Abstract
Bacillus probiotics have been shown to possess several advantages over conventional probiotics, including the capacity to withstand heat during feed manufacturing processes and to be stored for a long time without losing viability, as well as the ability to survive and function in the acidic environment of the chicken gut. However, there are inconsistent results on the effect of Bacillus on growth performance of broiler chickens. The objective of this meta-analysis was to assess the effect of dietary Bacillus supplementation on feed intake (FI), feed conversion efficiency (FCE), and average daily gain (ADG) in broiler chickens. PubMed, Google Scholar, and Scopus databases were searched for studies that fed diets with and without Bacillus to broilers. Pooled estimation revealed that Bacillus supplementation improved FCE (standardised mean difference [SMD] = −0.33, 95% confidence interval (CI) −0.39 to −0.28) and increased ADG (SMD = 0.37 g/bird/day, 95% CI 0.28–0.46). In contrast, feed intake (SMD) = 0.03 g/bird/day, 95% CI −0.03 to 0.09) was not significantly different from controls. Subanalysis revealed that broilers fed with Bacillus at 0.1–0.5 × 106 colony-forming unit (cfu)/g had higher ADG than controls. There is evidence of significant heterogeneity (inconsistency index [I 2] = 80–93%) among the studies included in the meta-analysis. Meta-regression showed that studied moderators (i.e., Bacillus spp., duration of supplementation, and broiler strain) explained most of the effect. In conclusion, our results suggest that Bacillus supplementation at 0.1–0.5 × 106 cfu/g improved FCE and ADG in broiler chickens. However, it is recommended that more research be conducted to determine the Bacillus supplementation dose that optimises growth performance indices in broiler chickens.
1 Introduction
Chicken production has undergone many changes over the years, moving from free range system to an intensive system. Although intensive chicken production is economical, it is associated with increased stress in birds, leading to impaired immunity and productivity, thus necessitating the use of several growth promoters such as in-feed antibiotics and probiotics to improve chicken and livestock performance [1,2,3,4]. Antibiotics are added in animal feed at lower concentrations to enhance growth performance parameters in chickens [5]. However, the ban on the use of antibiotics in chicken rations in many parts of the world because of growing problem of antimicrobial resistance, together with the deposition of antibiotic residues in meat and eggs has led to the search for alternatives. Probiotics is one such alternative, which are live micro-organisms that have the potential to improve host health when given at the right doses [1,2,3]. Conventional probiotics used in broiler chicken production include Lactobacillus spp., Saccharomyces spp., Enterococcus spp., and Bifidobacteria [1,4,5]. However, the use of conventional probiotics in the poultry industry is still problematic due to their inability to withstand heat during pelleting, poor storage life, and their low viability in the harsh environment of chicken gut, all of which led to the search for better probiotics for use in broiler chicken production [6].
The use of Bacillus in the broiler chicken industry is on the increase as it has features that address some of the limitations of conventional probiotics. Bacillus is a gram-positive bacterium with the potential to form endospores. Bacillus is moderate in minerals, amino acids, and vitamins [7,8], and has the ability to survive the low pH of the chicken gut [9]. Furthermore, Bacillus is stable and viable during feed processing, storage, and gut transit due to its endospore forming capability [8,10,11]. The probable mechanisms by which Bacillus spp. limit the proliferation of pathogens include competition for adhesion sites, production of organic acids leading to a reduction in gut pH, and maintenance of normal gut microbiota via competitive exclusion and antagonisms [12,13,14]. It may also achieve this by production of antimicrobial compounds, improvement in oxidative stability, modulation of immune systems, increase in digestive enzyme activity, and competition for nutrients [12,13,14].
Bacillus has been demonstrated to increase growth rate and efficiency of digestion in broilers by lowering gut oxygen concentrations [10], inhibiting bacterial metabolism, and increasing nutrient uptake in the small intestine [15]. On the other hand, influence of Bacillus on growth rate in broilers are not consistent. Some authors [16,17,18] found that Bacillus enhance growth performance in broilers, while others [19,20,21] state otherwise. This variation may be due to study design, broiler strain, inclusion level, and duration of Bacillus supplementation as reported by Ogbuewu et al. [22]. Currently, there is no study on the impact of Bacillus on the performance of broiler chickens using the outcomes of published studies.
One method for combining the results of published studies that assessed the same research questions is meta-analysis [22,23,24]. It is a statistical method used to resolving disagreements among studies and identifying research gaps and patterns that would not normally be visible in a single study [25,26]. In order to bridge the identified research gaps, the authors quantitatively pool and analyse the results of original investigations that evaluated the effect of Bacillus supplementation on growth performance of broiler chickens.
2 Materials and methods
2.1 Data source and search strategy
This meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Review and meta-analyses. PubMed, Google Scholar, and Scopus databases were searched for published studies that evaluated the impact of Bacillus supplementation on growth performance of broiler chickens. The reference list of retrieved studies was also searched for related articles. There was no date and language restriction in our systematic search since there is no published meta-analysis in this area in the literature. The search words were broiler chickens, Bacillus, feed intake, FCE, and ADG.
2.2 Eligibility criteria
One hundred and ninety-eight articles were retrieved in a systematic search performed in PubMed, Google Scholar, and Scopus databases and two additional studies were identified from the search performed on the reference list of the retrieved articles. Two hundred published articles were identified and 42 articles satisfied the eligibility conditions for the study as illustrated in Figure 1. To be added in the study, articles must have assessed at least one of the measured outcomes (feed intake (FI), feed conversion efficiency [FCE], or average daily gain [ADG]) in broiler chickens along with a measure of variance such as standard deviation (SD), standard error (SE), or p-value. In addition, Bacillus should be the only supplement added to the diet. The details of the 42 articles that met the inclusion criteria for the meta-analysis are shown in Table 1.

