How the cation size impacts on the relaxational and diffusional dynamics of supercooled butylammonium-based ionic liquids: DPEBA–TFSI versus BTMA–TFSI
Abstract
Li-bis(trifluoromethylsulfonyl)imide based ionic liquids with either butyl-trimethylammonium or N,N-dimethyl-N-(2-(propionyloxy)-ethyl)butan-1-ammonium as the anion were studied using proton and fluorine relaxometry as well as using field-gradient diffusometry to gain separate access to cation and anion dynamics in these compounds. The transport parameters obtained for these ionic liquids are compared with the estimates based on the conductivity data from literature and from the present work. The impact of cation size on correlation effects, the latter parameterized in terms of various Haven ratios, is mapped out.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: We thank the Deutsche Forschungsgemeinschaft for funding this work under project No. 396060266.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
Appendix: Radio-frequency electric impedance experiments on BTMA–TFSI
For BTMA–TFSI low-temperature electrical impedance data are obviously not available. Therefore, we carried out coaxial high-frequency reflectometry as well as low-frequency impedance measurements and present our results in Figure 6 in the modulus format. The complex electrical modulus is M* = M′ + iM" = 1/ε* = iωε 0/σ* where σ* designates the complex conductivity, ε* the complex dielectric constant, and ε 0 the permittivity of free space. From the maxima of M" conductivity relaxation times for the liquid and for the crystalline phase were determined and added to Figure 5. The σ 0 data obtained in this work are given in Table 1. For the liquid, the dc conductivities determined from the data are included in Figure 4 as well. From microwave experiments for BTMA–TFSI it was reported that σ 0 = 1.72 mS cm−1 at 25 °C [21].
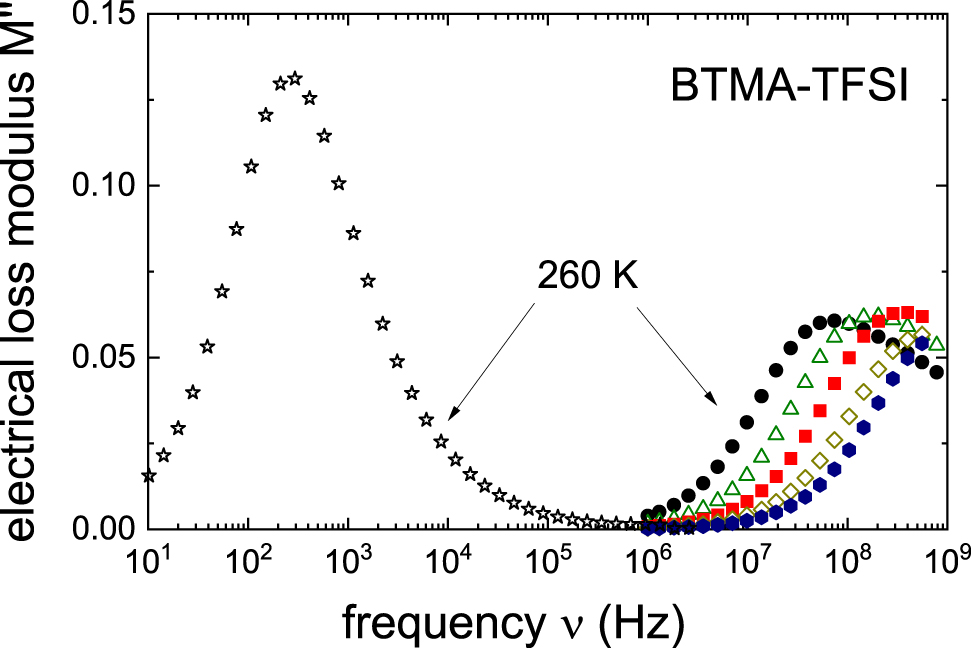
The imaginary part of the electrical modulus M" spectra of BTMA–TFSI is shown. For 310–240 K the data are recorded upon cooling in steps of 10 K. After the sample had crystallized, a few additional modulus spectra were taken. As an example, here we show an M" spectrum acquired at 260 K. Clearly, as compared to the liquid, in the crystal a much slower conductivity response (lower peak frequency and larger peak amplitude) is observed.
