Abstract
C24H22N8O8ClFe, triclinic, P1̄ (no. 2), a = 10.4568(4) Å, b = 10.5977(4) Å, c = 12.0793(6) Å, α = 82.119(4)°, β = 83.277(4)°, γ = 87.206(3)°, Z = 2, V = 1316.11(9) Å3, Rgt(F) = 0.0565, wRref(F2) = 0.1609, T = 173(10) K.
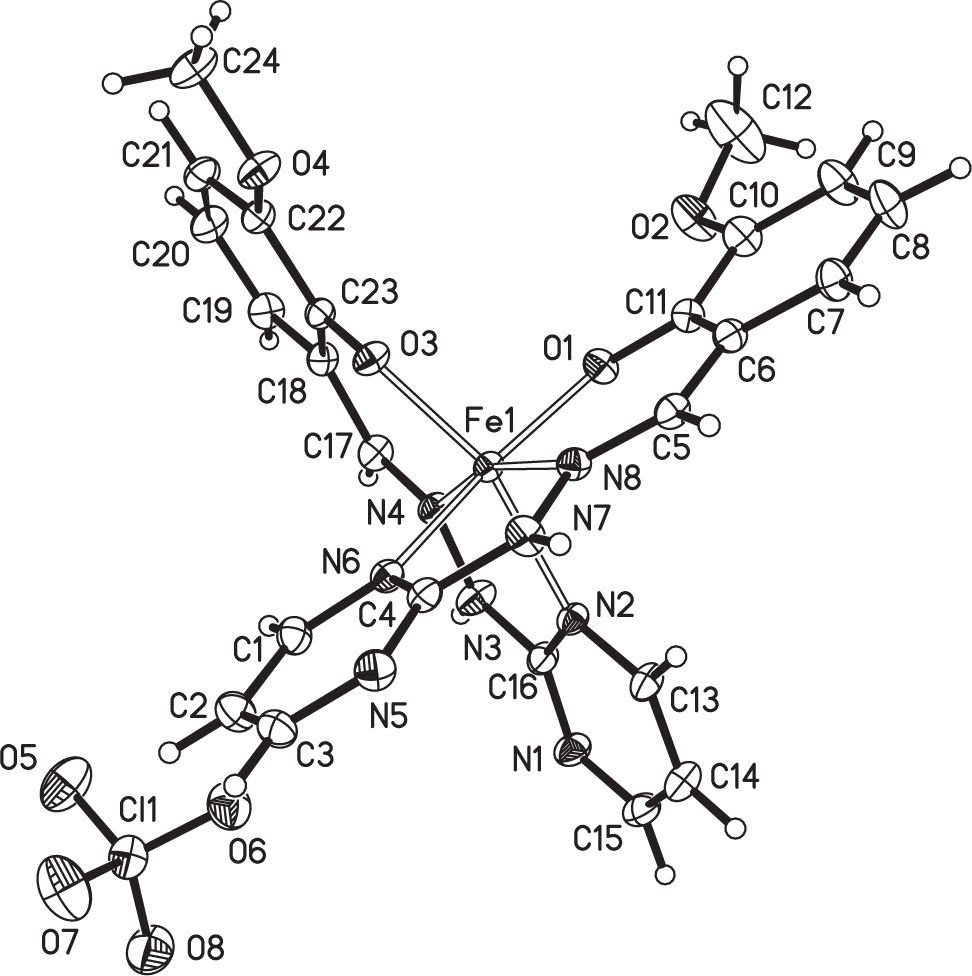
The crystal structure is shown in the figure. Tables 1 and 2 contain details on crystal structure and measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Brown cube |
| Size: | 0.40 × 0.20 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 6.11 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 70.0°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 8616, 4936, 0.048 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4310 |
| N(param)refined: | 381 |
| Programs: | CrysAlisPRO [1], OLEX2 [2], SHELX [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Fe1 | 0.47233(4) | 0.44331(4) | 0.27461(4) | 0.02041(17) |
| O1 | 0.6275(2) | 0.5249(2) | 0.21089(19) | 0.0276(5) |
| O2 | 0.7826(3) | 0.6853(3) | 0.0807(2) | 0.0468(7) |
| O3 | 0.3741(2) | 0.5990(2) | 0.29073(19) | 0.0264(5) |
| O4 | 0.3119(3) | 0.8236(2) | 0.3545(2) | 0.0344(6) |
| N1 | 0.4693(3) | 0.1274(3) | 0.0816(3) | 0.0344(7) |
| N2 | 0.5179(3) | 0.2576(3) | 0.2185(2) | 0.0249(5) |
| N3 | 0.4027(3) | 0.3376(3) | 0.0720(3) | 0.0343(7) |
| H3 | 0.3735 | 0.3336 | 0.0071 | 0.041* |
| N4 | 0.3861(3) | 0.4463(3) | 0.1234(2) | 0.0274(6) |
| N5 | 0.3177(3) | 0.2196(3) | 0.5844(3) | 0.0329(6) |
| N6 | 0.3371(3) | 0.3399(3) | 0.4000(2) | 0.0249(5) |
| N7 | 0.5050(3) | 0.3162(3) | 0.5079(2) | 0.0297(6) |
| H7 | 0.5407 | 0.2869 | 0.5692 | 0.036* |
| N8 | 0.5729(3) | 0.3888(3) | 0.4189(2) | 0.0243(5) |
| C1 | 0.2156(3) | 0.3109(3) | 0.3893(3) | 0.0304(7) |
| H1 | 0.1801 | 0.3413 | 0.3213 | 0.036* |
| C2 | 0.1418(4) | 0.2389(4) | 0.4732(3) | 0.0369(8) |
| H2 | 0.0558 | 0.2198 | 0.4651 | 0.044* |
| C3 | 0.1974(4) | 0.1947(4) | 0.5708(3) | 0.0341(8) |
| H3A | 0.1476 | 0.1447 | 0.6303 | 0.041* |
| C4 | 0.3817(3) | 0.2916(3) | 0.4977(3) | 0.0259(6) |
| C5 | 0.6923(3) | 0.4075(3) | 0.4293(3) | 0.0255(6) |
| H5 | 0.7255 | 0.3684 | 0.4961 | 0.031* |
| C6 | 0.7772(3) | 0.4818(3) | 0.3488(3) | 0.0261(6) |
| C7 | 0.9023(4) | 0.4978(4) | 0.3761(3) | 0.0339(8) |
| H7A | 0.9279 | 0.4552 | 0.4448 | 0.041* |
