Abstract
To understand the growth and survival during the postlarval stage of Macrobrachium rosenbergii, commonly known as the giant freshwater prawn, two experiments were performed which explored the differences in growth and survival rates between wild and captive postlarvae and the effect of temperature on survival and growth. The postlarvae reared at 27 and 30°C grew significantly throughout the experimental periods. The survival rates in the wild and captive postlarvae were similar at approximately 60%. The highest survival rate of 58% was found at 27°C.
1 Introduction
The giant freshwater prawn Macrobrachium rosenbergii population is distributed in tropical and subtropical areas throughout the Indo-Pacific region [1,2]. Among approximately 200 species of the genus Macrobrachium, the most important culturable species is M. rosenbergii. Macrobrachium rosenbergii is an amphidromous species living in various water environments, such as fresh and estuarine waters connected to the sea [3]. Matured females migrate from freshwater environments to estuarine environments for spawning. Larval development occurs in estuaries and moves to freshwater, and the adults spend their lives in freshwater [1].
In Brunei Darussalam, giant freshwater prawns can be found widely in rivers, streams, estuaries, and mangrove areas. It is locally known as “Udang Galah” and is a popular delicacy of the country. The market price of the prawn is approximately 10–15 USD per kilogram, depending on its body size. Hatchery production of M. rosenbergii in Brunei Darussalam began in 1982 [4], though the current hatchery and production rate for the species are not well developed. Currently, the species is captured in the wild and sold in local markets due to the unstable culturing production of the species in Brunei Darussalam. Therefore, it was found that the prawn landing has declined drastically from 600 to 70 kg per day [5]. This decline is detrimental because the country continues to obtain seeds from the wild, especially berried females, which could ultimately lead to a decline in the wild population [6,7]. Therefore, it is important to examine baseline information such as growth and survival in aquaculture and the enhancement of M. rosenbergii in Brunei Darussalam.
Environmental factors such as temperature and salinity play important roles in the development and growth of decapod crustaceans [8,9,10,11,12,13,14]. Macrobrachium rosenbergii can tolerate a wide range of temperatures (14–35°C) and salinities (0–25 ppt) [13,14,15]. The geographical distribution, acclimatization of ectothermic organisms, and range of thermal tolerance are influenced by temperature [11,12,13,14,16,17]. Ectotherms have a limited range of thermal tolerance in tropical regions because of limited seasonal fluctuation, causing them to be vulnerable to global warming circumstances [17]. High temperatures can impact egg development in M. americanum [18] and growth and survival in M. amazonicum [11]. In M. rosenbergii, the survival, growth, and molting are influenced by salinity, temperature, and pH [9,13]. The higher temperatures result in an increased larval heartbeat, while larval activity decreased at higher temperatures and salinities in M. rosenbergii [14]. However, there is still limited information available on the effect of temperature on the survival and growth of M. rosenbergii during the postlarval stage [19,20]. Furthermore, little information is also available on differences in growth and survival between wild and captive M. rosenbergii.
In the present study, two independent experiments were performed to understand the growth and survival of the giant freshwater prawn M. rosenbergii; the first experiment examined differences in growth and survival rates between wild and captive postlarvae, and the second experiment determined the effect of temperature on survival and growth under laboratory conditions. These experiments provide a baseline data for the aquaculture of the giant freshwater prawn M. rosenbergii and other related species.
2 Materials and methods
2.1 Experimental specimens and acclimation
To examine differences in growth and survival rates between captive and wild postlarvae, freshwater giant prawns were collected from the Tutong River (4°48′16″N, 114°39′40″E) and from a local hatchery in Brunei Darussalam (4°22′05″N, 114°27′40″E). Two types of postlarvae were collected: (1) second-generation captive postlarvae and (2) wild postlarvae. A total of 200 second-generation captive postlarvae were collected on December 5, 2018. A total of 30 individuals of wild postlarvae were collected on December 4, 2018. All captive and wild postlarvae were transported using polyethylene plastic bags with aerated water and were placed in styrofoam boxes to prevent excess shaking during transport to the Universiti Brunei Darussalam (Gadong, Brunei Darussalam) for experiments and then transferred to temporary tanks (130 cm × 80 cm × 46 cm, 270 L) for 11–12 days at the ambient temperature of 27°C for acclimation. Postlarvae were initially fed with Artemia and gradually shifted to artificial pellets (Star Feed 5004-S, Charoen Pokphand Malaysia, containing 40% protein) twice daily (9:00 and 16:00 h).
Furthermore, to determine the effect of temperature on the survival and growth, a total of 200 captive postlarvae were compared with the first experiment with acclimation under the same conditions. Experiments were performed by subdividing postlarvae into three tanks during the experimental period.
2.2 Differences in growth and survival rates between wild and captive postlarvae
A total of 12 captive postlarvae and 14 wild postlarvae were transferred to the experimental tanks and reared at 27°C (±1°C) with 0 psu in salinity (freshwater) and were observed for 80 days (December 2018 to March 2019). The optimal feeding rate of all crustaceans, including postlarvae, is 10–20% of their biomass [1]. The feeding rate of the postlarvae was calculated as follows:
The feeding rate was further split into 40 and 60% for the morning (9:00) and afternoon (16:00) routines, respectively. During the observation period, the dissolved oxygen (DO) (YSI EcoSense DO200, Yellow Springs, USA), water temperature (YSI EcoSense DO200), and pH (Campbell Scientific ISFET pH Probe, Logan, USA) were measured twice daily.
The growth rates of all the individuals were examined by measuring their weight and length every 20 days. The body length (BL) of each specimen was measured from the tip of the rostrum to the telson to the nearest 0.01 mm. The body weight (BW) of each specimen was examined to the nearest 0.01 g. The weight gain (WG) and survival rate of the prawns were calculated by using the following formulae:
where Wf is the final wet weight and Wi is the initial wet weight.
2.3 Effect of temperature on survival and growth
Three temperature regimes, 27, 30, and 33°C (±1°C), with 0 psu in salinity (freshwater) were chosen for the thermal tolerance experiment. The DO and pH values were 6.41 ± 0.73 (mean ± SD) mg L−1 and 6.66 ± 0.95, respectively. The temperature in each tank was controlled using a thermostat. Each tank was aerated in every corner of the tank with a total of 6 aeration tubes. A color stabilizer (blue) was added to each tank to mimic the natural environment for postlarvae and to prevent stress and cannibalism. In each tank, 3 nylon nets (51 cm × 44 cm × 35 cm) were placed to prevent predation and cannibalism among postlarvae. Nylon substrates were added to each net to increase the surface area and provide hiding spots during the molting period. Thirty-six postlarvae were assigned to each net, and 12 post-larvae were further separated into 3 nets (0.04 individual cm−3).
The BW and BL of each specimen were examined every 20 days for 80 days (December 2018 to March 2019). The average daily growth (ADG) (g d−1) and specific growth rate (SGR) (g d−1) were calculated by using the following formulae:
2.4 Statistical analysis
Differences in BL and BW between wild and captive postlarvae for every 20 days were examined by means of the Student’s t-test. Differences in BW between each measurement every 20 days were also examined by means of the Student’s t-test. Analysis of covariance (ANCOVA) was used to compare two regression lines by testing the effect of a categorical factor on a dependent variable in the growth patterns of BL and BW between wild and captive post larvae and BW between 27 and 30°C.
-
Ethical approval: Our protocols followed the ethical guidelines for the use of animals of Universiti Brunei Darussalam (UBD) and were approved by the animal ethics committee at UBD.
3 Results
3.1 Differences in survival rates and growth between wild and captive postlarvae
During the initial experimental period between 0 and 20 days, the survival rates of wild and captive postlarvae decreased from 100% (n = 14) to 64.3% (n = 9) and 100% (n = 12) to 58.3% (n = 7), respectively (Figure 1). Thereafter, the survival rates remained constant at 64.3% in wild and 58.3% in the captive postlarvae throughout the experiment, dropping slightly to 57.1% in the wild postlarvae at 80 days (Figure 1). There was no significant difference in survival rates between wild and captive prawns throughout the experiment (p > 0.05).