Flow diagram of the paper selection process used for the meta-analysis.
Characteristics of studies included in the meta-analysis of effect of dietary Bacillus supplementation in broiler chickens
| Ref. | Locations | Datasets | Explanatory variable | Response variables | |||
|---|---|---|---|---|---|---|---|
| Bacillus spp. | SL (×106 cfu/g) | DOS (days) | Broiler strain | ||||
| [16] | North Korea | 5 | amyloliquefaciens | 0.1–2.0 | 1–35 | Ross | FI, FCE, and ADG |
| [17] | Germany | 2 | subtilis | 0.8 | 1–42 | Cobb | FI and ADG |
| [19] | Korea | 2 | subtilis | 1.0 | 1–28 | Ross | FI, FCE, and ADG |
| [20] | USA | 2 | subtilis | 3.0 | 1–28 | Cobb | FI, FCE, and ADG |
| [21] | Brazil | 3 | subtilis | 3.0–6.0 | 1–35/1–42 | Ross | FI, FCE, and ADG |
| [28] | Korea | 3 | subtilis | 0.3–0.6 | 1–35 | Ross | FI, FCE, and ADG |
| [29] | Iran | 2 | subtilis | 0.2 | 1–49 | Ross | FI and FCE |
| [30] | China | 2 | coagulans | 1.0 | 1–42 | AA | FI, FCE, and ADG |
| [31] | Tunisia | 2 | subtilis | 1.0 | 1–35 | AA | FI |
| [32] | Korea | 2 | subtilis | 1.0 | 1–35 | Ross | FI, FCE, and ADG |
| [18] | Poland | 2 | subtilis | 2.5 | 1–42 | Ross | FI, FCE, and ADG |
| [33] | Brazil | 2 | subtilis | 0.2 | 1–42 | Cobb | FI, FCE, and ADG |
| [34] | China | 2 | amyloliquefaciens | 0.2 | 1–35 | Cobb | FCE and ADG |
| [35] | Poland | 2 | licheniformis | 0.5 | 1–36 | Ross | FI, FCE, and ADG |
| [36] | Indonesia | 2 | subtilis | 0.2 | 1–56 | — | FI, FCE, and ADG |
| [37] | Australia | 2 | amyloliquefaciens | 1.0 | 1–35 | Ross | FI, FCE, and ADG |
| [38] | Denmark | 2 | subtilis | 1.0 | 1–42 | Cobb | FI, FCE, and ADG |
| [39] | China | 5 | subtilis | 0.2–0.5 | 1–42 | AA | FI, FCE, and ADG |
| [40] | USA | 2 | subtilis | 0.5 | 1–42 | Cobb | FI, FCE, and ADG |
| [41] | USA | 2 | subtilis | 0.3 | 1–42 | — | FCE |
| [42] | USA | 2 | subtilis | 3.4 | 1–41 | — | FI and FCE |
| [43] | Korea | 2 | subtilis | 0.1 | 1–35 | AA | FI, FCE, and ADG |
| [44] | China | 5 | subtilis | 0.1–0.25 | 1–42 | AA | FI, FCE, and ADG |
| [45] | Iran | 2 | subtilis | 0.5 | 1–42 | Arian | FI and FCE |
| [46] | Jordan | 2 | subtilis | 1.0 | 1–35 | Hubbard | FI, FCE, and ADG |
| [47] | Italy | 2 | coagulans | 0.25 | 1–49 | Ross | FI and ADG |
| [48] | China | 3 | licheniformis | 1.0–2.0 | 1–42 | — | ADG |
| [49] | Australia | 4 | coagulans | 0.1–0.25 | 1–42 | AA | FI, FCE, and ADG |
| [50] | Korea | 4 | subtilis | 0.15–0.45 | 1–35 | Ross | FI, FCE, and ADG |
| [51] | Indonesia | 3 | subtilis | 1.0–2.0 | 1–42 | — | FI and FCE |
| [52] | Malaysia | 2 | subtilis | 1.0 | 1–28 | AA | FCE and ADG |
| [53] | China | 3 | subtilis | 0.4 | 1–21 | AA | FI, FCE, and ADG |
| [54] | Hungary | 2 | subtilis | 0.5 | 1–42 | — | FI and FCE |
| [55] | China | 5 | subtilis | 0.2–0.5 | 1–21 | AA | FI and FCE |
| [56] | Denmark | 2 | subtilis | 0.5 | 1–42 | Ross | FI, FCE, and ADG |
| [57] | India | 2 | subtilis | 0.4 | 1–35 | Cobb | FI |
| [58] | Taiwan | 3 | licheniformis | 1.0 – 3.0 | 1–35 | Ross | FI, FCE, and ADG |
| [59] | USA | 2 | subtilis | 0.5 | 1–42 | Ross | FI, FCE, and ADG |
| [60] | Singapore | 2 | subtilis | 1.0 | 1–21/1–42 | Ross | FI, FCE, and ADG |
| [61] | China | 2 | subtilis | 1.0 | 1–21/1–42 | AA | FI, FCE, and ADG |
| [62] | China | 3 | amyloliquefaciens | 3.0–6.0 | 1–21/1–42 | AA | FI, FCE, and ADG |
| [63] | China | 2 | * | 1.0 | 1–21/1–42 | Ross | FI, FCE, and ADG |
*subtilis, licheniformis and cereus; AA – Arbor acres; DOS – duration of study; FI – feed intake; FCE – feed conversion efficiency; ADG – average daily gain.