| C8 | 0.9872(4) | 0.5731(4) | 0.3058(4) | 0.0424(9) |
| H8 | 1.0719 | 0.5811 | 0.3246 | 0.051* |
| C9 | 0.9494(4) | 0.6386(4) | 0.2059(4) | 0.0428(9) |
| H9 | 1.0073 | 0.6941 | 0.1584 | 0.051* |
| C10 | 0.8279(4) | 0.6230(4) | 0.1756(3) | 0.0350(8) |
| C11 | 0.7404(3) | 0.5408(3) | 0.2444(3) | 0.0250(6) |
| C12 | 0.8610(6) | 0.7842(7) | 0.0182(5) | 0.086(2) |
| H12A | 0.8139 | 0.8309 | −0.0410 | 0.130* |
| H12B | 0.9408 | 0.7462 | −0.0159 | 0.130* |
| H12C | 0.8819 | 0.8430 | 0.0690 | 0.130* |
| C13 | 0.5836(3) | 0.1573(3) | 0.2692(3) | 0.0314(7) |
| H13 | 0.6247 | 0.1675 | 0.3335 | 0.038* |
| C14 | 0.5926(4) | 0.0412(4) | 0.2302(3) | 0.0363(8) |
| H14 | 0.6377 | −0.0294 | 0.2670 | 0.044* |
| C15 | 0.5339(4) | 0.0307(4) | 0.1357(4) | 0.0372(8) |
| H15 | 0.5396 | −0.0491 | 0.1076 | 0.045* |
| C16 | 0.4660(3) | 0.2367(3) | 0.1259(3) | 0.0284(7) |
| C17 | 0.3286(3) | 0.5428(3) | 0.0685(3) | 0.0297(7) |
| H17 | 0.3045 | 0.5332 | −0.0033 | 0.036* |
| C18 | 0.2996(3) | 0.6618(3) | 0.1094(3) | 0.0263(7) |
| C19 | 0.2387(4) | 0.7578(4) | 0.0379(3) | 0.0323(7) |
| H19 | 0.2234 | 0.7415 | −0.0348 | 0.039* |
| C20 | 0.2018(4) | 0.8721(4) | 0.0711(3) | 0.0353(8) |
| H20 | 0.1612 | 0.9351 | 0.0218 | 0.042* |
| C21 | 0.2234(4) | 0.8980(3) | 0.1782(3) | 0.0326(7) |
| H21 | 0.1962 | 0.9779 | 0.2018 | 0.039* |
| C22 | 0.2848(3) | 0.8066(3) | 0.2499(3) | 0.0266(7) |
| C23 | 0.3224(3) | 0.6854(3) | 0.2177(3) | 0.0235(6) |
| C24 | 0.2742(5) | 0.9434(4) | 0.3923(3) | 0.0417(9) |
| H24A | 0.3149 | 1.0123 | 0.3395 | 0.063* |
| H24B | 0.3014 | 0.9448 | 0.4670 | 0.063* |
| H24C | 0.1803 | 0.9552 | 0.3962 | 0.063* |
| Cl1 | −0.06798(9) | 0.14698(10) | 0.25431(8) | 0.0403(2) |
| O5 | −0.1315(4) | 0.2575(4) | 0.2010(3) | 0.0650(10) |
| O6 | 0.0664(3) | 0.1687(3) | 0.2515(3) | 0.0525(8) |
| O7 | −0.1251(4) | 0.1236(5) | 0.3686(3) | 0.0733(12) |
| O8 | −0.0846(4) | 0.0407(4) | 0.1951(4) | 0.0660(10) |
Source of material
All starting materials are commercially available, and are used without further purification. 2-Hydrazinopyrimidine and 2-methoxy-6-(pyrimidin-2-ylhydrazonomethyl)-phenol (H2L) were prepared following reported procedures [5]. Perchlorate salts of metal complexes with organic ligands are potentially explosive and should be handled in small quantities with care.
