoven, 2004.Suche in Google Scholar
10 Smart T., Lomas H., Massignani M., Flores-Merino M.V., Perez L.R., Battaglia G., Block copolymer nanostructures. Nano Today, 2008, 3(3-4), 38-46.10.1016/S1748-0132(08)70043-4Suche in Google Scholar
11 Krishnamoorthy S., Hinderling C., Heinzelmann H., Nanoscale patterning with block copolymers. Mater. Today, 2006, 9(9), 40-47.10.1016/S1369-7021(06)71621-2Suche in Google Scholar
12 Ballauff M., Spherical polyelectrolyte brushes. Prog. Polym. Sci., 2007, 32(10), 1135-1151.10.1002/3527603824.ch12Suche in Google Scholar
13 Zheltonozhskaya T.B., Fedorchuk S.V., Syromyatnikov V.G., Processes for obtaining linear block copolymers. Russ. Chem. Rev., 2007, 76(8), 731-765.10.1070/RC2007v076n08ABEH003696Suche in Google Scholar
14 Kizhnyaev V.N., Pokatilov F.A., Zhitov R.G., Proidakov A.G., Krakhotkina E.A., Forced Blending of Poly(5-vinyltetrazole) with Vinyl Polymers. Polym. Sci. Ser. B+, 2015, 57(5), 504-511.10.1134/S1560090415050073Suche in Google Scholar
15 Erdodi G., Kennedy J.P., Amphiphilic conetworks: Definition, synthesis, applications. Progr. Polym. Sci., 2006, 31(1), 1-18.10.1016/j.progpolymsci.2005.11.001Suche in Google Scholar
16 Bel’nikevich N.G., Budtova T.V., Nikolaeva O.V., Vesnebolotskaya S.A., The correctness of using the viscometric method as a test on interpolymer complex formation in polymer mixtures. Polym. Sci. Ser. B+, 2002, 44(1-2), 27-31.Suche in Google Scholar
17 Nesterov A.E., Reference Book of Polymer Physical Chemistry. Properties of Polymer Solutions and blends. Naukova dumka, Kiev, 1984.Suche in Google Scholar
18 Gantchev B., Mihailov M., Single phase polystyrene-polyacrylonitrile-dimethylformamide system studied by light scattering. Polym. Bull., 1987, 17(2), 181-187.10.1007/BF00256886Suche in Google Scholar
19 Kizhnyaev V.N., Vereshchagin L.I., Vinyltetrazoles: Synthesis and properties. Russ. Chem. Rev., 2003, 72(2), 143-164.10.1070/RC2003v072n02ABEH000731Suche in Google Scholar
20 Bartenev G.M., Frenkel S.Y., Physics of Polymers. Khimiya, Leningrad, 1990.Suche in Google Scholar
21 Macosko C.W., Jeon H.R., Hoye T.R., Reactions at polymer-polymer interfaces for blend compatibilization. Prog. Polym. Sci., 2005, 30(8-9), 939-947.10.1016/j.progpolymsci.2005.06.003Suche in Google Scholar
22 Plate N.A., Litmanovich A.D., Kudryavtsev Y.V., Macromolecular Reactions in Polymer Melts and Blends. Nauka, Moscow, 2008.Suche in Google Scholar
23 Golobokova T.V., Vereshchagin L.I., Ratovskii G.V., Proidakov A.G., Kizhnyaev V.N., Synthesis of N-(oxiran-2-ylmethyl)-5-phenyltetrazole and its reactions with nitrogen nucleophiles. Russ. J. Org. Chem., 2016, 52(7), 1039-1042.10.1134/S1070428016070216Suche in Google Scholar
24 Kent M.S., Tirrell M., Lodge T.P., Properties of polystyrene‐ poly(methylmethacrylate) random and diblock copolymers in dilute and semidilute solutions. J. Polym. Sci. Part B+, 1994, 32(11), 1927-1941.10.1002/polb.1994.090321110Suche in Google Scholar
25 Yoon B.K., Hwang W., Park Y.J., Hwang J., Park Ch., Chang J., Direct Patterning of Self Assembled Nano-Structures of Block Copolymers via Electron Beam Lithography. Macromol. Res., 2005, 13(5), 435-440.10.1007/BF03218477Suche in Google Scholar
26 Hershkovits E., Tannenbaum A., Tannenbaum R., Adsorption of block copolymers from selective solvents on curved surfaces. Macromolecules, 2008, 41(9), 3190-3198.10.1021/ma702706pSuche in Google Scholar PubMed PubMed Central
27 Nicaise S.M., Chemical and Physical Methods of the Templated Direction of Block Copolymers. Massachusetts Institute of Technology, Massachusetts, 2012.Suche in Google Scholar
28 Gottlieb S., Lorenzoni M., Evangelio L., Fernández-Regúlez M., Ryu Y.K., Rawlings C., et al., Thermal scanning probe lithography for the directed self-assembly of block copolymers. Nanotechnology, 2017, 28(17), 175301.10.1088/1361-6528/aa673cSuche in Google Scholar PubMed
29 Seguini G., Zanenga F., Giammaria T.J., Ceresoli M., Sparnacci K., Antonioli D., et al., Enhanced Lateral Ordering in Cylinder Forming PS-b-PMMA Block Copolymers Exploiting the Entrapped Solvent. ACS Appl. Mater. Inter., 2016, 8(12), 8280-8288.10.1021/acsami.6b00360Suche in Google Scholar PubMed
30 Giammaria T.J., Ferrarese F., Stguini G., Sparnacci K., Antonioli D., Gianotti V., et al., Effect of Entrapped Solvent on the Evolution of Lateral Order in Self-Assembled P(S-r-MMA)/PS-b-PMMA Systems with Different Thicknesses. ACS Appl. Mater. Inter., 2017, 9(37), 31215-31223.10.1021/acsami.6b14332Suche in Google Scholar PubMed
31 Miyazoe H., Jagtiani A.V., Tsai H.-Y., Engelmann S.U., Joseph E.A., Highly selective dry etching of polystyrene-poly(methyl methacrylate) block copolymer by gas pulsing carbon monoxide-based plasmas. J. Phys. D. Appl. Phys., 2017, 50(20), 204001.10.1088/1361-6463/aa68c6Suche in Google Scholar
© 2019 Kizhnyaev et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Artikel in diesem Heft
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