2.3 Data extraction and processing
Data on means of FI, FCE, and ADG for the control and treatment groups as well as their measures of variance from each of the 42 studies that met the inclusion criteria were extracted. In addition, information was extracted on the following modifiers: Bacillus spp. (B. subtilis, B. coagulans, B. amyloliquefaciens, B. licheniformis, and B. cereus), duration of supplementation (DOS) of Bacillus (1–21, 1–28, 1–35, 1–36, 1–41, 1–42, and 1–49 days), broiler strains (Ross, Cobb, Arbor Acres, Arian, and Hubbard), and supplementation level (SL) of Bacillus (0.1–0.5, 0.6–1.0, and >1.0 × 106 cfu/g) that we considered a priori to influence trial outcomes of the study for subgroup and meta-regression analyses, where it was provided. The supplementation dose level was categorised based on the level included in the individual studies used for the meta-analysis. When a trial reported SE instead of SD, SD was calculated using the equation (SD = SE × √n) as reported by Higgins and Deeks [27], where “n = number of chickens.” In studies with multiple comparisons, the control group was compared with each treatment group separately. A database of 42 articles that met the selection conditions for meta-analysis was created as shown in Table 1.
2.4 Statistical analysis
Results were combined using the standardised mean difference (SMD) for random-effects model and presented as 95% CI for each study outcome according to the method of Borenstein et al. [64]. Statistical analysis was performed on outcomes of interest using Open Meta-analyst for Ecology and Evolution (OpenMEE) software [65]. Articles were aggregated using inverse variance method [66]. Bar graphs of publication year were created in Microsoft Excel 2010. SMD was considered significant when the lower and upper CIs did not include zero [25]. SMD values of 0.2, 0.5, and 0.8 were considered as low, moderate, and large, respectively [67]. Subanalysis with fewer than three studies was not reported because of low statistical power. Chi-square (Q) test and the I 2 statistic were used to assess heterogeneity [68]. The I 2 values of 25, 50, and 75% indicate low, moderate, and substantial heterogeneity, respectively [69]. Meta-regression results were considered significant at 5% probability level [70]. Sensitivity analysis was performed using the method of Lean et al. [71] whereas publication bias was examined using funnel plots and Rosenberg’s fail-safe number (Nfs). Nfs indicates the number of non-significant, unpublished (or missing) articles that will be required to reduce the overall statistically significant observed result to non-significance. However, according to Rosenberg [72], the results of a meta-analysis is deemed robust regardless of the presence of publication bias when Nfs is greater than “5(n) + 10,” where n = number of studies included in the meta-analysis.
3 Results
3.1 Features of studies included in the meta-analysis
Studies which were review papers (n = 10), non-randomised studies (n = 5), and studies on diseased broilers (n = 39) were excluded as shown in Figure 1. Studies not conducted in broiler chickens (n = 77) and trials that had no extractable data (n = 3) were discarded. Studies were also removed if they fed Bacillus in combination with other growth promoters (n = 8) and did not report any outcome of interest (n = 7). The characteristics of studies included in the meta-analysis as presented in Table 1 revealed that studies used for the meta-analysis span for 25 years (1995–2020) with 88% of the articles published between 2011 and 2020. In addition, broiler chickens utilised for the meta-analysis were aged between 1 and 49 days. Bacillus supplementation doses were ranged from 0.1 to 6.0 × 106 cfu/g feed (Table 1). Thirty-eight studies were used to evaluate the effect of Bacillus on FI, whereas, 37 and 33 trials were included to assess the effect of dietary Bacillus supplementation on FCE and ADG, respectively. The spatial distribution of studies by country revealed that studies used for this analysis were conducted in 18 countries (Figure 2), with China having the highest number followed by North Korea and USA (Figure 2).

Plots of number of studies from each country.
3.2 Probiotic effect
The pooled effect size of 96 datasets, with 29,940 broiler chickens (20,241 for treatment group and 9,699 for control group) revealed that Bacillus had no effect on FI (SMD = 0.03 g/bird/day, 95% CI −0.03 to0.09; Figure 3). In contrast, the meta-analysis of 95 datasets, with 17,887 chickens (12,113 for treatment group and 5,774 for control group) suggested that dietary Bacillus supplementation significantly improved FCE in comparison with controls (SMD = −0.33, 95% CI −0.39 to −0.28; Figure 4). The analysis of 89 datasets with 18,147 broiler chickens (12,525 and 5,622 for Bacillus and control groups, respectively) significantly increased ADG (SMD = 0.37 g/bird/day, 95% CI 0.28–0.46; Figure 5) compared to controls. The magnitude of effect estimate was higher in ADG (0.37) than in FCE (0.33) in the present meta-analysis.

Influence of Bacillus supplementation on feed intake in broiler chickens. Pooled estimation (SMD) = 0 (thick line) suggests no effect, SMD > 0 suggests an increase in variables of interest over the controls, and SMD < 0 denotes a decline in variables of interest over the controls. The dotted line with a diamond denotes the cumulative effect size across all studies used for the meta-analysis.