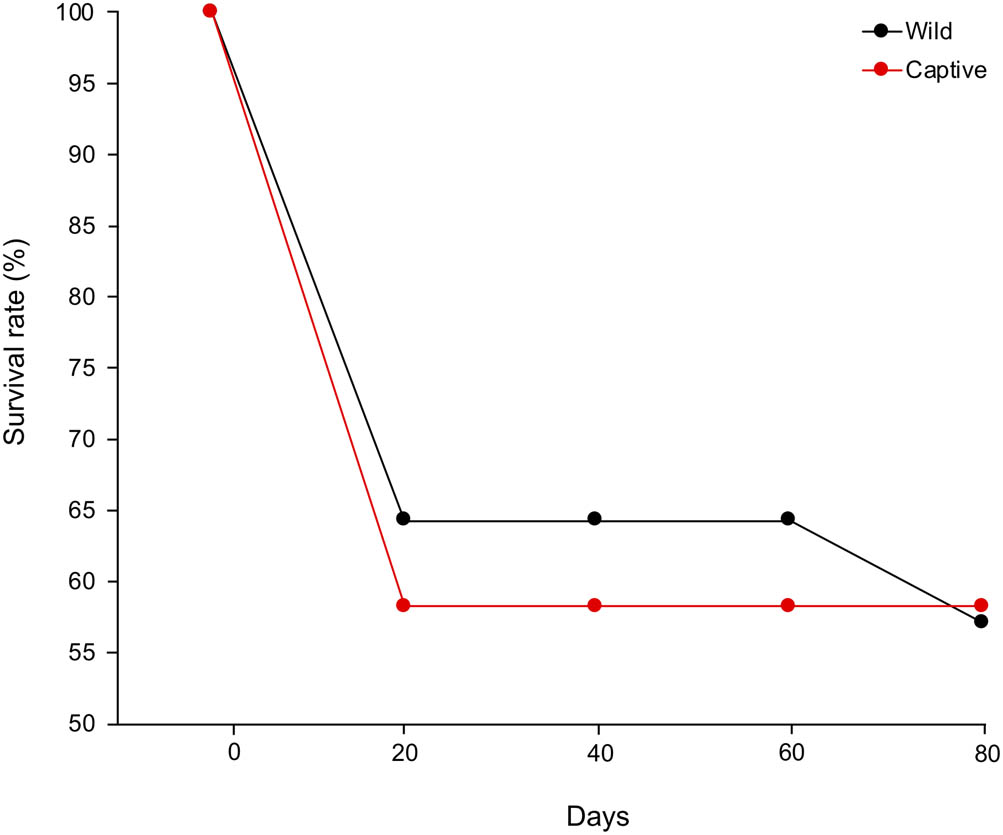
Survival rates in wild (red line) and captive (black line) postlarvae in the giant freshwater prawn Macrobrachium rosenbergii.
The initial and final BLs in wild prawns were 1.70 ± 0.68 cm (mean ± SD) and 3.35 ± 0.68 cm, respectively, and the initial and final BLs in captive prawns were 1.72 ± 0.83 and 3.81 ± 0.83 cm, respectively. The initial and final BWs in wild and captive prawns were 0.01 ± 0.003 g (mean ± SD) and 0.0575 ± 0.192 g and 0.289 ± 0.09 g and 0.509 ± 0.157 g, respectively. There were significant differences in BLs and BWs between the initial and final stages of the experiment (p < 0.0001). The BLs and BWs tend to increase throughout the experimental period (p < 0.0001) (Figure 2). There were no significant differences found in BLs between wild and captive postlarvae every 20 days (0, 20, 40, 60, 80 days) (p > 0.05) (Figure 2a). Significant differences were found in BW between wild and captive postlarvae every 20 days (p < 0.05–0.0001) (Figure 2b). No significant differences were found in regression slopes in BW and BL between wild and captive postlarvae (p > 0.05).
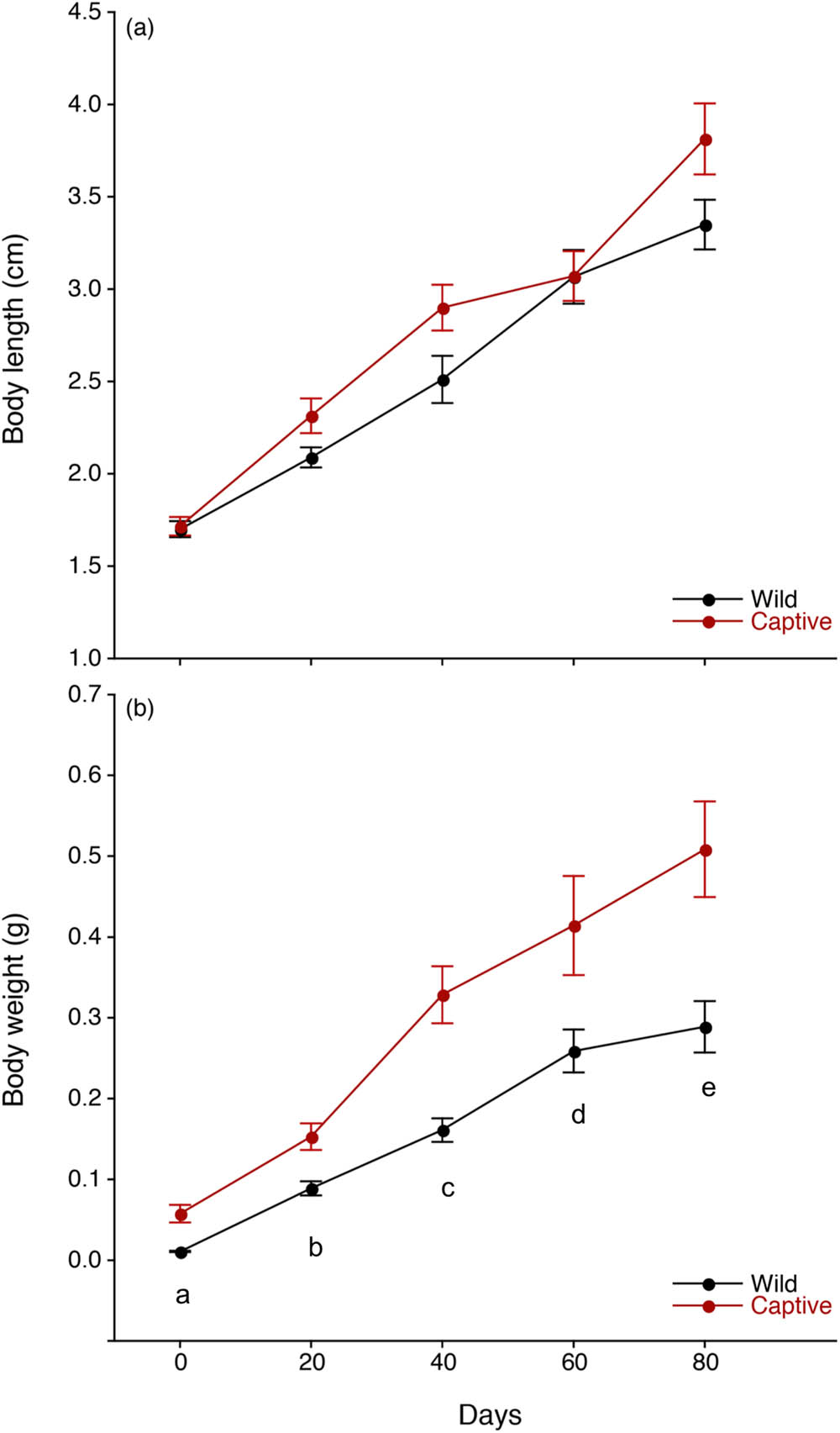
Growth patterns of body length (a) and body weight (b) in wild (black line) and captive (red line) postlarvae in the giant freshwater prawn Macrobrachium rosenbergii. The results are expressed as the mean ± standard error (SE). The letters (a–e) indicate a statistically significant difference at p < 0.05 between wild and captive postlarvae.