The title compound [Fe(L)2]ClO4 was obtained according to the following procedure. 2-Methoxy-6-(pyrimidin-2-ylhydrazonomethyl)-phenol (0.1 mmol, 21.4 mg) was dissolved in the methanol (15 mL) under stirring, and then filtered to give a light yellow solution. Afterwards, Fe(ClO4)2⋅6 H2O (0.05 mmol, 18.1 mg) in deionized water (10 mL) was slowly diffused to the above solution in a sealed monotube. After several days, brown cube-shaped crystals were obtained. Yield: ca. 80%.
Experimental details
The H atoms were added using riding models. Their Uiso values were set to 1.2Ueq of the parent C and N atoms, and 1.5Ueq of the parent O atoms.
Comment
Pyrimidine derivatives are important pharmaceutical intermediates, because of the antitumor, bactericidal, and plant growth promoting properties [6], [7]. After coordination to metal ions, pyrimidine derivatives can not only enhance the activity of the original drugs but also reduce their toxicity and side effects [8]. Many Schiff bases have antibacterial and antitumor activities that can be enhanced after forming complexes with transition metals. The Schiff base complexes have strong cell penetration and fat solubility, which makes the antibacterial range of the complexes expand, and it is not easy to produce drug resistance [9], [10], [11], [12].
The title complex is a mononuclear iron(III) complex. The iron(III) ion in [Fe(L)2]+ is hexa-coordinated by four nitrogen atoms and two oxygen atoms from two ligands to form a N4O2 octahedral configuration. The Fe—N and Fe—O bond distances are in the range of 2.125(3)–2.182(3) Å, and 1.906(2)–1.921(2) Å, respectively. There is apparent intermolecular hydrogen bonding interaction between the methoxy oxygen and the amide H—N group (O2⋯N3 = 2.866(4) Å, O4⋯N7 = 2.901(4) Å). The hydrogen bonds link adjacent molecules into a 1D supramolecular chain structure.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: U1504829
Funding statement: This work was supported by the National Natural Science Foundation of China (Project No. U1504829), the project of scientific and technological in Henan Province (Project No. 172102310433), the key scientific research project of colleges and universities in Henan Province (Project No. 16A350006) and the Provincial special Scientific Research Fund (Project No. 2014KYYWF-ZZCX3-06).
References
Agilent Technologies: CrysAlis PRO Software system, version 1.171.38.43f, Rigaku Oxford Diffraction (2015).Suche in Google Scholar
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar
Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar
Yuan, J.; Chu, Y.-X.; Kou, H.-Z.: Synthesis, crystal structures and magnetic properties of methoxo-bridged dinuclear MnIII complexes derived from tri-dentate chelating ligands. J. Coord. Chem. 69 (2016) 1218–1225.10.1080/00958972.2016.1166217Suche in Google Scholar
Anneheim-Herbelin, G.; Perrèe-Fauvet, M.; Gaudemer, A.; Helissey, P.; Giorgi-Renault, S.: Porphyrin-netropsin: a potential ligand of DNA. Tetrahedron 34 (1993) 7263–7266.10.1016/S0040-4039(00)79304-XSuche in Google Scholar
Li, B.; Lin, B.-D.; Liu, C.-L.; Liu, W.-C.: Synthesis and fungicidal activities of 2-substituted amino-4,6-dichloropyrimidines. Chin. J. Synth. Chem. 4 (1996) 176–179.Suche in Google Scholar
Zhang, P.-Z.; Wu, J.; Gong, Y.-Q.: Synthesis and bioactivity of pyrimidine complexes with Cu(II) and Zn(II). Chin. J. Appl. Chem. 17 (2000) 558–560.Suche in Google Scholar