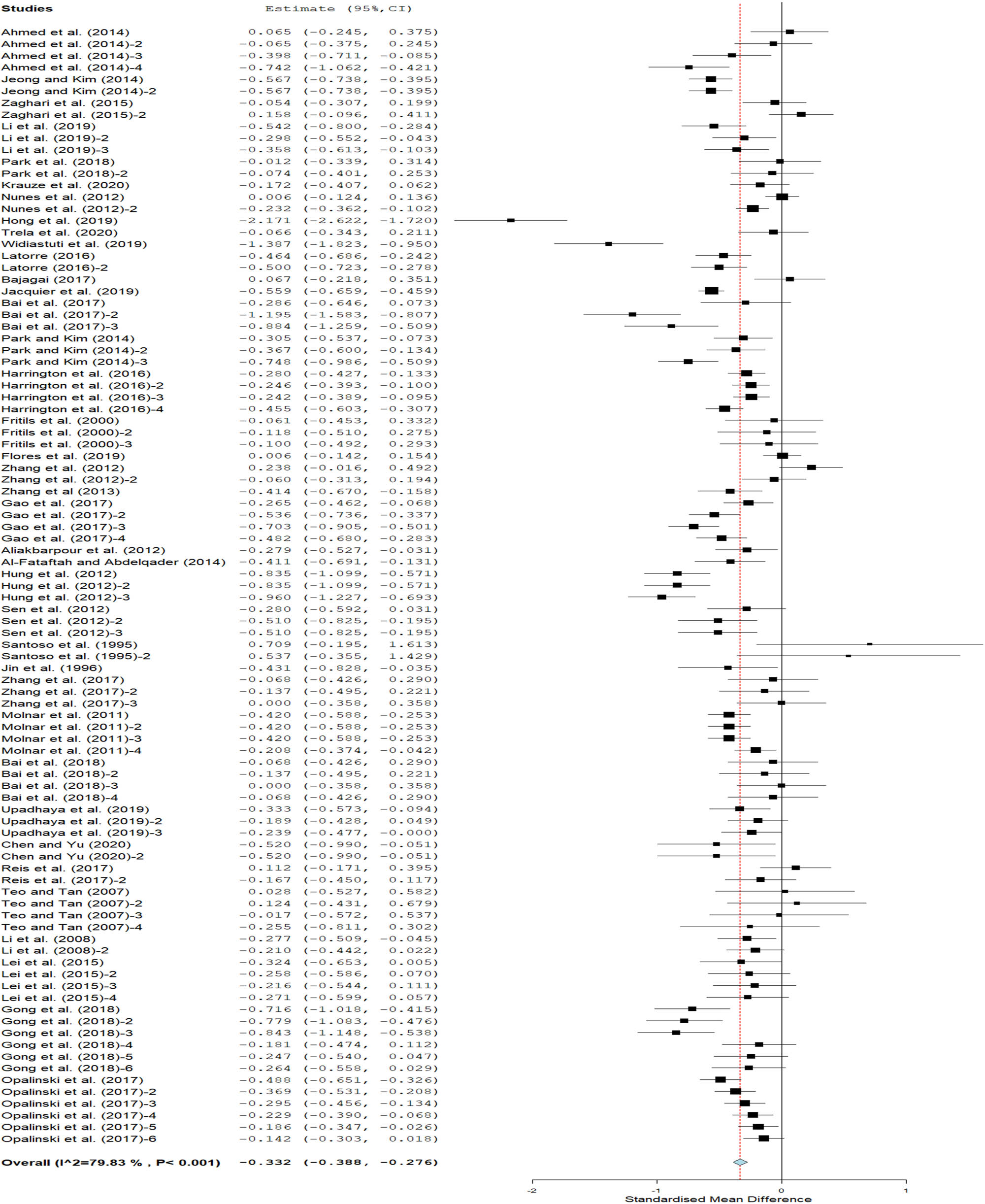
Effect of Bacillus supplementation on FCE in broiler chickens. Pooled estimation (SMD) = 0 (thick line) suggests no effect, SMD > 0 suggests an increase in variables of interest over the controls and SMD < 0 denotes a decline in variables of interest over the controls. The dotted line with a diamond denotes the cumulative effect size across all studies used for the meta-analysis.

Effect of Bacillus supplementation on ADG in broiler chickens. Pooled estimation (SMD) = 0 (thick line) suggests no effect, SMD > 0 suggests an increase in variables of interest over the controls and SMD < 0 denotes a decline in variables of interest over the controls. The dotted line with a diamond denotes the cumulative effect size across all studies used for the meta-analysis.
3.3 Stratification analysis
Subanalysis of the effect of studied moderators on FI, FCE, and ADG in broiler chickens on dietary Bacillus are presented in Tables 2–4. Results indicate that Cobb strain fed with Bacillus supplemented diets at 0.6–1.0 cfu/g (moderate dose) and > 1.0 × 106 cfu/g (high dose) for 28 days had significantly reduced FI compared to controls (SMD = −0.09, 95% CI −0.17 to −0.02). Cobb strain fed with Bacillus supplemented diets at moderate and high doses for 28 days had significantly reduced feed intake compared to controls. In contrast, Arbor Acres and Ross strains fed with Bacillus supplemented diets had similar FI with the controls. Similarly, Arbor Acres and Ross strains fed with Bacillus supplemented diets for 1–21, 1–35, 1–42, and 1–49 day had similar FI with the controls. There was no effect of Bacillus spp. on FI in broiler chickens. In contrast, Ross, Arbor Acres, and Cobb strains fed with B. subtilis, B. amyloliquefaciens, B. coagulans, and B. licheniformis at lower (0.1–0.5 × 106 cfu/g), moderate, and higher doses for 1–21 days, 1–28 days, 1–35 days, and 1–42 day had better FCE than controls (Table 3). Broilers fed with B. subtilis differed significantly from those offered with B. coagulans, but similar to those given B. amyloliquefaciens, and B. licheniformis. Ross, Arbor Acres, and Cobb strains fed with B. subtilis, B. amyloliquefaciens, B. coagulans, and B. licheniformis at 0.1 – 0.5 × 106 cfu/g for 1–35 days and 1–42 days had higher ADG than controls. In converse, broilers fed with moderate doses of Bacillus spp. for 1–28 days had comparable FCE with controls.