In wild prawns, significant increases in BW were found between 0 days (0.01 ± 0.003 g; mean ± SD) and 20 days (0.089 ± 0.03 g), between 20 days and 40 days (0.161 ± 0.04 g) and between 40 days and 60 days (0.259 ± 0.08 g) (p < 0.01–0.0005), while there was no significant difference in BW between 60 days and 80 days (0.289 ± 0.09 g) (p > 0.05). There were significant increases in BW between 0 days (0.0575 ± 0.04 g; mean ± SD) and 20 days (0.153 ± 0.04 g) and between 20 days and 40 days (0.329 ± 0.09 g) (p < 0.05–0.0005), while there were no significant differences in BW between 40 days and 60 days (0.414 ± 0.16 g) or between 60 days and 80 days (0.509 ± 0.16 g) (p > 0.05) in captive prawns. The WG based on the mean BW in wild and captive prawns between 0 and 20 days, between 20 and 40 days, between 40 and 60 days, and between 60 and 80 days was 0.078 and 0.095 g, 0.072 and 0.176 g, 0.098 and 0.086 g, and 0.030 and 0.094 g, respectively. WG in captive prawns was higher than that in wild prawns for all periods except between 40 and 60 days.
3.2 Effect of temperature on survival and growth
The survival reduction was found during the initial experimental period between 0 and 20 days from 100% (n = 36) to 63.9% (n = 23) at 27°C and 100% (n = 36) to 58.3% (n = 21) at 30°C (Figure 3). All postlarvae died within 7 days during the initial 20 days at 33°C (Figure 3). There was no survival reduction found, and the survival rate was constant (58.3%) throughout the experiment after a slight reduction at 20 days (61.1%) at 27°C (Figure 3). In contrast, a survival reduction was found at 60 days (n = 19, 52.8%) and the end of the experiment at 80 days (n = 15, 41.7%) at 30°C (Figure 3).
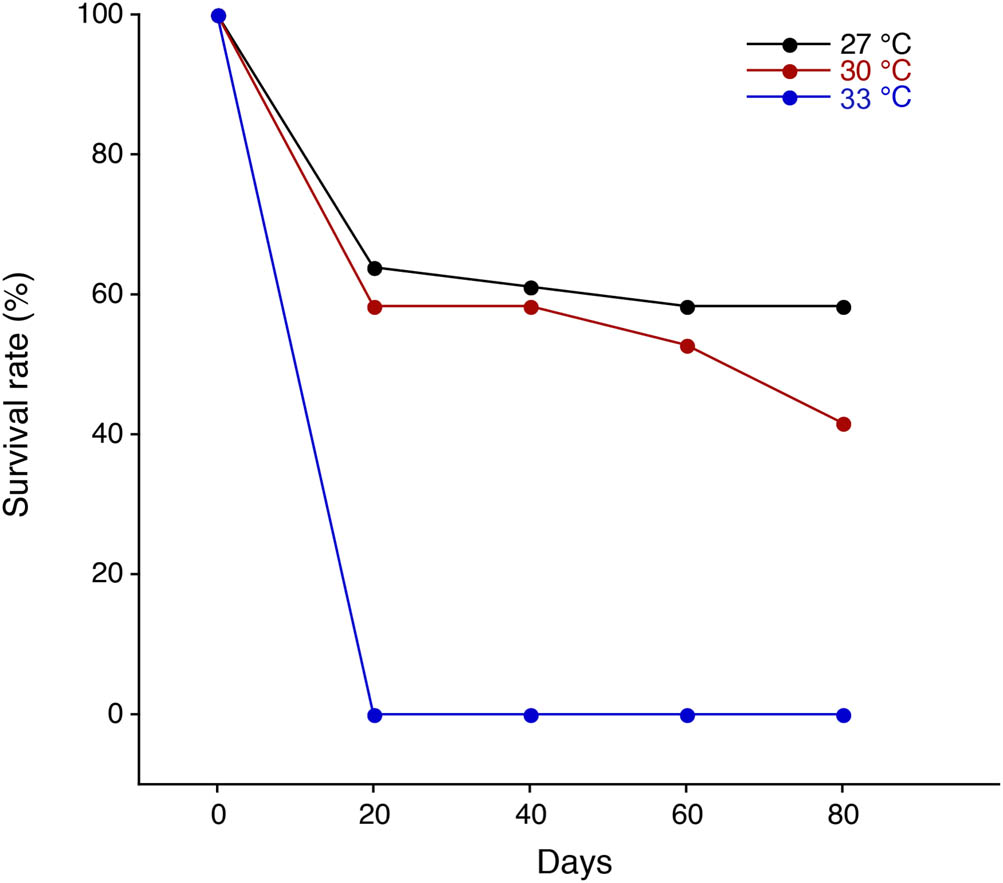
Survival rates of postlarvae in the giant freshwater prawn Macrobrachium rosenbergii reared in different temperature regimes of 27°C (black), 30°C (red), and 33°C (blue).
The BWs at 27, 30, and 33°C at the beginning of the experiment were 0.16 ± 0.13 (mean ± SD) g, 0.13 ± 0.10 and 0.13 ± 0.11, respectively (Table 1). There were no significant differences in initial BW among the three temperature regimes (p > 0.05). Significant differences were found in BW between each experimental period (p < 0.05–0.0001), except between 40 days and 60 days (p > 0.05) at 27°C (Table 2), suggesting successive growth throughout the experiment (Figure 4). There were significant differences in BW between each experimental period (p < 0.01–0.0001), except between 60 days and 80 days (p > 0.05) at 30°C (Table 3, Figure 4). The final BWs at 27 and 30°C were 2.14 ± 1.67 and 2.37 ± 1.68 g, respectively. There was no significant difference in the final BW between these two temperature regimes (p > 0.05). WG, ADG, and SGR during 80 days at 27 and 30°C were 1.98 (g), 0.02 (g d−1), and 3.24 (g d−1) and 2.24 (g), 0.03 (g d−1), and 3.63 (g d−1), respectively (Table 1).
Production parameters of the giant freshwater prawn Macrobrachium rosenbergii reared in different temperature ranges for 80 days
| Temperature | |||
|---|---|---|---|
| 27°C | 30°C | 33°C | |
| Initial weight | 0.16 ± 0.13 (n = 36) | 0.13±0.10 (n = 36) | 0.13 ± 0.11 (n = 36) |
| Final weight | 2.14 ± 1.67 (n = 21) | 2.37 ± 1.68 (n = 15) | n.d (n = 0) |
| Weight gain | 1.98 | 2.24 | n.d |
| Average daily growth | 0.02 | 0.03 | n.d |
| Specific growth rate | 3.24 | 3.63 | n.d |
n – number of specimens; n.d – no data due to all specimens died within 7 days.