Liu, Y. C.; Yang, Z. Y.: Antioxidation and DNA-binding properties of binuclear Er(III) complexes with Schiff-base ligands derived from 8-hydroxyquinoline-2-carboxyaldehyde and four aroylhydrazides. Inorg. Chem. Commun. 12 (2009) 704–706.10.1016/j.inoche.2009.05.020Suche in Google Scholar
Creaven, B. S.; Duff, B.; Egan, D. A.; Kavanagh, K.; Rosair, G.; Thangella, V. R.; Walsh, M.: Anticancer and antifungal activity of copper(II) complexes of quinolin-2(1H)-one-derived Schiff bases. Inorg. Chim. Acta 363 (2010) 4048–4058.10.1016/j.ica.2010.08.009Suche in Google Scholar
Chakraborty, A.; Kumar, P.; Ghosh, K.; Roy, P.: Evaluation of a Schiff base copper complex compound as potent anticancer molecule with multiple targets of action. Eur. J. Pharmacol. 647 (2010) 1–12.10.1016/j.ejphar.2010.08.003Suche in Google Scholar PubMed
Qiao, X.; Ma, Z. Y.; Xie, C. Z.; Xue, F.; Zhang, Y. W.; Xu, J. Y.; Qiang, Z. Y.; Lou, J. S.; Chen, G. J.; Yan, S. P.: Study on potential antitumor mechanism of a novel Schiff base copper(III) complex: synthesis, crystal structure, DNA binding, cytotoxicity and apoptosis induction activity. J. Inorg. Biochem. 105 (2011) 728–737.10.1016/j.jinorgbio.2011.01.004Suche in Google Scholar PubMed
©2018 Jun-Ying Song et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of bis(6,6′-((ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2,4-diiodophenolato)-κ4N,N′,O,O′)cer(IV), C32H20CeI8N4O4
- Crystal structure of (6,6′-((ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato-κ2N,O))copper(II), C16H12Cl2CuN2O2
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)samarium(III)], C26H18N2O9Sm
- Crystal structure of 1-{4-[(2-hydroxy-5-nitrobenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15N3O4
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis(μ2-1-(8-(2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)naphthalen-2-olato-κ6O:O,N,N′,O′:O′)trizinc(II), C44H38Zn3N4O12
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)–bis(μ2–(8-(2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)naphthalen-2-olato-κ6O:O,N,N′,O′:O′)tricobalt(II), C44H38Co3N4O12
- Crystal structure of poly[aqua(μ4-2,2′,2′′-nitrilotriacetato-κ6O1,O3,O5:O2:O4:O6)-yttrium(III)], C6H8NO7Y
- Crystal structure of catena-poly[bromido-(μ2-pyrazine-2-carboxylato-κ3N:N′,O)copper(II)], C10H6N4Cu2O4Br
- Crystal structure of poly[dodekais(μ2-2,6-difluorobenzoato-κ2O:O′)-bis((μ3-hexamethylenetetramine-κ3N:N′:N′′)hexacopper(II)], C48H30N4O12F12Cu3
- Crystal structure of methyl 4-(4-hydroxy-3-nitrophenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H18N2O6
- Crystal structure of N-phenyl-2-(propan-2-ylidene)hydrazine-1-carbothioamide, C10H13N3S
- Crystal structure of sodium caesium zinc phosphate, NaCsZnP2O7
- Crystal structure of methyl 4-(4-isopropylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C23H29NO3
- Crystal structure of catena-poly[bis(μ2-1,3-di(4H-1,2,4-triazol-4-yl)benzene-κ2N:N′)-bis(thiocyanato-κ1N)cobalt(II), C22H16CoN14S2
- Crystal structure of catena-poly[(μ2-2,5-di(pyridin-3-yl)-1,3,4-thiadiazole-κ2N:N′)-bis(2,5-di(pyridin-3-yl)-1,3,4-thiadiazole-κ1N)-bis(thiocyanato-κ1N)cobalt(II)], C38H24CoN14S5
- Crystal structure of bis(2,5-di(pyridin-2-yl)-1,3,4-thiadiazole-κ2N,N′)-bis(thiocyanato-κ1N)cobalt(II), C26H16CoN10S4
- Crystal structure of (bis((1H-benzo[d]imidazol2-yl)methyl)amine-κ3N,N′,N′′)-(pyridine-2,6-dicarboxylato-κ3N,O,O′)zinc(II) — methanol — water (1/2/1), C25H28N6O7Zn
- Crystal structure of catena-poly[triaqua-(μ2-5-methoxy-isophthalato-κ2O:O′)copper(II)], C9H12CuO8