Compilation of the dc-conductivities measured in the present work. The first four entries correspond to the crystallized sample, the others refer to the liquid state.
| T (K) | 245 | 250 | 255 | 260 | 260 | 270 | 280 | 290 | 300 |
|---|---|---|---|---|---|---|---|---|---|
| log10[σ 0/(S cm−1)] | −11.7 | −11.1 | −10.3 | −9.45 | −3.84 | −3.46 | −3.15 | −2.91 | −2.69 |
References
1. Heitjans, P. (guest editor) Themed issue: mobility of ions in solids. Z. Phys. Chem. 2017, 231, 1211ff.https://doi.org/10.1515/zpch-2017-5000.Suche in Google Scholar
2. Bauer, T., Köhler, M., Lunkenheimer, P., Loidl, A., Angell, C. A. Relaxation dynamics and ionic conductivity in a fragile plastic crystal. J. Chem. Phys. 2010, 133, 144509; https://doi.org/10.1063/1.3487521.Suche in Google Scholar PubMed
3. Geirhos, K., Lunkenheimer, P., Michl, M., Reuter, D., Loidl, A. Conductivity enhancement in plastic-crystalline solid-state electrolytes. J. Chem. Phys. 2015, 143, 081101; https://doi.org/10.1063/1.4929554.Suche in Google Scholar PubMed
4. Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., Sangoro, J. R. Deep eutectic solvents: a review of fundamentals and applications. Chem. Rev. 2021, 121, 1232; https://doi.org/10.1021/acs.chemrev.0c00385.Suche in Google Scholar PubMed
5. Bocharova, V., Sokolov, A. P. Perspectives for polymer electrolytes: a view from fundamentals of ionic conductivity. Macromolecules 2020, 53, 4141; https://doi.org/10.1021/acs.macromol.9b02742.Suche in Google Scholar
6. Paluch, M., Ed. Dielectric Properties of Ionic Liquids; Springer: Switzerland, 2016.10.1007/978-3-319-32489-0Suche in Google Scholar
7. Wang, Y.-L., Li, B., Sarman, S., Mocci, F., Lu, Z.-Y., Yuan, J., Laaksonen, A., Fayer, M. D. Microstructural and dynamical heterogeneities in ionic liquids. Chem. Rev. 2020, 120, 5798; https://doi.org/10.1021/acs.chemrev.9b00693.Suche in Google Scholar PubMed PubMed Central
8. Eftekhari, A., Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245; https://doi.org/10.1016/j.eurpolymj.2017.03.033.Suche in Google Scholar
9. Habaski, J., Leon, C., Ngai, K. L. Dynamics of Glassy, Crystalline and Liquid Ionic Conductors; Springer: Switzerland, 2017. And references cited therein.10.1007/978-3-319-42391-3Suche in Google Scholar
10. Heitjans, P., Kärger, J., Eds. Diffusion in Condensed Matter: Methods, Materials, Models; Springer: Berlin, 2005.10.1007/3-540-30970-5Suche in Google Scholar
11. Loidl, A., Kremer, F., Eds. The Scaling of Relaxation Processes; Springer: Cham, 2018.10.1007/978-3-319-72706-6Suche in Google Scholar
12. Chandran, C. V., Heitjans, P. Solid-state NMR studies of lithium ion dynamics across materials classes. Annu. Rep. NMR Spectrosc. 2016, 89, 1.10.1016/bs.arnmr.2016.03.001Suche in Google Scholar
13. Pecher, O., Carretero-González, J., Griffith, K. J., Grey, C. P. Materials’ methods: NMR in battery research. Chem. Mater. 2017, 29, 213.10.1021/acs.chemmater.6b03183Suche in Google Scholar
14. Böhmer, R., Storek, M., Vogel, M. NMR studies of ionic dynamics. In Modern Methods in Solid-State NMR: A Practitioners’ Guide; Hodgkinson, P., Ed.; Royal Society of Chemistry, 2018, pp. 193–230, Chapter 7.10.1039/9781788010467-00193Suche in Google Scholar
15. Edward, J. T. Molecular volumes and Stokes–Einstein equation. J. Chem. Educ. 1970, 47, 261.10.1021/ed047p261Suche in Google Scholar