Statistical results (p-value) of body weight in the freshwater giant prawn Macrobrachium rosenbergii between 20-day intervals reared at 27°C
| Day 0 | Day 20 | Day 40 | Day 60 | Day 80 | |
|---|---|---|---|---|---|
| Day 0 | 1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Day 20 | 1 | <0.0005 | <0.0005 | <0.0001 | |
| Day 40 | 1 | 0.388 | <0.05 | ||
| Day 60 | 1 | <0.05 | |||
| Day 80 | 1 |
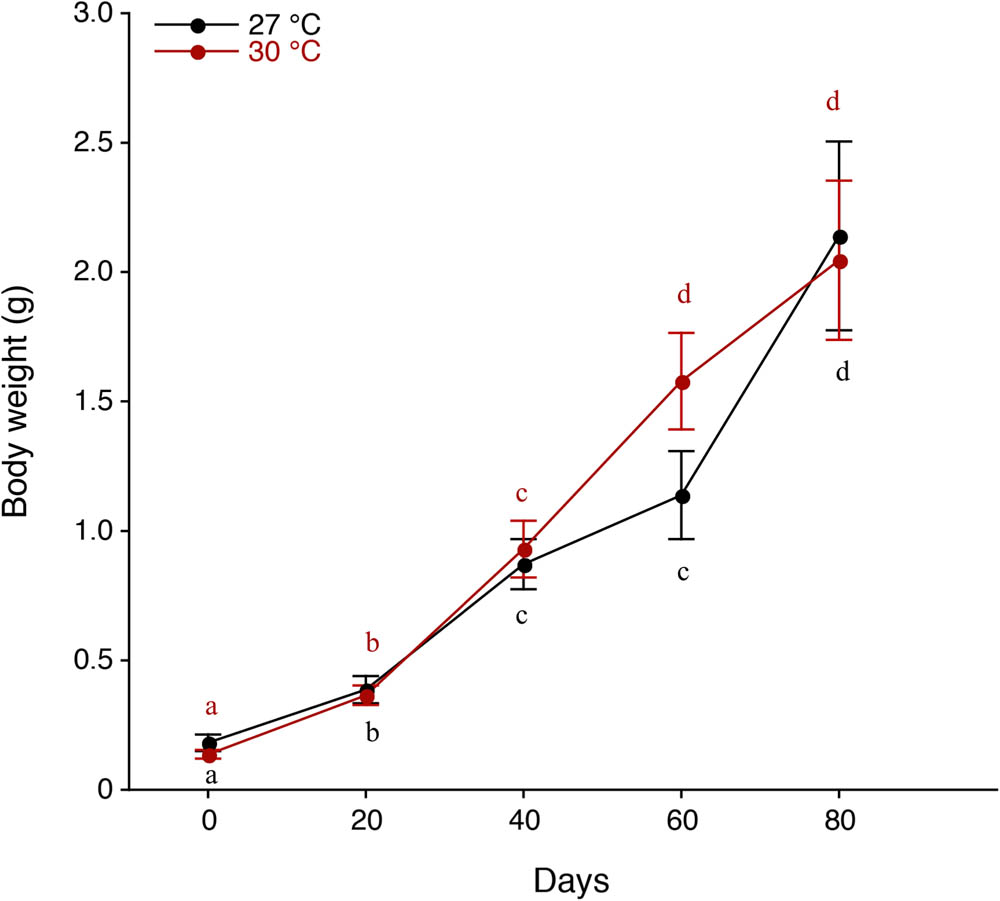
Growth patterns of body weight of postlarvae in the giant freshwater prawn Macrobrachium rosenbergii reared in different temperature regimes of 27°C (black) and 30°C (red). The results are expressed as the mean ± standard error (SE). The letters a, b, c, and d indicate a statistically significant difference at p < 0.05 between each experimental period in different temperature regimes of 27°C (black) and 30°C (red).
Statistical results (p-value) of body weight in the freshwater giant prawn Macrobrachium rosenbergii between 20-day intervals reared at 30°C
| Day 0 | Day 20 | Day 40 | Day 60 | Day 80 | |
|---|---|---|---|---|---|
| Day 0 | 1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Day 20 | 1 | <0.0005 | <0.0001 | <0.0001 | |
| Day 40 | 1 | <0.01 | <0.0005 | ||
| Day 60 | 1 | 0.206 | |||
| Day 80 | 1 |
4 Discussion
4.1 Differences in growth and survival rates between wild and captive postlarvae
There was no difference between the survival rates of wild and captive postlarvae. The cultural environment can create significant selection pressures that can affect population growth and reproduction rates, collectively known as “fitness” [21]. The giant freshwater prawns are generally opportunistic omnivores that eat a wide range of food items efficiently [22]. Wild prawns feed on algae, molluscs, aquatic insects, worms, aquatic plants, and other crustaceans [23]. The cultivation of live feed to support aquaculture is costly and unpredictable, and hence, the usage of artificial feed has become popular, particularly in shrimp culture [24]. Macrobrachium rosenbergii is a nonactive hunter and appears to capture food only by chance [25,26]. The captive postlarvae might be more acclimatized than the wild postlarvae. Environmental factors such as nutrition, social stress, or fear were found to play an important role in shaping the traits and phenotypes across several offspring generations; an effect is known as transgenerational epigenetic inheritance [27]. This suggests that the captive postlarvae would be more acclimatized than the wild postlarvae under experimental and cultural conditions. The captive postlarvae might also be acclimatized to artificial foods providing regular instead of predator–prey interactions in the wild. However, wild postlarvae might be undomesticated compared to captive larvae. Adult female prawns might experience less stressful conditions in the wild, and thus, transgenerational epigenetic information might not be transferred to their offspring [28]. Therefore, the wild postlarvae might need to adjust artificial pellets across generations. Moreover, the growth rate in captive prawns might be higher than that in wild postlarvae. The growth patterns were not significantly different between wild and captive postlarvae in BL and BW (Figure 2), and no significant differences were found in BLs between wild and captive postlarvae through the experimental periods (Figure 2a). However, the initial BW was different between wild and captive postlarvae, and significant differences were found in BW between wild and captive postlarvae through the experimental period (Figure 2b). These results suggest that wild postlarvae might be less nourished than captive postlarvae and that condition might be maintained throughout their growth period. Another explanation is that wild postlarvae might not be acclimatized to food-hunting behavior for artificial foods while maintaining opportunistic behavior in the wild. Furthermore, in relation to energy partitioning, the wild postlarvae adjusting to new captive environments might need to increase energy supply to acclimatize to new conditions from wild to experimental conditions. It is suggested that the wild prawn subsequently depletes the energy reserved for growth [29]. These results might suggest a reduction in growth rates, such as WG. Further studies would be required using wild and captive postlarvae from various environments in the wild and multiple hatcheries, respectively, to enable broader conclusions to be drawn.
Specific competitive capacities between wild and captive-bred animals can occur by means of several factors. In captive-bred animals, there are fewer competitors than in wild animals [30]. The high rearing densities and the absence of predatory stimuli in captive environments may lead to aggressive foraging behavior [30]. Density-dependent survival in response to food limitation has been observed in Penaeus esculentus and P. setiferus [31,32], although no study has examined competition in M. rosenbergii between wild and captive breeds. We conducted the experiment using similar numbers of wild and captive postlarvae at the same densities with sufficient food. Therefore, the survival rate might not differ between wild and captive postlarvae in the same experimental environment.
4.2 Effect of temperature on survival and growth
The giant freshwater prawn is susceptible to various factors, such as ambient temperature, stocking density, physiochemical parameters, transport, and storage in aquaculture [13,14,33]. Temperature is an important abiotic factor that can affect the survival and growth of aquatic organisms. The regulation of temperature by the growth rate is the main factor in determining the economic performance of prawn farming [34]. Macrobrachium rosenbergii could acclimate to a wide range of temperatures, with an optimal temperature range between 26 and 31°C [13,14,35]. Successive growth was found at 27 and 30°C, while their growth rates did not differ significantly in the present study. This study suggests that 27 and 30°C would be the optimal temperatures in M. rosenbergii.
There was a sudden decrease in the survival rates at 27 and 30°C over 20 days. The reduction in survival rates occurred after prawns were stocked even when the conditions were ideal [1]. The sudden change in temperature and pH during the stocking period can cause an increase in mortality in prawns [1]. It was also noted that the prawns reared at 27°C had the highest survival rate of 58%, while those reared at 30°C had the second-highest survival rate of 41% throughout the experiment. Paul et al. [36] found a survival rate of 60% after initial stocking days and a survival rate of 55% after 90 days in M. rosenbergii reared at 28°C. The current results and that of the previous study were similar in the survival rate in M. rosenbergii, so a survival rate of 60 % might be common in M. rosenbergii under culturing conditions at the optimal temperature.
The lethal thermal tolerance of M. rosenbergii is less than 14°C and more than 35°C [37]. However, the postlarvae reared at 33°C did not survive within a week after the experiment was initiated in the present study. This result suggests that the temperature range was the lethal thermal tolerance for the postlarvae to live during the rearing experiment. The postlarvae reared at 33°C may represent a predeath thermal point in which the locomotory movements became impaired because of presynaptic failure, and postlarvae might not tolerate such a temperature regime [38,39]. In addition, the higher respiratory rate at increased temperature may also contribute to mortality.