- Structure and photochromism of 1-[2-methyl-5-phenyl-3-thienyl]-2-[2-methyl-5-(4-chlorophenyl)-3-thienyl]3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16ClF6S2
- Crystal structure of methyl 4-(3-phenoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C26H27NO4
- Crystal structure of poly[diaqua-di-μ2-hydroxido-(μ4-3,4,5,6-tetrafluoro-1,2-phthalato-κ4O:O′:O′′:O′′′)-(μ4-3,4,5,6-tetrafluoro-1,2-phthalato-κ5O,O:O′:O′′:O′′′)disamarium(III)] – bipyridine (2/1), C21H11NF16O12Sm2
- Crystal structural of diethyl 4-(3,5-dibromo-4-hydroxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C19H21Br2NO5
- Crystal structure of catena-poly[tetraaqua(μ2-4-(1H-imidazol-1-yl)pyridine-κ2N:N′))zinc(II)] thiophene-2,5-dicarboxylate, C14H17N3O8ZnS
- Crystal structure of (20S)-20-(dimethylamino)-3-(tigloylamino)-5α-pregn-2,16-dien-4-one, C28H42N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-diiodosalicylidene)-1,2-cyclohexanediamine, C20H18I4N2O2
- Crystal structure of 2-bromo-7-(4-(dimethylamino)phenyl)-10-methylacridin-9 (10H)-one, C22H19BrN2O
- Crystal structure of catena-poly[(μ2-pentane-1,5-dicarboxylato-κ2O:O′)-(μ2-2-[(1H-imidazol-1-yl)methyl]-6-methyl-1H-benzimidazole-κ2N:N′)zinc(II)] sesquihydrate, C17H21N4O5.5Zn
- Crystal structure of ethyl 2-amino-4-(3,4-difluorophenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C21H15F2NO5
- Crystal structure of 9-(5-methylthiophen-2-yl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione, C18H18O3S
- Crystal structure of [5,5′-((propane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-2-ylidene))bis(3-(ethoxycarbonyl)-2,4-dimethylpyrrol-1-ido)-κ4N,N′,N′′,N′′′]nickel(II), C23H30N4O4Ni
- Crystal structure of (Z)-2-(2-(1,3-dioxo-1-(phenylamino)butan-2-ylidene)hydrazineyl) terephthalic acid-dimethylsulfoxide (1/1), C18H15N3O6 ⋅ C2H6OS
- Crystal structure of (2E,4Z)-dimethyl 4-((phenylamino)methylene)pent-2-enedioate, C14H15N1O4
- Crystal structure of (2Z,2′Z)-1,1′-(pyridine-2,6-diyl) bis(3-hydroxy-3-phenylprop-2-en-1-one), C23H17NO4
- Crystal structure of ethyl 2-amino-4-(3-bromophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C21H16BrNO5
- Crystal structure of bis(μ2-3,5-dichloro-2-oxidobenzoato-κ4O,O′:O′,O′′)-hexakis(μ2-pivalato-κ2O:O′)-bis(pivalato-κ2O,O′)-tetrakis(1,10-phenanthroline-κ2N,N′)tetragadolinium(III), C102H108Cl4Gd4N8O22
- Crystal structure of catena-poly[aqua-bis(formato-κ1O)-(μ2-1,1′-(oxybis(1,4-phenylene))-bis(1H-1,2,4-triazole)-κ2N:N′)copper(II)]hydrate, C18H16CuN6O6
- Crystal structure of 5-bromo-2-(naphthalen-6-yl)pyridine, C15H10BrN
- Crystal structure of N-(4-methoxybenzyl)pyridazin-3-amine- a rare Z′ = 4 structure, C12H13N3O
- Crystal structure of bis(perchlorato-κ1O)-bis(3,4,5-trimethoxy-N-(pyridin-2-yl)benzamide-κ2N,O)copper(II), C32H30Cl2CuN4O16
- Crystal structure of methyl 4-methyl-2,5-di(pyridin-4-yl)-1H-pyrrole-3-carboxylate monohydrate C17H15N3O2⋅H2O
- Crystal structure of dimethyl 5-(10-(methoxycarbonyl)anthracen-9-yl) isophthalate,C26H20O6
- Crystal structure of tert-butyl (R)-(1-(benzylamino)-3-methoxy-1-oxopropan-2-yl)carbamate, C16H24N2O4
- Crystal structure of aqua-bis(1,5-dimethyl-2-phenyl-4-(((E)-4-pyridylmethylene)amino)pyrazolidin-3-one-κN)-(nitrato-κO)-(nitrato-κ2O,O′)zinc(II), C34H34N10O9Zn
- Crystal structure of catena-poly[dichlorido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)cobalt(II)] – methanol (1/1), C18H20Cl2CoN4O2
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)]bis(2-carboxybenzoate) dihydrate, MnC40H40N10O12
