Abstract
C46H38N10S2, triclinic,
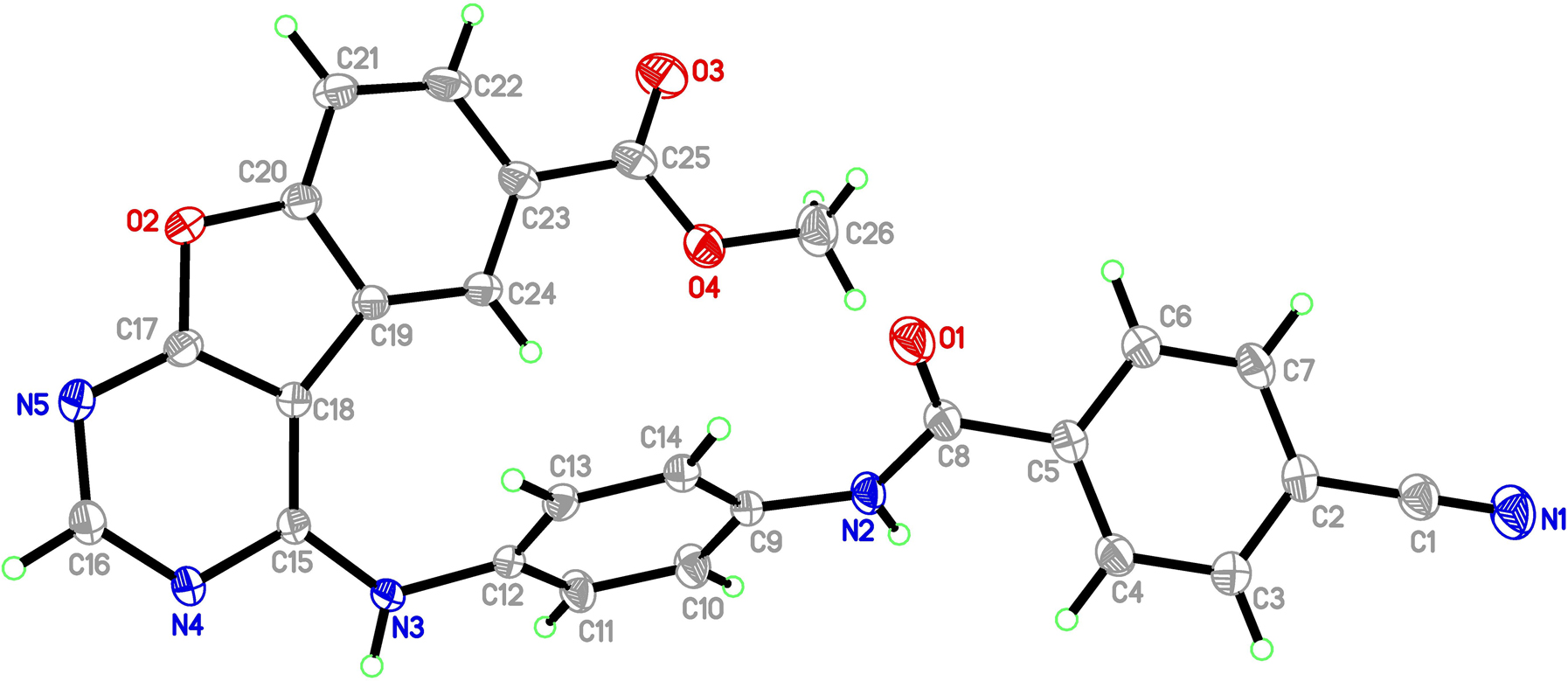
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.26 × 0.23 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 7604, 3800, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2865 |
| N(param)refined: | 317 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2075 (3) | 0.9636 (2) | 0.48804 (17) | 0.0437 (5) |
| C2 | 0.2247 (3) | 0.85162 (19) | 0.52004 (16) | 0.0384 (5) |
| C3 | 0.1096 (3) | 0.7784 (2) | 0.57219 (16) | 0.0409 (5) |
| H3 | 0.0218 | 0.8009 | 0.5863 | 0.049* |
| C4 | 0.1272 (2) | 0.6717 (2) | 0.60290 (16) | 0.0399 (5) |
| H4 | 0.0517 | 0.6233 | 0.6391 | 0.048* |
| C5 | 0.2565 (2) | 0.63591 (19) | 0.58034 (15) | 0.0338 (4) |
| C6 | 0.3689 (2) | 0.7075 (2) | 0.52555 (16) | 0.0398 (5) |
| H6 | 0.4535 | 0.6820 | 0.5081 | 0.048* |
| C7 | 0.3545 (3) | 0.8161 (2) | 0.49726 (16) | 0.0421 (5) |
| H7 | 0.4317 | 0.8659 | 0.4628 | 0.050* |
| C8 | 0.2721 (2) | 0.5168 (2) | 0.60996 (16) | 0.0352 (4) |
| C9 | 0.2368 (2) | 0.41242 (18) | 0.76391 (15) | 0.0306 (4) |
| C10 | 0.2532 (3) | 0.4488 (2) | 0.87935 (16) | 0.0390 (5) |
| H10 | 0.2806 | 0.5402 | 0.9207 | 0.047* |
| C11 | 0.2291 (3) | 0.35066 (19) | 0.93276 (16) | 0.0386 (5) |
| H11 | 0.2416 | 0.3764 | 1.0100 | 0.046* |
| C12 | 0.1865 (2) | 0.21431 (18) | 0.87256 (15) | 0.0299 (4) |
| C13 | 0.1700 (2) | 0.17780 (18) | 0.75761 (15) | 0.0308 (4) |
| H13 | 0.1401 | 0.0858 | 0.7165 | 0.037* |
| C14 | 0.1974 (2) | 0.27580 (18) | 0.70327 (15) | 0.0309 (4) |
| H14 | 0.1894 | 0.2505 | 0.6264 | 0.037* |
| C15 | 0.2079 (2) | 0.01272 (18) | 0.91373 (14) | 0.0285 (4) |
| C16 | 0.1607 (2) | −0.20249 (19) | 0.93002 (15) | 0.0363 (5) |
| H16 | 0.0955 | −0.2762 | 0.9522 | 0.044* |
| C17 | 0.3759 (2) | −0.10197 (19) | 0.86383 (14) | 0.0312 (4) |
| C18 | 0.3500 (2) | 0.01644 (18) | 0.87303 (14) | 0.0273 (4) |
| C19 | 0.4905 (2) | 0.10971 (18) | 0.83988 (14) | 0.0289 (4) |
| C20 | 0.5858 (2) | 0.03535 (19) | 0.81361 (15) | 0.0338 (4) |
| C21 | 0.7340 (2) | 0.0853 (2) | 0.78159 (17) | 0.0419 (5) |
| H21 | 0.7941 | 0.0326 | 0.7659 | 0.050* |
| C22 | 0.7884 (2) | 0.2173 (2) | 0.77403 (16) | 0.0419 (5) |