5 Conclusion
This study showed that a temperature range between 27 and 30°C is optimal for postlarvae-to-juvenile M. rosenbergii in terms of survival and growth rate. The prawns reared at 33°C did not survive throughout the experiment. This would suggest the lethal temperature for survival for postlarval to juvenile stages. Precautions of temperature fluctuations are essential to monitor in culturing the prawn, especially in the ponds, as they may affect the growth and survival rates.
The captive prawns exhibited significantly higher WG than the wild prawns. Although the wild prawns also showed WG throughout the entire experiment, it was significantly lower than that of the captive prawns. This weight reduction would be due to its parental lack of acclimation to captive conditions by means of a lack of transgenerational epigenetic inheritance [27]. The wild prawns exerted a lower growth rate due to their energy partitioning, whereby the energy used for growth might be replaced by acclimation to captive conditions.
Domesticated animals, such as farmed aquaculture crustaceans, have a higher survivability rate [30], while we found that there was no significant difference in the survival rates between wild and captive prawns. Our domesticated or farmed prawns might have similar survival rates because each prawn was reared at a density similar to that of sufficient food in this experiment.
This study might provide baseline information for further experiments to improve the aquaculture of the giant freshwater prawn M. rosenbergii and other prawns.
Acknowledgments
We are grateful to Aliyah Deli of Usaha Tani Deli Duman, a local farm company in Brunei Darussalam, for kindly providing the specimens for this study.
-
Funding information: This study was financially supported by the Universiti Brunei Darussalam under the Competitive Research Grant (No. UBD/OVACRI/CRGWG(003)) and the Faculty/Institute/Center Research Grant (No. UBD/RSCH/1.4/FICBF(b)/2020/029) and (No. UBD/RSCH/1.4/FICBF(b)/2021/037).
-
Author contributions: JT: data curation, formal analysis, and writing – original draft; AS: data curation, formal analysis, and writing – original draft; MA: data curation, formal analysis, and writing–original draft; NY: conceptualization and data curation; TA: supervision, conceptualization, writing – review and editing, and funding acquisition.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] New MB. Farming freshwater prawns: a manual for the culture of the giant river prawn (Macrobrachium rosenbergii). In FAO fisheries technical paper. Vol. 428. Rome: Food and Agriculture Organization of the United Nations; 2002. https://freshwater-aquaculture.extension.org/wp-content/uploads/2019/08/Farming_freshwater_prawns_a_manual_for_the_culture_of_giant_rive_prawn_Macorbrachium_rosenbergii.pdf.Search in Google Scholar
[2] New MB. Freshwater prawn farming: global status, recent research and a glance at the future. Aquacult Res. 2005;36:210–30. 10.1111/j.1365-2109.2005.01237.x.10.1111/j.1365-2109.2005.01237.xSearch in Google Scholar
[3] Jalihal D, Sankolli K, Shenoy S. Evolution of larval developmental patterns and the process of freshwaterization in the prawn genus Macrobrachium Bate. 1868 (Decapoda, Palaemonidae). Crustaceana. 1993;65:365–76. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Evolution+of+larval+developmental+patterns+and+the+process+of+freshwaterization+in+the+prawn+genus+Macrobrachium+Bate&btnG=.10.1163/156854093X00793Search in Google Scholar
[4] Hamid HLH. Current status of transboundary fish diseases in Brunei Darussalam: Occurrence, surveillance, research and training. In: Lavilla-Pitogo CR, Nagasawa K, editors, Transboundary Fish diseases in Southeast Asia: Occurence, surveillance, desearch and training. Proceedings of the Meeting on Current Status of Transboundary Fish Diseases in Southeast Asia. Tigbauan, Iloilo, Philippines: SEAFDEC Aquaculture Department; 2004. p. 77–83.Search in Google Scholar
[5] The Fish Site. Brunei govt supports freshwater aquaculture; 2009. https://thefishsite.com/articles/brunei-govt-supports-freshwater-aquaculture.Search in Google Scholar
[6] García-Guerrero MU, Romero R, Vega-Villasante F, Cortes-Jacinto E. Conservation and aquaculture of native freshwater prawns: the case of the cauque river prawn Macrobrachium americanum (Bate, 1868). Lat Am J Aquat Res. 2015;43:819–27. 10.3856/vol43-issue5-fulltext-2.Search in Google Scholar
[7] De Los Santos Romero R, Vega-Villasante F, Cortes-Jacinto E, García-Guerrero M. The culture potential and management problems of freshwater prawns (Macrobrachium americanum and Macrobrachium tenellum) in their native areas: the case for Mexico. Lat Am J Aquat Res. 2021;49:376–90. 10.3856/vol49-issue3-fulltext-2625.Search in Google Scholar
[8] Anger K. Salinity as a key parameter in the larval biology of decapod crustaceans. Invert Repr Dev. 2003;43:29–45. 10.1080/07924259.2003.9652520.10.1080/07924259.2003.9652520Search in Google Scholar
[9] Habashy M, Hassan M. Effects of temperature and salinity on growth and reproduction of the fresh water prawn, Macrobrachium rosenbergii (Crustacea-Decapoda) in Egypt. Int J Env Sci Eng. 2010;1:83–90. http://www.pvamu.edu/engineering/wp-content/uploads/sites/30/IJESE-vol-1-issue-8.pdf.Search in Google Scholar
[10] Chand BK, Trivedi RK, Dubey SK, Rout SK, Beg MM, Das UK. Effect of salinity on survival and growth of giant freshwater prawn Macrobrachium rosenbergii (de Man). Aquacult Rep. 2015;2:26–33. 10.1016/j.aqrep.2015.05.002 .10.1016/j.aqrep.2015.05.002Search in Google Scholar
[11] Bastos AM, Lima JF, Tavares-Dias M. Effect of increase in temperature on the survival and growth of Macrobrachium amazonicum (Palaemonidae) in the Amazon. Aquat Living Resour. 2018;31:21. 10.1051/alr/2018010.10.1051/alr/2018010Search in Google Scholar
[12] Hernández-Sandoval P, Díaz-Herrera F, Díaz-Gaxiola JM, Martínez-Valenzuela C, García-Guerrero M. Effect of temperature on growth, survival, thermal behavior, and critical thermal maximum in the juveniles of Macrobrachium occidentale (Holthuis, 1950) (Decapoda: Caridea: Palaemonidae) from Mexico. J Crusta Biol. 2018;38:483–8. 10.1093/jcbiol/ruy024.Search in Google Scholar
[13] Habashy MM, Sharshar KM. On some factors affecting molting and growth rate of the giant freshwater prawn, Macrobrachium rosenbergii (De Man, 1879). Egyp J Aquat Biol Fish. 2020;24:163–75. 10.21608/EJABF.2020.79317.Search in Google Scholar
[14] John J, Siva VS, Kumar A. Physiological tolerance of the early life history stages of fresh water prawn (Macrobrachium rosenbergii De Man, 1879) to environmental stress. Indian J Geo-Mar Sci. 2020;49:382–9. http://nopr.niscair.res.in/handle/123456789/54428.Search in Google Scholar