- The crystal structure of 4-((3,4-dichlorobenzylidene)amino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one, C18H15Cl2N3O
- Crystal structure of bis(2-methoxy-6-((2-(pyrimidin-2-yl)hydrazono)methyl)phenolato-κ3N,N′,O)iron(III) perchlorate, C24H22N8O8ClFe
- The crystal structure of 2-[4-hydroxy-3-methoxyphenyl]-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl, C14H19N2O4
- Crystal structure of diaqua-bis(3,3-dimethylacrylato-κ2O,O′)zinc(II), C10H18ZnO6
- Crystal structure of dichloro-tetrakis[(E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-ol-κN]cadmium (II), C60H74CdCl6N12O4
- Crystal structure of (20R)-20,25-epoxy-dammaran-3,12-dione, C30H48O3
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-((pyridin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C32H31N5O2
- The crystal structure of 2-(4-fluorophenyl)-1,3,4-oxadiazole, C8H5FN2O
- Crystal structure of (2,2′-bipyridine-κ2N,N′)bis(tri(p-tolyl)phosphine-κP)copper(I) tetrafluoroborate – 4,4′-bipyridine (2/1), C57H54BCuF4N3P2
- The crystal structure of 2,6-dimethyl-3,5-dinitrocyclohexa-2,5-diene-1,4-dione, C8H6N2O6
- The crystal structure of 2,3-dimethyl-1,4-dinitrobenzene – a Z′ = 4 structure, C8H8N2O4
- Crystal structure of [(1,2-η)-1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl] (1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-κ6N2,O4) rubidium (I), [Rb(diaza-18-crown-6)]Cp*, C22H41N2O4Rb
- Crystal structure of 2-(4-fluorophenyl)-N-phenyl-2-(phenylamino)ethanesulfonamide – toluene (1/0.5), C23.5H23FN2O2S
- Crystal structure of pyrene-4-aldehyde, C17H10O
- Crystal structure of 2-(furan-2-yl)-5-methyl-1,3-dioxane-5-carboxylic acid, C10H12O5
- Crystal structure of 2-(4-chlorophenyl)-3-phenyl-1,8-naphthyridine, C20H13N2Cl
- Crystal structure and photochromism of 1-(2-ethyl-5-formylthiophen-3yl)-2-(2-cyano-1,5-dimethyl-4-pyrrl)-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C19H14F6N2OS
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16BrIN2
- Crystal structure of 2-(4-(dimethylamino)phenyl)-10-methylacridin-9(10H)-one, C22H20N2O
- Crystal structure of 4-(acetoxymethyl)-6-(3-acetyl-3-(4-fluorophenyl)thioureido)cyclohex-4-ene-1,2,3-triyl triacetate, C24H26FN2O9S
Artikel in diesem Heft
- Cover and Frontmatter
- Crystal structure of bis(6,6′-((ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2,4-diiodophenolato)-κ4N,N′,O,O′)cer(IV), C32H20CeI8N4O4
- Crystal structure of (6,6′-((ethane-1,2-diylbis(azanylylidene))bis(methanylylidene))bis(2-chlorophenolato-κ2N,O))copper(II), C16H12Cl2CuN2O2
- Crystal structure of poly[aqua-(μ3-4-(pyridin-4-yl)isophthalato-κ4O,O′:O′′:O′′′)-(μ2-5-carboxy-2-(pyridin-4-yl)benzoato-κ2O:O′)samarium(III)], C26H18N2O9Sm
- Crystal structure of 1-{4-[(2-hydroxy-5-nitrobenzylidene)amino]phenyl}ethanone O-methyl oxime, C16H15N3O4
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)-bis(μ2-1-(8-(2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)naphthalen-2-olato-κ6O:O,N,N′,O′:O′)trizinc(II), C44H38Zn3N4O12
- Synthesis and crystal structure of bis(μ2-acetato-κ2O:O′)–bis(μ2–(8-(2-oxidophenyl)-3,6-dioxa-2,7-diazaocta-1,7-dien-1-yl)naphthalen-2-olato-κ6O:O,N,N′,O′:O′)tricobalt(II), C44H38Co3N4O12
- Crystal structure of poly[aqua(μ4-2,2′,2′′-nitrilotriacetato-κ6O1,O3,O5:O2:O4:O6)-yttrium(III)], C6H8NO7Y
- Crystal structure of catena-poly[bromido-(μ2-pyrazine-2-carboxylato-κ3N:N′,O)copper(II)], C10H6N4Cu2O4Br