Subgroup analysis of the effect of Bacillus probiotics on feed intake of broiler chickens
| Subgroups | Model results | Heterogeneity | |||
|---|---|---|---|---|---|
| SMD (95% CI) | SE | P < 0.05 | I 2 test (%) | P < 0.05 | |
| Broiler strain | |||||
| Cobb | −0.09 (−0.17 to −0.02) | 0.04 | 0.017 | 58.79 | 0.004 |
| Ross | 0.06 (−0.06 to 0.18) | 0.06 | 0.351 | 89.14 | <0.001 |
| Arbor acres | −0.01 (−0.10 to 0.08) | 0.05 | 0.850 | 86.65 | <0.001 |
| Bacillus spp. | |||||
| B. subtilis | 0.00 (−0.06 to 0.06) | 0.03 | 0.992 | 87.79 | <0.001 |
| B. amyloliquefaciens | 0.12 (−0.05 to 0.28) | 0.08 | 0.159 | 59.43 | 0.001 |
| B. coagulans | −0.22 (−0.56 to 0.13) | 0.18 | 0.218 | 86.09 | <0.001 |
| B. licheniformis | 0.40 (−0.02 to 0.81) | 0.21 | 0.051 | 85.16 | <0.001 |
| SL (106 cfu/g) | |||||
| 0.1–0.5 | 0.03 (−0.07 to 0.13) | 0.05 | 0.520 | 85 | <0.001 |
| 0.6–1.0 | 0.19 (0.06−0.32) | 0.07 | 0.004 | 48 | 0.032 |
| >1.0 | 0.19 (0.07−0.31) | 0.06 | 0.002 | 0 | 0.452 |
| DOS (days) | |||||
| 1–21 | 0.23 (−0.02 to 0.49) | 0.13 | 0.070 | 87.39 | <0.001 |
| 1–28 | −0.37 (−0.58 to −0.17) | 0.10 | <0.001 | 74.85 | 0.003 |
| 1–35 | 0.01 (−0.08 to 0.10) | 0.05 | 0.767 | 82.92 | <0.001 |
| 1–42 | −0.03 (−0.11 to 0.06) | 0.04 | 0.560 | 86.90 | <0.001 |
| 1–49 | 0.11 (−0.07 to 0.28) | 0.09 | 0.231 | 8.44 | 0.351 |
SMD – standardised mean difference; SE – standard error; I 2 – inconsistency index; cfu – colony forming unit; SL – supplementation level; DOS – duration of supplementation.
Subgroup analysis of the effect of Bacillus probiotics on FCE of broiler chickens
| Subgroups | Model results | Heterogeneity | |||
|---|---|---|---|---|---|
| SMD (95% CI) | SE | P <0.05 | I 2 test (%) | P <0.05 | |
| Broiler strain | |||||
| Cobb | −0.45 (−0.63 to −0.27) | 0.09 | <0.001 | 92.56 | <0.001 |
| Ross | −0.29 (−0.37 to −0.22) | 0.04 | <0.001 | 90.90 | <0.001 |
| Arbor Acres | −0.37 (−0.48 to −0.26) | 0.06 | <0.001 | 78.72 | <0.001 |
| Bacillus spp. | |||||
| B. subtilis | −0.29 (−0.35 to −0.24) | 0.03 | <0.001 | 75.58 | <0.001 |
| B. amyloliquefaciens | −0.41 (−0.74 to −0.09) | 0.16 | 0.012 | 89.75 | <0.001 |
| B. coagulans | −0.79 (−0.97 to −0.62) | 0.09 | <0.001 | 43.77 | 0.149 |
| B. licheniformis | −0.41 (−0.69 to −0.13) | 0.14 | 0.004 | 69.44 | 0.011 |
| SL (106 cfu/g) | |||||
| 0.1–0.5 | −0.06 (−0.45 to −0.27) | 0.05 | <0.001 | 84 | <0.001 |
| 0.6–1.0 | −0.30 (−0.40 to −0.19) | 0.05 | <0.001 | 20 | 0.247 |
| >1.0 | −0.28 (−0.49 to −0.08) | 0.11 | 0.008 | 56 | 0.025 |
| DOS (days) | |||||
| 1–21 | −0.26 (−0.42 to −0.10) | 0.08 | 0.001 | 68.64 | <0.001 |
| 1–28 | −0.47 (−0.60 to −0.34) | 0.07 | <0.001 | 37.36 | 0.157 |
| 1–35 | −0.36 (−0.51 to −0.21) | 0.08 | <0.001 | 85.46 | <0.001 |
| 1–42 | −0.34 (−0.41 to −0.28) | 0.03 | <0.001 | 76.76 | <0.001 |
SMD – standardised mean difference; SE – standard error; I 2 – inconsistency index; cfu – colony forming unit; SL – supplementation level; DOS – duration of supplementation.