[15] New MB. Status of freshwater prawn culture: A review. Aquacult Res. 1995;26:1–54. 10.1111/j.1365-2109.1995.tb00859.x.Search in Google Scholar
[16] Schmidt-Nielsen K. Animal physiology: adaptation and environment. Cambridge, New York: Cambridge University Press; 1997. https://scholar.google.com/scholar?hl=en&q=Animal+physiology:+adaptation+and+environment&as_sdt=0.10.1017/9780511801822Search in Google Scholar
[17] Sunday JM, Bates AE, Dulvy NK. Thermal tolerance and the global redistribution of animals. Nat Clim Change. 2012;2:686–90. 10.1038/nclimate1539.Search in Google Scholar
[18] Sainz-Hernández JC, Fierro-Coronado JA, Aguiñaga-Cruz JA, García-Rodríguez LD, Barraza-López JSA, Santamaría-Miranda A, et al. Effect of temperature on the morphometric development of eggs in the prawn Macrobrachium americanum (Caridea: Palaemonidae) and larval success under experimental conditions. Invertebr Repr Dev. 2016;60:194–200. 10.1080/07924259.2016.1186753.Search in Google Scholar
[19] Lal MM, Seeto J, Pickering TD, Hodge S. Salinity and temperature requirements for larviculture of the Monkey River prawn Macrobrachium lar (Fabricius, 1798) (Decapoda: Caridea: Palaemonidae). Aquaculture. 2012;366–367:1–8. 10.1016/j.aquaculture.2012.08.042.Search in Google Scholar
[20] Hosain ME, Nurul Amin SM, Kamarudin MS, Arshad A, Karim M, Romano N. Effect of salinity on growth, survival, and proximate composition of Macrobrachium rosenbergii post larvae as well as zooplankton composition reared in a maize starch based biofloc system. Aquaculture. 2021;533:736235. 10.1016/j.aquaculture.2020.736235.Search in Google Scholar
[21] Whitehead H, Laland KN, Rendell L, Thorogood R, Whiten A. The reach of gene–culture coevolution in animals. Nat Commun. 2019;10:2405. 10.1038/s41467-019-10293-y.Search in Google Scholar
[22] Tidwell JH, Schulmeister G, Mahl C, Coyle S. Growth, survival and biochemical composition of freshwater prawns, Macrobrachium rosenbergii fed natural food organisms under controlled conditions. J World Aquacult Soc. 1997;28:123–32. 10.1111/j.1749-7345.1997.tb00847.x.Search in Google Scholar
[23] Ismael D, New MB. Biology. In: New MB, Valenti WC, editors. freshwater prawn culture: the farming of Macrobrachium rosenbergii. Blackwell Science; 2000. p. 18–40. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Freshwater+Prawn+Culture%3A+the+farming+of+Macrobrachium+rosenbergii.&btnG=.10.1002/9780470999554.ch3Search in Google Scholar
[24] Jones DA, Kamarudin MS, Vay L. The potential for replacement of live feeds in larval culture. J World Aquacult Soc. 1993;24:199–210. 10.1111/j.1749-7345.1993.tb00009.x.Search in Google Scholar
[25] Moller TH. Feeding behaviour of larvae and postlarvae of Macrobrachium rosenbergii (De Man) (Crustacea, Palaemonidae). J Exp Mar Biol Ecol. 1978;35:251–8. 10.1016/0022-0981(78)90078-3.Search in Google Scholar
[26] Daniels WH, D’Abramo LR, Parseval LD. Design and management of a closed circulating clearwater hatchery system for freshwater prawns Macrobrachium rosenbergii. De Man, 1879. J Shellfish Res. 1992;11:65–73. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Design+and+management+of+a+closed+recirculating+clearwater+hatchery+system+for+freshwater+prawns+macrobrachium+rosenbergii+de+man+1879&btnG=.Search in Google Scholar
[27] Lim JP, Brunet A. Bridging the transgenerational gap with epigenetic memory. Trends Genet. 2013;29:176–86. 10.1016/j.tig.2012.12.008.Search in Google Scholar
[28] GranadaL Lemos MFL, Cabral HN, Novais Bossier P. SC Epigenetics in aquaculture–the last frontier. Rev Aquacult. 2018;10:994–1013. 10.1111/raq.12219.Search in Google Scholar
[29] Mu Y, Wang F, Dong S, Huang G, Dong S. Effects of salinity fluctuation pattern on growth and energy budget of juvenile shrimp Fenneropenaeus chinensis. J Shellfish Res. 2005;24:1217–21. 10.2983/0730-8000(2005)24[1217:EOSFPO]2.0.CO;2.Search in Google Scholar
[30] Weber ED, Fausch KD. Interactions between hatchery and wild salmonids in streams: differences in biology and evidence for competition. Can J Fish Aquat Sci. 2003;60:1018–36. 10.1139/f03-087.Search in Google Scholar
[31] Williams AS, Davis DA, Arnold CR. Density-dependent growth and survival of Penaeus setiferus and Penaeus vannamei in a semi-closed recirculating system. J World Aquacult Soc. 1996;27:107–12. 10.1111/j.1749-7345.1996.tb00600.x.Search in Google Scholar
[32] Arnold SJ, Sellars MJ, Crocos PJ, Coman GJ. Response of juvenile brown tiger shrimp (Penaeus esculentus) to intensive culture conditions in a flow through tank system with three-dimensional artificial substrate. Aquaculture. 2005;246:231–8. 10.1016/j.aquaculture.2005.01.020.Search in Google Scholar
[33] Liu B, Xie J, Ge X, Xu P, Wang A, He Y, et al. Effects of anthraquinone extract from Rheum officinale Bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol. 2010;29:49–57. 10.1016/j.fsi.2010.02.018.Search in Google Scholar
[34] Wyban JA, Ogle J, Pruder GD, Rowland LW, Leung PS. Design, operation, and comparative financial analysis of shrimp farms in Hawaii and Texas. Technical Report 86-6. Honolulu, Hawaii, USA: Ocean Institute; 1987. https://scholar.google.com/scholar?hl=en&q=Design,+operation,+and+comparative+financial+analysis+of+shrimp+farms+in+Hawaii+and+Texas.&as_sdt=0.Search in Google Scholar
[35] Davassi LA. Survival and Growth of the Freshwater Prawn Macrobrachium rosenbergii in Relation to Different Nutrients Composition. J Fish Aquat Sci. 2011;6:649–54. 10.3923/jfas.2011.649.654.Search in Google Scholar
[36] Paul P, Hasan MT, Mazumder SK, Harun-Al-Rashid A, Rahman MA. Growth performance of prawn (Macrobrachium Rosenbergii) in relation to moulting in farmed condition in the Southeast part of Bangladesh. Bangladesh J Sci Res. 2013;25:88–93. https://scholar.google.com/scholar?hl=en&q=GROWTH+PERFORMANCE+OF+PRAWN+(Macrobrachium+rosenbergii)+IN+RELATION+TO+MOULTING+IN+FARMED+CONDITION+IN+THE+SOUTH-EAST+PART+OF+BANGLADESH&as_sdt=0.Search in Google Scholar
[37] New MB. Freshwater prawn culture: a review. Aquaculture. 1990;88:99–143. 10.1016/0044-8486(90)90288-X.Search in Google Scholar
[38] Beitinger TL, Bennett WA, McCauley RW. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Env Biol Fish. 2000;58:237–75. 10.1023/A:1007676325825.Search in Google Scholar
[39] Ern R, Phuong NT, Madsen PT, Wang T, Bayley M. Some like it hot: thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Sci Rep. 2015;5:10743. 10.1038/srep10743.Search in Google Scholar PubMed PubMed Central
© 2022 Jolene Tay et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Foliar application of boron positively affects the growth, yield, and oil content of sesame (Sesamum indicum L.)