- Crystal structure of poly[dodekais(μ2-2,6-difluorobenzoato-κ2O:O′)-bis((μ3-hexamethylenetetramine-κ3N:N′:N′′)hexacopper(II)], C48H30N4O12F12Cu3
- Crystal structure of methyl 4-(4-hydroxy-3-nitrophenyl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C18H18N2O6
- Crystal structure of N-phenyl-2-(propan-2-ylidene)hydrazine-1-carbothioamide, C10H13N3S
- Crystal structure of sodium caesium zinc phosphate, NaCsZnP2O7
- Crystal structure of methyl 4-(4-isopropylphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxylate, C23H29NO3
- Crystal structure of catena-poly[bis(μ2-1,3-di(4H-1,2,4-triazol-4-yl)benzene-κ2N:N′)-bis(thiocyanato-κ1N)cobalt(II), C22H16CoN14S2
- Crystal structure of catena-poly[(μ2-2,5-di(pyridin-3-yl)-1,3,4-thiadiazole-κ2N:N′)-bis(2,5-di(pyridin-3-yl)-1,3,4-thiadiazole-κ1N)-bis(thiocyanato-κ1N)cobalt(II)], C38H24CoN14S5
- Crystal structure of bis(2,5-di(pyridin-2-yl)-1,3,4-thiadiazole-κ2N,N′)-bis(thiocyanato-κ1N)cobalt(II), C26H16CoN10S4
- Crystal structure of (bis((1H-benzo[d]imidazol2-yl)methyl)amine-κ3N,N′,N′′)-(pyridine-2,6-dicarboxylato-κ3N,O,O′)zinc(II) — methanol — water (1/2/1), C25H28N6O7Zn
- Crystal structure of catena-poly[triaqua-(μ2-5-methoxy-isophthalato-κ2O:O′)copper(II)], C9H12CuO8
- Structure and photochromism of 1-[2-methyl-5-phenyl-3-thienyl]-2-[2-methyl-5-(4-chlorophenyl)-3-thienyl]3,3,4,4,5,5-hexafluorocyclopent-1-ene, C27H16ClF6S2
- Crystal structure of methyl 4-(3-phenoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate, C26H27NO4
- Crystal structure of poly[diaqua-di-μ2-hydroxido-(μ4-3,4,5,6-tetrafluoro-1,2-phthalato-κ4O:O′:O′′:O′′′)-(μ4-3,4,5,6-tetrafluoro-1,2-phthalato-κ5O,O:O′:O′′:O′′′)disamarium(III)] – bipyridine (2/1), C21H11NF16O12Sm2
- Crystal structural of diethyl 4-(3,5-dibromo-4-hydroxyphenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate, C19H21Br2NO5
- Crystal structure of catena-poly[tetraaqua(μ2-4-(1H-imidazol-1-yl)pyridine-κ2N:N′))zinc(II)] thiophene-2,5-dicarboxylate, C14H17N3O8ZnS
- Crystal structure of (20S)-20-(dimethylamino)-3-(tigloylamino)-5α-pregn-2,16-dien-4-one, C28H42N2O2
- Crystal structure of rac-trans-N,N′-bis(3,5-diiodosalicylidene)-1,2-cyclohexanediamine, C20H18I4N2O2
- Crystal structure of 2-bromo-7-(4-(dimethylamino)phenyl)-10-methylacridin-9 (10H)-one, C22H19BrN2O
- Crystal structure of catena-poly[(μ2-pentane-1,5-dicarboxylato-κ2O:O′)-(μ2-2-[(1H-imidazol-1-yl)methyl]-6-methyl-1H-benzimidazole-κ2N:N′)zinc(II)] sesquihydrate, C17H21N4O5.5Zn
- Crystal structure of ethyl 2-amino-4-(3,4-difluorophenyl)-5-oxo-4H,5H-pyrano[3,2-c] chromene-3-carboxylate, C21H15F2NO5
- Crystal structure of 9-(5-methylthiophen-2-yl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione, C18H18O3S
- Crystal structure of [5,5′-((propane-1,3-diylbis(azanylylidene))bis(ethan-1-yl-2-ylidene))bis(3-(ethoxycarbonyl)-2,4-dimethylpyrrol-1-ido)-κ4N,N′,N′′,N′′′]nickel(II), C23H30N4O4Ni
- Crystal structure of (Z)-2-(2-(1,3-dioxo-1-(phenylamino)butan-2-ylidene)hydrazineyl) terephthalic acid-dimethylsulfoxide (1/1), C18H15N3O6 ⋅ C2H6OS
- Crystal structure of (2E,4Z)-dimethyl 4-((phenylamino)methylene)pent-2-enedioate, C14H15N1O4
- Crystal structure of (2Z,2′Z)-1,1′-(pyridine-2,6-diyl) bis(3-hydroxy-3-phenylprop-2-en-1-one), C23H17NO4
- Crystal structure of ethyl 2-amino-4-(3-bromophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carboxylate, C21H16BrNO5
- Crystal structure of bis(μ2-3,5-dichloro-2-oxidobenzoato-κ4O,O′:O′,O′′)-hexakis(μ2-pivalato-κ2O:O′)-bis(pivalato-κ2O,O′)-tetrakis(1,10-phenanthroline-κ2N,N′)tetragadolinium(III), C102H108Cl4Gd4N8O22