16. Haynes, W. M., Lide, D. R., Bruno, T. J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Florida, 2017.10.1201/9781315380476Suche in Google Scholar
17. Wieland, F., Sokolov, A. P., Böhmer, R., Gainaru, C. Transient nonlinear response of dynamically decoupled ionic conductors. Phys. Rev. Lett. 2018, 121, 064503.10.1103/PhysRevLett.121.064503Suche in Google Scholar PubMed
18. Wieland, F., Bocharova, V., Münzner, P., Hiller, W., Sakrowski, R., Sternemann, C., Böhmer, R., Sokolov, A. P., Gainaru, C. Structure and dynamics of small-chain polymerized ionic liquids. J. Chem. Phys. 2019, 151, 034903.10.1063/1.5109228Suche in Google Scholar PubMed
19. Jacquemin, J., Husson, P., Padua, A. A. H., Majer, V. Density and viscosity of several pure and water-saturated ionic liquids. Green Chem 2006, 8, 172.10.1039/B513231BSuche in Google Scholar
20. Cimini, A., Palumbo, O., Simonetti, E., De Francesco, M., Appetecchi, G. B., Fantini, S., Lin, R., Falgayrat, A., Paolone, A. Decomposition temperatures and vapour pressures of selected ionic liquids for electrochemical applications. J. Therm. Anal. Calorim. 2020, 142, 1791.10.1007/s10973-020-10334-5Suche in Google Scholar
21. Weingärtner, H., Sasisanker, P., Daguenet, C., Dyson, P. J., Krossing, I., Slattery, J. M., Schubert, T. The dielectric response of room-temperature ionic liquids: effect of cation variation. J. Phys. Chem. B 2007, 111, 4775.10.1021/jp0671188Suche in Google Scholar PubMed
22. Lima, T. A., Paschoal, V. H., Faria, L. F. O., Ribeiro, M. C. C., Giles, C. Comparing two tetraalkylammonium ionic liquids. I. Liquid phase structure. J. Chem. Phys. 2016, 144, 224504.10.1063/1.4953414Suche in Google Scholar
23. Lima, T. A., Paschoal, V. H., Faria, L. F. O., Ribeiro, M. C. C., Ferreira, F. F., Costa, F. N., Giles, C. Comparing two tetraalkylammonium ionic liquids. II. Phase transitions. J. Chem. Phys. 2016, 144, 224505.10.1063/1.4953415Suche in Google Scholar
24. Lima, T. A., Li, Z., Tyagi, M., Ribeiro, M. C. C. Spatial and thermal signatures of alpha and beta-relaxations in glassy and glacial aliphatic ionic liquids. J. Chem. Phys. 2019, 150, 144506.10.1063/1.5081684Suche in Google Scholar
25. Lima, T. A., Paschoal, V. H., Freitas, R. S., Faria, L. F. O., Li, Z., Tyagi, M., Ribeiro, M. C. C. An inelastic neutron scattering, Raman, far-infrared, and molecular dynamics study of the intermolecular dynamics of two ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 9074–9085.10.1039/D0CP00374CSuche in Google Scholar
26. Tiyapiboonchaiya, C., MacFarlane, D. R., Sun, J., Forsyth, M. Polymer-in-ionic-liquid electrolytes. Macromol. Chem. Phys. 2002, 203, 1906.10.1002/1521-3935(200209)203:13<1906::AID-MACP1906>3.0.CO;2-ISuche in Google Scholar
27. Fan, F., Wang, W., Holt, A. P., Feng, H., Uhrig, D., Lu, X., Hong, T., Wang, Y., Kang, N.-G., Mays, J., Sokolov, A. P. Effect of molecular weight on the ion transport mechanism in polymerized ionic liquids. Macromolecules 2016, 49, 4557.10.1021/acs.macromol.6b00714Suche in Google Scholar
28. Fleischer, G., Fujara, F. NMR as a generalized incoherent scattering experiment. In Diehl, P., Fluck, E., Günther, H., Kosfeld, P., Seelig, J. NMR – Basic Principles and Progress; Springer: Berlin, Vol. 30, 1994, pp. 159–207.10.1007/978-3-642-78483-5_4Suche in Google Scholar
29. Holz, M., Heil, S., Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740.10.1039/b005319hSuche in Google Scholar