- Impacts of adopting specialized agricultural programs relying on “good practice” – Empirical evidence from fruit growers in Vietnam
- Evaluation of 11 potential trap crops for root-knot nematode (RKN) control under glasshouse conditions
- Technical efficiency of resource-poor maize farmers in northern Ghana
- Bulk density: An index for measuring critical soil compaction levels for groundnut cultivation
- Efficiency of the European Union farm types: Scenarios with and without the 2013 CAP measures
- Participatory validation and optimization of the Triple S method for sweetpotato planting material conservation in southern Ethiopia
- Selection of high-yield maize hybrid under different cropping systems based on stability and adaptability parameters
- Soil test-based phosphorus fertilizer recommendation for malting barley production on Nitisols
- Effects of domestication and temperature on the growth and survival of the giant freshwater prawn (Macrobrachium rosenbergii) postlarvae
- Influence of irrigation regime on gas exchange, growth, and oil quality of field grown, Texas (USA) olive trees
- Present status and prospects of value addition industry for agricultural produce – A review
- Competitiveness and impact of government policy on chili in Indonesia
- Growth of Rucola on Mars soil simulant under the influence of pig slurry and earthworms
- Effect of potassium fertilizer application in teff yield and nutrient uptake on Vertisols in the central highlands of Ethiopia
- Dissection of social interaction and community engagement of smallholder oil palm in reducing conflict using soft system methodology
- Farmers’ perception, awareness, and constraints of organic rice farming in Indonesia
- Improving the capacity of local food network through local food hubs’ development
- Quality evaluation of gluten-free biscuits prepared with algarrobo flour as a partial sugar replacer
- Effect of pre-slaughter weight on morphological composition of pig carcasses
- Study of the impact of increasing the highest retail price of subsidized fertilizer on rice production in Indonesia
- Agrobiodiversity and perceived climatic change effect on family farming systems in semiarid tropics of Kenya
- Influences of inter- and intra-row spacing on the growth and head yield of cabbage (Brassica oleracea var. capitata) in western Amhara, Ethiopia
- The supply chain and its development concept of fresh mulberry fruit in Thailand: Observations in Nan Province, the largest production area
- Toward achieving sustainable development agenda: Nexus between agriculture, trade openness, and oil rents in Nigeria
- Phenotyping cowpea accessions at the seedling stage for drought tolerance in controlled environments
- Apparent nutrient utilization and metabolic growth rate of Nile tilapia, Oreochromis niloticus, cultured in recirculating aquaculture and biofloc systems
- Influence of season and rangeland-type on serum biochemistry of indigenous Zulu sheep
- Meta-analysis of responses of broiler chickens to Bacillus supplementation: Intestinal histomorphometry and blood immunoglobulin
- Weed composition and maize yield in a former tin-mining area: A case study in Malim Nawar, Malaysia
- Strategies for overcoming farmers’ lives in volcano-prone areas: A case study in Mount Semeru, Indonesia
- Principal component and cluster analyses based characterization of maize fields in southern central Rift Valley of Ethiopia
- Profitability and financial performance of European Union farms: An analysis at both regional and national levels
- Analysis of trends and variability of climatic parameters in Teff growing belts of Ethiopia
- Farmers’ food security in the volcanic area: A case in Mount Merapi, Indonesia
- Strategy to improve the sustainability of “porang” (Amorphophallus muelleri Blume) farming in support of the triple export movement policy in Indonesia
- Agrarian contracts, relations between agents, and perception on energy crops in the sugarcane supply chain: The Peruvian case
- Factors influencing the adoption of conservation agriculture by smallholder farmers in KwaZulu-Natal, South Africa
- Meta-analysis of zinc feed additive on enhancement of semen quality, fertility and hatchability performance in breeder chickens
- Meta-analysis of the potential of dietary Bacillus spp. in improving growth performance traits in broiler chickens
- Biocomposites from agricultural wastes and mycelia of a local mushroom, Lentinus squarrosulus (Mont.) Singer
- Cross transferability of barley nuclear SSRs to pearl millet genome provides new molecular tools for genetic analyses and marker assisted selection
- Detection of encapsulant addition in butterfly-pea (Clitoria ternatea L.) extract powder using visible–near-infrared spectroscopy and chemometrics analysis
- The willingness of farmers to preserve sustainable food agricultural land in Yogyakarta, Indonesia
- Transparent conductive far-infrared radiative film based on polyvinyl alcohol with carbon fiber apply in agriculture greenhouse
- Grain yield stability of black soybean lines across three agroecosystems in West Java, Indonesia
- Forms of land access in the sugarcane agroindustry: A comparison of Brazilian and Peruvian cases
- Assessment of the factors contributing to the lack of agricultural mechanization in Jiroft, Iran
- Do poor farmers have entrepreneurship skill, intention, and competence? Lessons from transmigration program in rural Gorontalo Province, Indonesia
- Communication networks used by smallholder livestock farmers during disease outbreaks: Case study in the Free State, South Africa
- Sustainability of Arabica coffee business in West Java, Indonesia: A multidimensional scaling approach
- Farmers’ perspectives on the adoption of smart farming technology to support food farming in Aceh Province, Indonesia
- Rice yield grown in different fertilizer combination and planting methods: Case study in Buru Island, Indonesia
- Paclobutrazol and benzylaminopurine improve potato yield grown under high temperatures in lowland and medium land
- Agricultural sciences publication activity in Russia and the impact of the national project “Science.” A bibliometric analysis
- Storage conditions and postharvest practices lead to aflatoxin contamination in maize in two counties (Makueni and Baringo) in Kenya
- Relationship of potato yield and factors of influence on the background of herbological protection
- Biology and life cycle Of Diatraea busckella (Lepidoptera: Crambidae) under simulated altitudinal profile in controlled conditions
- Evaluation of combustion characteristics performances and emissions of a diesel engine using diesel and biodiesel fuel blends containing graphene oxide nanoparticles
- Effect of various varieties and dosage of potassium fertilizer on growth, yield, and quality of red chili (Capsicum annuum L.)
- Review Articles
- Germination ecology of three Asteraceae annuals Arctotis hirsuta, Oncosiphon suffruticosum, and Cotula duckittiae in the winter-rainfall region of South Africa: A review
- Animal waste antibiotic residues and resistance genes: A review
- A brief and comprehensive history of the development and use of feed analysis: A review
- The evolving state of food security in Nigeria amidst the COVID-19 pandemic – A review
- Short Communication
- Response of cannabidiol hemp (Cannabis sativa L.) varieties grown in the southeastern United States to nitrogen fertilization
- Special Issue on the International Conference on Multidisciplinary Research – Agrarian Sciences
- Special issue on the International Conference on Multidisciplinary Research – Agrarian Sciences: Message from the editor
- Maritime pine land use environmental impact evolution in the context of life cycle assessment
- Influence of different parameters on the characteristics of hazelnut (var. Grada de Viseu) grown in Portugal
- Organic food consumption and eating habit in Morocco, Algeria, and Tunisia during the COVID-19 pandemic lockdown
- Customer knowledge and behavior on the use of food refrigerated display cabinets: A Portuguese case
- Perceptions and knowledge regarding quality and safety of plastic materials used for food packaging
- Understanding the role of media and food labels to disseminate food related information in Lebanon
- Liquefaction and chemical composition of walnut shells
- Validation of an analytical methodology to determine humic substances using low-volume toxic reagents
- Special Issue on the International Conference on Agribusiness and Rural Development – IConARD 2020
- Behavioral response of breeder toward development program of Ongole crossbred cattle in Yogyakarta Special Region, Indonesia
- Special Issue on the 2nd ICSARD 2020
- Perceived attributes driving the adoption of system of rice intensification: The Indonesian farmers’ view
- Value-added analysis of Lactobacillus acidophilus cell encapsulation using Eucheuma cottonii by freeze-drying and spray-drying
- Investigating the elicited emotion of single-origin chocolate towards sustainable chocolate production in Indonesia
- Temperature and duration of vernalization effect on the vegetative growth of garlic (Allium sativum L.) clones in Indonesia
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Prediction model for agro-tourism development using adaptive neuro-fuzzy inference system method
- Special Issue of International Web Conference on Food Choice and Eating Motivation
- Can ingredients and information interventions affect the hedonic level and (emo-sensory) perceptions of the milk chocolate and cocoa drink’s consumers?
Articles in the same Issue
- Regular Articles
- Foliar application of boron positively affects the growth, yield, and oil content of sesame (Sesamum indicum L.)