- Crystal structure of catena-poly[aqua-bis(formato-κ1O)-(μ2-1,1′-(oxybis(1,4-phenylene))-bis(1H-1,2,4-triazole)-κ2N:N′)copper(II)]hydrate, C18H16CuN6O6
- Crystal structure of 5-bromo-2-(naphthalen-6-yl)pyridine, C15H10BrN
- Crystal structure of N-(4-methoxybenzyl)pyridazin-3-amine- a rare Z′ = 4 structure, C12H13N3O
- Crystal structure of bis(perchlorato-κ1O)-bis(3,4,5-trimethoxy-N-(pyridin-2-yl)benzamide-κ2N,O)copper(II), C32H30Cl2CuN4O16
- Crystal structure of methyl 4-methyl-2,5-di(pyridin-4-yl)-1H-pyrrole-3-carboxylate monohydrate C17H15N3O2⋅H2O
- Crystal structure of dimethyl 5-(10-(methoxycarbonyl)anthracen-9-yl) isophthalate,C26H20O6
- Crystal structure of tert-butyl (R)-(1-(benzylamino)-3-methoxy-1-oxopropan-2-yl)carbamate, C16H24N2O4
- Crystal structure of aqua-bis(1,5-dimethyl-2-phenyl-4-(((E)-4-pyridylmethylene)amino)pyrazolidin-3-one-κN)-(nitrato-κO)-(nitrato-κ2O,O′)zinc(II), C34H34N10O9Zn
- Crystal structure of catena-poly[dichlorido-(μ2-1,5-dimethyl-2-phenyl-4-((pyridin-4-ylmethylene)amino)-1,2-dihydro-3H-pyrazol-3-one-κ2N:O)cobalt(II)] – methanol (1/1), C18H20Cl2CoN4O2
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)]bis(2-carboxybenzoate) dihydrate, MnC40H40N10O12
- The crystal structure of 4-((3,4-dichlorobenzylidene)amino)-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one, C18H15Cl2N3O
- Crystal structure of bis(2-methoxy-6-((2-(pyrimidin-2-yl)hydrazono)methyl)phenolato-κ3N,N′,O)iron(III) perchlorate, C24H22N8O8ClFe
- The crystal structure of 2-[4-hydroxy-3-methoxyphenyl]-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl, C14H19N2O4
- Crystal structure of diaqua-bis(3,3-dimethylacrylato-κ2O,O′)zinc(II), C10H18ZnO6
- Crystal structure of dichloro-tetrakis[(E)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pent-1-en-3-ol-κN]cadmium (II), C60H74CdCl6N12O4
- Crystal structure of (20R)-20,25-epoxy-dammaran-3,12-dione, C30H48O3
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-((pyridin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C32H31N5O2
- The crystal structure of 2-(4-fluorophenyl)-1,3,4-oxadiazole, C8H5FN2O
- Crystal structure of (2,2′-bipyridine-κ2N,N′)bis(tri(p-tolyl)phosphine-κP)copper(I) tetrafluoroborate – 4,4′-bipyridine (2/1), C57H54BCuF4N3P2
- The crystal structure of 2,6-dimethyl-3,5-dinitrocyclohexa-2,5-diene-1,4-dione, C8H6N2O6
- The crystal structure of 2,3-dimethyl-1,4-dinitrobenzene – a Z′ = 4 structure, C8H8N2O4
- Crystal structure of [(1,2-η)-1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl] (1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-κ6N2,O4) rubidium (I), [Rb(diaza-18-crown-6)]Cp*, C22H41N2O4Rb
- Crystal structure of 2-(4-fluorophenyl)-N-phenyl-2-(phenylamino)ethanesulfonamide – toluene (1/0.5), C23.5H23FN2O2S
- Crystal structure of pyrene-4-aldehyde, C17H10O
- Crystal structure of 2-(furan-2-yl)-5-methyl-1,3-dioxane-5-carboxylic acid, C10H12O5
- Crystal structure of 2-(4-chlorophenyl)-3-phenyl-1,8-naphthyridine, C20H13N2Cl
- Crystal structure and photochromism of 1-(2-ethyl-5-formylthiophen-3yl)-2-(2-cyano-1,5-dimethyl-4-pyrrl)-3,3,4,4,5,5-hexafluorocyclopent-1-ene, C19H14F6N2OS
- Crystal structure of 2-(4-bromophenyl)-1,3-dimethyl-1H-perimidin-3-ium iodide, C19H16BrIN2
- Crystal structure of 2-(4-(dimethylamino)phenyl)-10-methylacridin-9(10H)-one, C22H20N2O
- Crystal structure of 4-(acetoxymethyl)-6-(3-acetyl-3-(4-fluorophenyl)thioureido)cyclohex-4-ene-1,2,3-triyl triacetate, C24H26FN2O9S