30. Böhmer, R., Maglione, M., Lunkenheimer, P., Loidl, A. Radio-frequency dielectric measurements at temperatures from 10 to 450 K. J. Appl. Phys. 1989, 65, 901.10.1063/1.342990Suche in Google Scholar
31. Abragam, A. The Principles of Nuclear Magnetism; Clarendon: Oxford, 1961; p. 289 ff. Chapter VIII.E.Suche in Google Scholar
32. Davidson, D. W., Cole, R. H. Dielectric relaxation in glycerol, propylene glycol, and n-propanol. J. Chem. Phys. 1951, 19, 1484.10.1063/1.1748105Suche in Google Scholar
33. Beckmann, P. A. Spectral densities and nuclear spin relaxation in solids. Phys. Rep. 1988, 171, 85.10.1016/0370-1573(88)90073-7Suche in Google Scholar
34. Bloembergen, N., Purcell, E. M., Pound, R. V. Relaxation effects in nuclear magnetic resonance absorption. Phys. Rev. 1948, 73, 679.10.1142/9789814540223_0039Suche in Google Scholar
35. Wieland, F. Chain-Length Dependence of Charge Transport and Viscoelasticity in poly[N,N-Dimethyl-N-(2-(Propionyloxy)Ethyl)Butan-1-Ammonium]+[TFSI]– Ionic Melts. Master Thesis, TU Dortmund University, 2017.Suche in Google Scholar
36. Böhmer, R., Ngai, K. L., Angell, C. A., Plazek, D. J. Non-exponential relaxations in strong and fragile glass-formers. J. Chem. Phys. 1993, 99, 4201.10.1063/1.466117Suche in Google Scholar
37. Sippel, P., Lunkenheimer, P., Krohns, S., Thoms, E., Loidl, A. Importance of liquid fragility for energy applications of ionic liquids. Sci. Rep. 2015, 5, 13922.10.1038/srep13922Suche in Google Scholar
38. Kircher, O., Böhmer, R., Alba-Simionesco, C. Reorientations and translations in a fragile glass-former: magnetic resonance studies of meta-fluoroaniline. J. Mol. Struct. 1999, 479, 195.10.1016/S0022-2860(98)00870-9Suche in Google Scholar
39. Schildmann, S., Reiser, A., Gainaru, R., Gainaru, C., Böhmer, R. Nuclear magnetic resonance and dielectric noise study of spectral densities and correlation functions in the glass forming monoalcohol 2-ethyl-1-hexanol. J. Chem. Phys. 2011, 135, 174511.10.1063/1.3647954Suche in Google Scholar
40. Geil, B. Measurement of translational molecular diffusion using ultrahigh magnetic field gradient NMR. Concepts Magn. Reson. 1998, 10, 299.10.1002/(SICI)1099-0534(1998)10:5<299::AID-CMR3>3.0.CO;2-SSuche in Google Scholar
41. Angell, C. A., Ngai, K. L., McKenna, G. B., McMillan, P. F., Martin, S. W. Relaxation in glassforming liquids and amorphous solids. J. Appl. Phys. 2000, 88, 3113.10.1063/1.1286035Suche in Google Scholar
42. Lima, T. A., Faria, L. F. O., Paschoal, V. H., Ribeiro, M. C. C. Communication: glass transition and melting lines of an ionic liquid. J. Chem. Phys. 2018, 148, 171101.10.1063/1.5030083Suche in Google Scholar
43. Isard, J. O. The Haven ratio in glasses. J. Non-Cryst. Solids 1999, 246, 16.10.1016/S0022-3093(99)00036-8Suche in Google Scholar
44. Gainaru, C., Stacy, E. W., Bocharova, V., Gobet, M., Holt, A. P., Saito, T., Greenbaum, S., Sokolov, A. P. Mechanism of conductivity relaxation in liquid and polymeric electrolytes: direct link between conductivity and diffusivity. J. Phys. Chem. B 2016, 120, 11074.10.1021/acs.jpcb.6b08567Suche in Google Scholar PubMed
45. Gainaru, C., Ahlmann, S., Röwekamp, L. S., Moch, K., Bierwirth, S. P., Böhmer, R. Rheology based estimates of self- and collective diffusivities in viscous liquids. J. Chem. Phys. 2021, 155, 011101.10.1063/5.0055811Suche in Google Scholar PubMed