| H22 | 0.8884 | 0.2553 | 0.7532 | 0.050* |
| C23 | 0.6967 (2) | 0.2958 (2) | 0.79697 (15) | 0.0352 (5) |
| C24 | 0.5483 (2) | 0.24295 (19) | 0.83136 (14) | 0.0310 (4) |
| H24 | 0.4889 | 0.2960 | 0.8483 | 0.037* |
| C25 | 0.7596 (2) | 0.4320 (2) | 0.77734 (16) | 0.0407 (5) |
| C26 | 0.6936 (3) | 0.6120 (2) | 0.7482 (2) | 0.0580 (6) |
| H26A | 0.8013 | 0.6784 | 0.7937 | 0.087* |
| H26B | 0.6130 | 0.6483 | 0.7622 | 0.087* |
| H26C | 0.6971 | 0.5940 | 0.6706 | 0.087* |
| N1 | 0.1922 (3) | 1.0516 (2) | 0.46162 (16) | 0.0588 (5) |
| N2 | 0.2515 (2) | 0.51604 (15) | 0.71448 (13) | 0.0361 (4) |
| H2 | 0.2468 | 0.5890 | 0.7560 | 0.043* |
| N3 | 0.15244 (19) | 0.11254 (15) | 0.92789 (13) | 0.0363 (4) |
| H3A | 0.0904 | 0.1148 | 0.9750 | 0.044* |
| N4 | 0.11724 (18) | −0.09785 (15) | 0.94456 (13) | 0.0326 (4) |
| N5 | 0.2863 (2) | −0.21529 (16) | 0.88753 (13) | 0.0361 (4) |
| O1 | 0.30078 (19) | 0.42996 (15) | 0.54474 (12) | 0.0510 (4) |
| O2 | 0.51634 (16) | −0.09451 (13) | 0.82680 (11) | 0.0377 (3) |
| O3 | 0.89676 (19) | 0.49024 (17) | 0.76328 (16) | 0.0688 (5) |
| O4 | 0.64762 (17) | 0.48432 (14) | 0.77534 (12) | 0.0472 (4) |
Source of material
The methyl 4-[(4-aminophenyl)amino]benzofuro[2,3-d] pyrimidine-6-carboxylate 7 (2.0 g, 6.0 mmol), 4-cyanobenzoic acid (1.15 g, 7.8 mmol), HATU (systematic name : 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate; 2.96 g, 7.8 mmol) and Et3N (1.2 g, 12.0 mmol) were stirred together in dimethylformamide (15 mL) at room temperature for 7 h. Upon completion of the reaction, the mixture was diluted with saturated sodium carbonate solution (50 mL) and subsequently extracted with dichloromethane. The extracts were combined, washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. Purification by silica-gel column chromatography provided 2.13 g the desired product. After slowly evaporating the solvents for several days, some light yellow single crystals were obtained.
Experimental details
The structure was solved by direct methods and refined with the SHELX crystallographic software package [1]. The hydrogen atoms were placed at calculated positions and refined as riding atoms.