Subgroup analysis of the effect of Bacillus probiotics on ADG of broiler chickens
| Subgroups | Model results | Heterogeneity | |||
|---|---|---|---|---|---|
| SMD (95% CI) | SE | P < 0.05 | I 2 test (%) | P < 0.05 | |
| Broiler strain | |||||
| Cobb | 0.25 (0.06–0.43) | 0.09 | 0.009 | 93.81 | <0.001 |
| Ross | 0.36 (0.22–0.51) | 0.07 | <0.001 | 91.68 | <0.001 |
| Arbor acres | 0.41 (0.28–0.55) | 0.07 | <0.001 | 84.31 | <0.001 |
| Bacillus spp. | |||||
| B. subtilis | 0.31 (0.21–0.41) | 0.05 | <0.001 | 91.44 | <0.001 |
| B. amyloliquefaciens | 0.35 (0.08–0.63) | 0.14 | 0.011 | 85.80 | <0.001 |
| B. coagulans | 0.45 (0.15–0.75) | 0.16 | 0.004 | 81.70 | <0.001 |
| B. licheniformis | 0.45 (0.11–0.88) | 0.20 | 0.011 | 97.25 | <0.001 |
| SL (106 cfu/g) | |||||
| 0.1–0.5 | 0.44 (0.22– 0.66) | 0.11 | <0.001 | 89 | <0.001 |
| 0.6–1.0 | 0.26 (−0.08 to 0.61) | 0.18 | 0.135 | 91 | <0.001 |
| DOS (days) | |||||
| 1–21 | 0.45 (−0.05 to 0.94) | 0.25 | 0.075 | 95.38 | <0.001 |
| 1–28 | 0.14 (−0.05 to 0.33) | 0.10 | 0.153 | 73.04 | <0.001 |
| 1–35 | 0.36 (0.21–0.52) | 0.08 | <0.001 | 85.05 | 0.002 |
| 1–42 | 0.33 (0.21– 0.45) | 0.06 | <0.001 | 93.94 | <0.001 |
SMD – standardised mean difference; SE – standard error; I 2 – inconsistency index; cfu – colony forming unit; SL – supplementation level; DOS – duration of supplementation.
3.4 Analysis of heterogeneity, meta-regression, and publication bias
Substantial heterogeneity (I 2 = 79.83–92.84%) was observed among the studies used for the analysis (Figures 3–5). In addition, results of subanalysis as shown in Tables 2–4 found that studied moderators did not eliminate the problems of substantial heterogeneity. Table 5 shows that Bacillus spp. and supplementation dose were significant predictors of the effect of Bacillus on FI in broiler chickens and accounted for approximately 39% of the sources of heterogeneity. Broiler strain and DOS explained about 26% of the factors that led to the inconsistent results among studies on the effect of Bacillus on FCE. 12% of the sources of heterogeneity among investigators on the effect of Bacillus supplementation on ADG were explained by broiler strain and DOS. Visual examination of the funnel plots as displayed in Figure 6a–c revealed the presence of publication bias among the trials included in the meta-analysis to evaluate the impact of Bacillus supplementation on growth performance indices of broiler chickens. The funnel plots were asymmetrical. However, this is not a problem as the Rosenberg’s Nfs for the database was 593 (FI), 18,298 (FCE), and 14,592 (ADG) which were 3, 38, and 32 folds above the threshold of 200 (5 × 38 + 10), 195 (5 × 37 + 10), and 175 (5 × 33 + 10) needed to proclaim the mean effect size significant, despite the possibility of publication bias [72].
Meta-regression of the moderator variables
| Parameter | Moderators | Q M | df | P < 0.05 | R 2 (%) |
|---|---|---|---|---|---|
| FI | Bacillus spp. | 18.00 | 4 | 0.001 | 17.19 |
| Dosage | 2.75 | 2 | 0.253 | 4.00 | |
| Broiler strain | 2.13 | 4 | 0.711 | 0.00 | |
| Duration of study | 27.90 | 7 | <0.001 | 21.67 | |
| FCE | Bacillus spp. | 2.77 | 4 | 0.597 | 0.00 |
| Dosage | 0.74 | 2 | 0.690 | 0.00 | |
| Broiler strain | 13.20 | 4 | 0.010 | 12.93 | |
| Duration of study | 17.60 | 7 | 0.014 | 12.68 | |
| ADG | Bacillus spp. | 8.55 | 4 | 0.073 | 5.44 |
| Dosage | 0.70 | 2 | 0.705 | 0.00 | |
| Broiler strain | 1.28 | 3 | 0.733 | 0.00 | |
| Duration of study | 19.90 | 7 | 0.006 | 12.29 |
R 2 – amount of heterogeneity accounted for; df – degree of freedom; Q M – coefficient of moderators; Q M – was considered significant at P < 0.05.

Funnel graphs of the effect of dietary Bacillus supplementation on (a) feed intake; (b) FCE; and (c) ADG in broiler chickens.