- Impacts of adopting specialized agricultural programs relying on “good practice” – Empirical evidence from fruit growers in Vietnam
- Evaluation of 11 potential trap crops for root-knot nematode (RKN) control under glasshouse conditions
- Technical efficiency of resource-poor maize farmers in northern Ghana
- Bulk density: An index for measuring critical soil compaction levels for groundnut cultivation
- Efficiency of the European Union farm types: Scenarios with and without the 2013 CAP measures
- Participatory validation and optimization of the Triple S method for sweetpotato planting material conservation in southern Ethiopia
- Selection of high-yield maize hybrid under different cropping systems based on stability and adaptability parameters
- Soil test-based phosphorus fertilizer recommendation for malting barley production on Nitisols
- Effects of domestication and temperature on the growth and survival of the giant freshwater prawn (Macrobrachium rosenbergii) postlarvae
- Influence of irrigation regime on gas exchange, growth, and oil quality of field grown, Texas (USA) olive trees
- Present status and prospects of value addition industry for agricultural produce – A review
- Competitiveness and impact of government policy on chili in Indonesia
- Growth of Rucola on Mars soil simulant under the influence of pig slurry and earthworms
- Effect of potassium fertilizer application in teff yield and nutrient uptake on Vertisols in the central highlands of Ethiopia
- Dissection of social interaction and community engagement of smallholder oil palm in reducing conflict using soft system methodology
- Farmers’ perception, awareness, and constraints of organic rice farming in Indonesia
- Improving the capacity of local food network through local food hubs’ development
- Quality evaluation of gluten-free biscuits prepared with algarrobo flour as a partial sugar replacer
- Effect of pre-slaughter weight on morphological composition of pig carcasses
- Study of the impact of increasing the highest retail price of subsidized fertilizer on rice production in Indonesia
- Agrobiodiversity and perceived climatic change effect on family farming systems in semiarid tropics of Kenya
- Influences of inter- and intra-row spacing on the growth and head yield of cabbage (Brassica oleracea var. capitata) in western Amhara, Ethiopia
- The supply chain and its development concept of fresh mulberry fruit in Thailand: Observations in Nan Province, the largest production area
- Toward achieving sustainable development agenda: Nexus between agriculture, trade openness, and oil rents in Nigeria
- Phenotyping cowpea accessions at the seedling stage for drought tolerance in controlled environments
- Apparent nutrient utilization and metabolic growth rate of Nile tilapia, Oreochromis niloticus, cultured in recirculating aquaculture and biofloc systems
- Influence of season and rangeland-type on serum biochemistry of indigenous Zulu sheep
- Meta-analysis of responses of broiler chickens to Bacillus supplementation: Intestinal histomorphometry and blood immunoglobulin
- Weed composition and maize yield in a former tin-mining area: A case study in Malim Nawar, Malaysia
- Strategies for overcoming farmers’ lives in volcano-prone areas: A case study in Mount Semeru, Indonesia
- Principal component and cluster analyses based characterization of maize fields in southern central Rift Valley of Ethiopia
- Profitability and financial performance of European Union farms: An analysis at both regional and national levels
- Analysis of trends and variability of climatic parameters in Teff growing belts of Ethiopia
- Farmers’ food security in the volcanic area: A case in Mount Merapi, Indonesia
- Strategy to improve the sustainability of “porang” (Amorphophallus muelleri Blume) farming in support of the triple export movement policy in Indonesia
- Agrarian contracts, relations between agents, and perception on energy crops in the sugarcane supply chain: The Peruvian case
- Factors influencing the adoption of conservation agriculture by smallholder farmers in KwaZulu-Natal, South Africa
- Meta-analysis of zinc feed additive on enhancement of semen quality, fertility and hatchability performance in breeder chickens
- Meta-analysis of the potential of dietary Bacillus spp. in improving growth performance traits in broiler chickens
- Biocomposites from agricultural wastes and mycelia of a local mushroom, Lentinus squarrosulus (Mont.) Singer
- Cross transferability of barley nuclear SSRs to pearl millet genome provides new molecular tools for genetic analyses and marker assisted selection
- Detection of encapsulant addition in butterfly-pea (Clitoria ternatea L.) extract powder using visible–near-infrared spectroscopy and chemometrics analysis
- The willingness of farmers to preserve sustainable food agricultural land in Yogyakarta, Indonesia
- Transparent conductive far-infrared radiative film based on polyvinyl alcohol with carbon fiber apply in agriculture greenhouse
- Grain yield stability of black soybean lines across three agroecosystems in West Java, Indonesia
- Forms of land access in the sugarcane agroindustry: A comparison of Brazilian and Peruvian cases
- Assessment of the factors contributing to the lack of agricultural mechanization in Jiroft, Iran
- Do poor farmers have entrepreneurship skill, intention, and competence? Lessons from transmigration program in rural Gorontalo Province, Indonesia
- Communication networks used by smallholder livestock farmers during disease outbreaks: Case study in the Free State, South Africa
- Sustainability of Arabica coffee business in West Java, Indonesia: A multidimensional scaling approach
- Farmers’ perspectives on the adoption of smart farming technology to support food farming in Aceh Province, Indonesia
- Rice yield grown in different fertilizer combination and planting methods: Case study in Buru Island, Indonesia
- Paclobutrazol and benzylaminopurine improve potato yield grown under high temperatures in lowland and medium land
- Agricultural sciences publication activity in Russia and the impact of the national project “Science.” A bibliometric analysis
- Storage conditions and postharvest practices lead to aflatoxin contamination in maize in two counties (Makueni and Baringo) in Kenya
- Relationship of potato yield and factors of influence on the background of herbological protection
- Biology and life cycle Of Diatraea busckella (Lepidoptera: Crambidae) under simulated altitudinal profile in controlled conditions
- Evaluation of combustion characteristics performances and emissions of a diesel engine using diesel and biodiesel fuel blends containing graphene oxide nanoparticles
- Effect of various varieties and dosage of potassium fertilizer on growth, yield, and quality of red chili (Capsicum annuum L.)
- Review Articles
- Germination ecology of three Asteraceae annuals Arctotis hirsuta, Oncosiphon suffruticosum, and Cotula duckittiae in the winter-rainfall region of South Africa: A review
- Animal waste antibiotic residues and resistance genes: A review
- A brief and comprehensive history of the development and use of feed analysis: A review
- The evolving state of food security in Nigeria amidst the COVID-19 pandemic – A review
- Short Communication
- Response of cannabidiol hemp (Cannabis sativa L.) varieties grown in the southeastern United States to nitrogen fertilization
- Special Issue on the International Conference on Multidisciplinary Research – Agrarian Sciences
- Special issue on the International Conference on Multidisciplinary Research – Agrarian Sciences: Message from the editor
- Maritime pine land use environmental impact evolution in the context of life cycle assessment
- Influence of different parameters on the characteristics of hazelnut (var. Grada de Viseu) grown in Portugal
- Organic food consumption and eating habit in Morocco, Algeria, and Tunisia during the COVID-19 pandemic lockdown
- Customer knowledge and behavior on the use of food refrigerated display cabinets: A Portuguese case
- Perceptions and knowledge regarding quality and safety of plastic materials used for food packaging
- Understanding the role of media and food labels to disseminate food related information in Lebanon
- Liquefaction and chemical composition of walnut shells
- Validation of an analytical methodology to determine humic substances using low-volume toxic reagents
- Special Issue on the International Conference on Agribusiness and Rural Development – IConARD 2020
- Behavioral response of breeder toward development program of Ongole crossbred cattle in Yogyakarta Special Region, Indonesia
- Special Issue on the 2nd ICSARD 2020
- Perceived attributes driving the adoption of system of rice intensification: The Indonesian farmers’ view
- Value-added analysis of Lactobacillus acidophilus cell encapsulation using Eucheuma cottonii by freeze-drying and spray-drying
- Investigating the elicited emotion of single-origin chocolate towards sustainable chocolate production in Indonesia
- Temperature and duration of vernalization effect on the vegetative growth of garlic (Allium sativum L.) clones in Indonesia
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Prediction model for agro-tourism development using adaptive neuro-fuzzy inference system method
- Special Issue of International Web Conference on Food Choice and Eating Motivation
- Can ingredients and information interventions affect the hedonic level and (emo-sensory) perceptions of the milk chocolate and cocoa drink’s consumers?