Comment
Cancer is one of the most health problems in the world; although many classes of drugs were used for the treatment, the needs for safe and effective anticancer compounds are still significant target. Heterocyclic building blocks represent useful scaffolds for applications in medicinal chemistry. To our knowledge, 4-anilinopyrimidine derivatives often have a wide range of biological activities, especially in antitumor drugs [4, 5]. Some of them have been developed as the important prescription antitumor drugs in the market, for example, Pazopanib [6], Ceritinib [7], Brigatinib [8], afatinib [9] and rociletinib [10] are all based on 4-anilinopyrimidine.
The title molecule (see the Figure) has a benzofuro[2,3-d]pyrimidine skeleton, in which all bond lengths and bond angles fall in normal ranges [11]. The dihedral angles between benzofuro[2,3-d]pyrimidine and two phenyl rings are 62.46° and 20.25° respectively. And the two phenyl planes are not coplanar with a dihedral angle of 64.08°. Furthermore, a mass of the intermolecular hydrogen bonds N(3)–H(3A) ⃛ N(4), N(3)–H(3A) ⃛ N(4), C(14)–H(14) ⃛ N(1), C(4)–H(4) ⃛ O(3) and C(11)–H(11) ⃛ O(4) are found in the title structure. It is worth noting that the crystal packing is further stabilized by weak p-p interactions (3.5142 Å). These interactions together with intermolecular hydrogen bond result in the formation of a three-dimensional framework.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlispro Software system (version 1.171.35.15); Agilent Technologies UK Ltd: Oxford, UK, 2011.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Abd, E. H. S. R., Lasheen, D. S., Hassan, M. A., Abouzid, K. A. M. Design and synthesis of 4-anilinothieno[2,3-d]pyrimidine-based compounds as dual EGFR/HER-2 inhibitors. Arch. Pharm. 2016, 349, 827–847; https://doi.org/10.1002/ardp.201600197.Search in Google Scholar PubMed
5. Ding, H.-G., Cai, Z.-Q., Hou, L., Hu, Z.-Q., Jin, Z.-S., Xu, D., Cao, H., Meng, M.-M., Xie, Y.-H., Zheng, D.-Q. Synthesis and evaluation of some novel 6-substituted quinazoline derivatives as antitumor agents. J. Chem. Soc. Pakistan 2014, 87, 782–793.Search in Google Scholar
6. Ghith, A., Youssef, K. M., Ismail, N. S. M., Abouzid, K. A. M. Design, synthesis and molecular modeling study of certain VEGFR-2 inhibitors based on thienopyrimidne scaffold as cancer targeting agents. Bioorg. Chem. 2019, 83, 111–128; https://doi.org/10.1016/j.bioorg.2018.10.008.Search in Google Scholar PubMed
7. Sailaja, T. G. G., Laxminarayanaa, E., Chary, M. T., Ramesh, M. Synthesis and antibacterial activity of novel 4-{4-(methylamino)thieno[3,2-d]pyrimidin-2-yl}-benzohydrazide derivatives. Russ. J. Gen. Chem. 2017, 87, 1275–1280.10.1134/S1070363217060238Search in Google Scholar
8. Mohan, C., Bhargava, G., Bedi, P. M. S. Thieno[3,2-d] pyrimidin-4-one derivatives as potential antibacterial agents. J. Life Sci. 2009, 2, 97–101; https://doi.org/10.1080/09751270.2009.11885139.Search in Google Scholar
9. Kang, D.-W., Fang, Z.-G., Li, Z.-Y., Huang, B.-S., Zhang, H., Lu, X.-Y., Xu, H.-R., Zhou, Z.-G., Ding, X., Daelemans, D., Clercq, E. D., Pannecouque, C., Zhan, P., Liu, X.-Y. Design, synthesis, and evaluation of thiophene[3,2-d] pyrimidine derivatives as HIV 1 non-nucleoside reverse transcriptase inhibitors with significantly improved drug resistance profiles. J. Med. Chem. 2016, 59, 7991–8007; https://doi.org/10.1021/acs.jmedchem.6b00738.Search in Google Scholar PubMed
10. Kang, D.-W., Fang, Z.-G., Huang, B.-S., Lu, X.-Y., Zhang, H., Xu, H.-R., Huo, Z.-P., Zhou, Z.-G., Yu, Z., Meng, Q., Wu, G.-C., Ding, X., Tian, Y., Daelemans, D., Clercq, E. D., Pannecouque, C., Zhan, P., Liu, X.-Y. Structure-based optimization of thiophene[3,2-d]pyrimidine derivatives as potent HIV 1 non-nucleoside reverse transcriptase inhibitors with improved potency against resistance-associated variants. J. Med. Chem. 2017, 60, 4424–4443; https://doi.org/10.1021/acs.jmedchem.7b00332.Search in Google Scholar PubMed
11. Sheng, J., Liu, Z., Yan, M., Zhang, X., Wang, D., Xu, J., Zhang, E., Zou, Y. Biomass-involved synthesis of N-substituted benzofuro[2,3-d] pyrimidine-4-amines and biological evaluation as novel EGFR tyrosine kinase inhibitors. Org. Biomol. Chem. 2017, 15, 4971–4977; https://doi.org/10.1039/c7ob00793k.Search in Google Scholar PubMed
© 2022 Pei-Xuan Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27