4 Discussion
4.1 Probiotic effect of Bacillus spp.
The results of this meta-analysis demonstrated the beneficial effect of dietary Bacillus supplementation on growth performance in broiler chickens. This is consistent with the findings of other authors that found beneficial effect of Bacillus supplementation on FCE and ADG in broiler chickens [3,17,18]. These findings also support Frizzo et al. [1] and Hu et al. [73], who reported a positive association between growth performance and probiotics in animals other than broiler chickens. Our meta-analysis results also revealed that broiler chickens fed with Bacillus supplemented diets gained weights at comparable FI with the controls. The mechanisms by which Bacillus improved growth performance in broiler chickens are not clear. However, the improved FCE and ADG obtained in broilers fed with diets supplemented with Bacillus in the present meta-analysis could be credited to the capability of Bacillus to limit proliferation of pathogens by competitive exclusion and antagonism and to improve immune systems of the host [9,13,14]. Bacillus has been reported to enhance digestive activity which can improve nutrient digestibility in broiler chickens [17,30,32].
4.2 Analysis of moderators
4.2.1 DOS
DOS was a limiting factor in the present meta-analysis. The effect of Bacillus on FI was found in studies that fed Bacillus for 1–28 days. FCE was enhanced in broilers fed with Bacillus for 1–21, 1–28, 1–35, and 1–42 days. Improvement in FCE in broilers fed with Bacillus for 1–21 days corroborated the results of Gaggìa et al. [74], who observed that probiotic action was evident in chickens during the first few days of life, when the gut microbiota has not been stabilised. Initial colonisation is very relevant to the host because bacteria can modulate the expression of genes in epithelial cells, thus creating a favourable habitat for themselves [75]. Probiotic effect on ADG was identified in trials that fed Bacillus for 1–35 and 1–42 days which agrees with Li et al. [30], who noticed significantly higher ADG in broiler chickens fed with Bacillus spp. at 1 × 106 cfu/g for 1–21 days. On the other hand, Ahmed et al. [16] revealed that broiler chickens fed with 2.0 × 106 cfu/g B. amyloliquefaciens for 1–21 days had no significant effect on ADG. The observed difference could be related to the species of Bacillus used as well as the amount added to the diet [5,9].
4.2.2 Broiler chicken strain
Nutrition accounts for about 70% of cost of poultry production under an intensive management system [76], and nutritional strategies that enhance FI and FCE are desirable in the face of rising prices of feed due to high cost of feedstuffs. Probiotic effect on feed intake was evident only in the experiments using Cobb, but not in the experiments using Ross and Arbor acres. This implies that Cobb fed with diets treated with Bacillus gained weight at a lower FI compared with controls. FCE is one of the important indices utilised to assess chicken performance. The lower the FCE, the more efficient feed digestion and nutrient utilisation are. Probiotic effect on FCE and ADG was demonstrated in Cobb, Ross, and Arbor Acre strains. The ability of the Cobb strain to gain weight with a reduced FI in this study is a welcome development as it may affect feed cost. However, we could not proceed to ascertain the economics of production of broiler chickens on dietary Bacillus in this meta-analysis as such information is lacking in the literature. There is a significant relationship between FCE and broiler strain, which is consistent with the findings of others [77,78,79]. Although the present study shows evidence of treatment effect on FCE in broiler strains, the influence of other factors such as feed composition, gut health, and indoor temperature known to regulate feed efficiency in chickens could not be ruled out in this meta-analysis [80].
4.2.3 Bacillus spp.
Meta-regression analysis demonstrated that the species of Bacillus is a limiting factor among the studies included in the analysis and led to the inconsistent results among studies that assessed the effect of Bacillus on FI. The non-significant effect of Bacillus spp. on FI as shown in our subanalysis results indicates that Bacillus spp. has a limited ability to stimulate appetite in broiler chickens. This finding is in harmony with Boroojeni et al. [17] who found that incorporation of B. subtilis at 1.6 × 106 cfu/g (starter diet) and 0.8 × 106 cfu/g (grower diet) had no significant effect on FI. In contrast, this result differs with the finding of Ahmed et al. [16], who reported that supplementation of B. amyloliquefaciens at 1, 5, 10, and 20 g/kg had no significant effect on FI in Ross 308 broiler chickens and Park et al. [19] who reported significantly increased FI in broilers fed with B. subtilis at 1.0 × 106 cfu/g for 1–28 days. The observed differences may be due to the quantity of Bacillus included in the diet and type of Bacillus species used. On the other hand, subanalysis results indicate that Bacillus spp. had beneficial effects on FCE and ADG in broiler chickens. The potential of Bacillus spp. to improve FCE and ADG in broilers at a comparable FI with the control supports the findings of Zaghari et al. [29], who found that feed cost per kilogram weight gain was lower in broiler fed with diet supplemented with 0.2 g/kg B. subtilis (4 × 109 cfu/g) than in broiler fed with the same diet without B. subtilis supplementation. The increased ADG in chickens fed with diets containing supplemental levels of Bacillus spp. when compared to control chickens could be credited to the capability of Bacillus to boost digestive enzyme activity in the gut, resulting in increased digestion and nutrient uptake, supporting the earlier findings of Mingmongkolchai and Panbangred [12] and Ogbuewu et al. [3] that Bacillus organisms enhance the production and secretion of digestive juices and enzymes in chickens.