46. Sangoro, J. R., Serghei, A., Naumov, S., Galvosas, P., Kärger, J., Wespe, C., Bordusa, F., Kremer, F. Charge transport and mass transport in imidazolium based ionic liquids. Phys. Rev. E 2008, 77, 051202.10.1103/PhysRevE.77.051202Suche in Google Scholar PubMed
47. Sangoro, J. R., Kremer, F. Charge transport and glassy dynamics in ionic liquids. Acc. Chem. Res. 2012, 45, 525.10.1021/ar2001809Suche in Google Scholar PubMed
48. Wang, Y., Sun, C.-N., Fan, F., Sangoro, J. R., Berman, M. B., Greenbaum, S. G., Zawodzinski, T. A., Sokolov, A. P. Examination of methods to determine free-ion diffusivity and number density from analysis of electrode polarization. Phys. Rev. E 2013, 87, 042308.10.1103/PhysRevE.87.042308Suche in Google Scholar PubMed
49. Vargas-Barbosa, N. M., Roling, B. Dynamic ion correlations in solid and liquid electrolytes: how do they affect charge and mass transport? ChemElectroChem 2020, 7, 367.10.1002/celc.201901627Suche in Google Scholar
50. Pfeifer, S., Ackermann, F., Sälzer, F., Schönhoff, M., Roling, B. Quantification of cation–cation, anion–anion and cation–anion correlations in Li salt/glyme mixtures by combining very-low-frequency impedance spectroscopy with diffusion and electrophoretic NMR. Phys. Chem. Chem. Phys. 2021, 23, 628.10.1039/D0CP06147FSuche in Google Scholar PubMed
51. Stacy, E. W., Gainaru, C. P., Gobet, M., Wojnarowska, Z., Bocharova, V., Greenbaum, S. G., Sokolov, A. P. Fundamental limitations of ionic conductivity in polymerized ionic liquids. Macromolecules 2018, 51, 8637.10.1021/acs.macromol.8b01221Suche in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Preface
- Special issue on the occasion of the 75th birthday of Paul Heitjans
- Contribution to Special Issue dedicated to Paul Heitjans
- Unusual cation coordination in nanostructured mullites
- A novel high entropy spinel-type aluminate MAl2O4 (M = Zn, Mg, Cu, Co) and its lithiated oxyfluoride and oxychloride derivatives prepared by one-step mechanosynthesis
- Two new quaternary copper bismuth sulfide halides: CuBi2S3Cl and CuBi2S3Br as candidates for copper ion conductivity
- Sintering behavior and ionic conductivity of Li1.5Al0.5Ti1.5(PO4)3 synthesized with different precursors
- Status and progress of ion-implanted βNMR at TRIUMF
- How Li diffusion in spinel Li[Ni1/2Mn3/2]O4 is seen with μ ±SR
- Nuclear magnetic resonance (NMR) studies of sintering effects on the lithium ion dynamics in Li1.5Al0.5Ti1.5(PO4)3
- Anion reorientations and cation diffusion in a carbon-substituted sodium nido-borate Na-7,9-C2B9H12: 1H and 23Na NMR studies
- Site preferences and ion dynamics in lithium chalcohalide solid solutions with argyrodite structure: I. A multinuclear solid state NMR study of the system Li6PS5-xSexI and of Li6AsS5I
- Site preferences and ion dynamics in lithium chalcohalide solid solutions with argyrodite structure: II. Multinuclear solid state NMR of the systems Li6PS5−x Se x Cl and Li6PS5−x Se x Br
- Independent component analysis combined with Laplace inversion of spectrally resolved spin-alignment echo/T 1 3D 7Li NMR of superionic Li10GeP2S12
- How the cation size impacts on the relaxational and diffusional dynamics of supercooled butylammonium-based ionic liquids: DPEBA–TFSI versus BTMA–TFSI
- Solid-state NMR studies of non-ionic surfactants confined in mesoporous silica
- Inorganic-organic hybrid materials based on the intercalation of radical cations: 2-(4-N-methylpyridinium)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl-3-N-oxide in fluoromica clay
- Lithium tracer diffusion in near stoichiometric LiNi0.5Mn1.5O4 cathode material for lithium-ion batteries
- On the CaF2-BaF2 interface
- The ionic conductivity of alkali aluminum germanium phosphate glasses – comparison of Plasma CAIT with two electrode DC measurements
- Thin-film chemical expansion of ceria based solid solutions: laser vibrometry study
- Predicting conductivities of alkali borophosphate glasses based on site energy distributions derived from network former unit concentrations
- Ionic transport in K2Ti6O13
- F anion transport in nanocrystalline SmF3 and in mechanosynthesized, vacancy-rich Sm1—x BaxF3—x
- An overview of thermotransport in fluorite-related ionic oxides
Artikel in diesem Heft
- Frontmatter
- Preface
- Special issue on the occasion of the 75th birthday of Paul Heitjans
- Contribution to Special Issue dedicated to Paul Heitjans
- Unusual cation coordination in nanostructured mullites
- A novel high entropy spinel-type aluminate MAl2O4 (M = Zn, Mg, Cu, Co) and its lithiated oxyfluoride and oxychloride derivatives prepared by one-step mechanosynthesis
- Two new quaternary copper bismuth sulfide halides: CuBi2S3Cl and CuBi2S3Br as candidates for copper ion conductivity
- Sintering behavior and ionic conductivity of Li1.5Al0.5Ti1.5(PO4)3 synthesized with different precursors
- Status and progress of ion-implanted βNMR at TRIUMF
- How Li diffusion in spinel Li[Ni1/2Mn3/2]O4 is seen with μ ±SR
- Nuclear magnetic resonance (NMR) studies of sintering effects on the lithium ion dynamics in Li1.5Al0.5Ti1.5(PO4)3
- Anion reorientations and cation diffusion in a carbon-substituted sodium nido-borate Na-7,9-C2B9H12: 1H and 23Na NMR studies
- Site preferences and ion dynamics in lithium chalcohalide solid solutions with argyrodite structure: I. A multinuclear solid state NMR study of the system Li6PS5-xSexI and of Li6AsS5I
- Site preferences and ion dynamics in lithium chalcohalide solid solutions with argyrodite structure: II. Multinuclear solid state NMR of the systems Li6PS5−x Se x Cl and Li6PS5−x Se x Br
- Independent component analysis combined with Laplace inversion of spectrally resolved spin-alignment echo/T 1 3D 7Li NMR of superionic Li10GeP2S12
- How the cation size impacts on the relaxational and diffusional dynamics of supercooled butylammonium-based ionic liquids: DPEBA–TFSI versus BTMA–TFSI
- Solid-state NMR studies of non-ionic surfactants confined in mesoporous silica
- Inorganic-organic hybrid materials based on the intercalation of radical cations: 2-(4-N-methylpyridinium)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl-3-N-oxide in fluoromica clay
- Lithium tracer diffusion in near stoichiometric LiNi0.5Mn1.5O4 cathode material for lithium-ion batteries
- On the CaF2-BaF2 interface
- The ionic conductivity of alkali aluminum germanium phosphate glasses – comparison of Plasma CAIT with two electrode DC measurements
- Thin-film chemical expansion of ceria based solid solutions: laser vibrometry study
- Predicting conductivities of alkali borophosphate glasses based on site energy distributions derived from network former unit concentrations
- Ionic transport in K2Ti6O13
- F anion transport in nanocrystalline SmF3 and in mechanosynthesized, vacancy-rich Sm1—x BaxF3—x
- An overview of thermotransport in fluorite-related ionic oxides