4.3 Supplementation dose
Meta-regression indicates significant relationship between SL and growth performance variables in broilers. Subanalysis results show that broilers offered with diets containing lower inclusion doses of Bacillus (0.1–0.5 × 106 cfu/g) had lower FI than birds given moderate and higher doses at 0.6–1.0 and >1.0 × 106 cfu/g compared to controls, showing that broilers fed with higher doses of probiotic Bacillus consumed more feed than control broilers. However, the higher FI in broilers fed with diets having moderate and high inclusion levels of Bacillus did not translate to higher ADG. Interestingly, broilers fed with lower doses of Bacillus gained more weight than the controls, implying that these birds were gaining weight at a FI similar to controls. This could be ascribed to the ability of Bacillus at certain supplementation dose levels to enhance the secretion of digestive enzymes which assist in feed digestion and nutrient absorption [14]. These findings are consistent with that of other authors [12,14,16], who noticed differences in growth traits between broiler chickens fed with diets supplemented with low and high inclusion levels of Bacillus. Taking into account the economic benefits of overall feeding costs in broiler chicken production, subanalysis revealed that SL of 0.1–0.5 × 106 cfu/g may be the optimal SL for broiler chickens. However, more research is required to determine the optimal supplementation dose of Bacillus in the chicken feed that optimised growth performance in broiler chickens using the regression analysis. Broilers offered with Bacillus supplemented diets had better FCE than the controls. The better FCE in group offered with Bacillus may be credited to the ability of the Bacillus to enhance the quality of the diets resulting in higher ADG especially in sub group fed with lower doses of Bacillus. The better FCE in broilers given Bacillus when compared to the controls supported the findings of other researchers [81,82,83] who discovered that Bacillus improves FCE in broiler chicken via improvement in gut health [83].
4.4 Heterogeneity and publication biases
Heterogeneity is one of the limiting factors in a meta-analysis which typically arises from differences in the study population, type of probiotics used, differences in dose level, and duration of study [3,26]. Our results revealed the existence of significant heterogeneity and this prompted sub group analysis to explain the likely sources of heterogeneity. However, large heterogeneity was still observed within all subgroups of the studied modifiers. Meta-regression showed effect for Bacillus spp. and duration of study as moderators for FI, and the duration of study and broiler strain for FCE, implying that not more than 26–39% of the variations across articles used for the current analysis were explained by these moderators, which is similar to the findings of others [3,26]. Meta-regression showed no effect of studied moderators on ADG, implying that none of the studied moderators is a significant predictor of the study effect. These results imply that modifiers other than those studied could be responsible for the unexplained heterogeneity. Thus, more studies are required to ascertain other factors responsible for the unexplained heterogeneity. However, studies included in the analysis were performed in 18 countries of the world showing the validity of our conclusions [25].
Publication bias is one major source of bias in meta-analysis. Even if a meta-analysis produces a mathematically accurate synthesis of the studies included in the analysis, if these studies are a biased sample of all relevant studies, the mean effect computed by the meta-analysis will reflect this bias [84]. The likely explanation for not including all relevant articles in meta-analysis can be the tendency for negative trials or small studies to not be published, either due to editorial bias to only publish results with significant results or authors’ aversion to publishing papers with negative results [85]. However, the minimal evidence of publication bias as observed in this meta-analysis is not a problem as the Nfs values were several folds above the thresholds needed to proclaim pooled estimates free from bias [72].
5 Conclusion
Meta-analysis results suggest that Bacillus supplementation improved ADG and feed FCE in broilers when compared to controls. Subanalysis showed significant differences among studied moderators (broiler strains, supplementation doses, Bacillus species, and DOS). There is a high degree of heterogeneity among studies included in the meta-analysis which could not be removed by the subanalysis. Meta-regression analysis revealed that studied moderators accounted for about 77% of the sources of variation, implying the presence of factors other than those studied in the current meta-analysis. It is therefore recommended that the effect of factors such as indoor rearing temperature, ventilation rate, and relative humidity of the poultry house, among other variables known to influence growth performance in broiler chickens be reported as these factors were not stated in about 90% of studies included in the meta-analysis. This study has standardised the study design for future experiment on the impact of Bacillus on broiler chicken productivity.
Acknowledgments
Authors are sincerely grateful to Agriculture for Food Security (AgriFoSe2030) for granting the first author a training fellowship on Introduction to Meta-analysis.
-
Funding information: The authors state no funding involved.
-
Author contributions: I.P.O. and C.A.M. conceived, designed the methodology, and prepared the manuscript. I.P.O. did data extraction, analysis, and visualisation. C.A.M. revised the draft, and made the manuscript ready for journal submission. All authors read and approved the final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Frizzo LS, Zbrun MV, Soto LP, Signorini ML. Effects of probiotics on growth performance in young calves: A meta-analysis of randomized controlled trials. Anim Feed Sci and Techn. 2011;169:147–56.10.1016/j.anifeedsci.2011.06.009Search in Google Scholar
[2] Ogbuewu IP, Okoro VM, Mbajiorgu EF, Mbajiorgu CA. Yeast (Saccharomyces cerevisiae) and its effect on production indices of livestock and poultry – a review. Comp Clin Pathol. 2019;51:669–77.10.1007/s00580-018-2862-7Search in Google Scholar
[3] Ogbuewu IP, Okoro VMO, Mbajiorgu CA. Probiotic-yeast improves performance indicators in broiler chickens: Evidence from meta-analysis. Appld Ecol and Environ Res. 2020;18:2823–43.10.15666/aeer/1802_28232843Search in Google Scholar
[4] Patterson J, Burkholder K. Application of prebiotics and probiotics in poultry production. Poult Sci. 2003;82:627–31.10.1093/ps/82.4.627Search in Google Scholar
[5] Kabir S. The role of probiotics in the poultry industry. Int J Mol Sci. 2009;10:3531–46.10.3390/ijms10083531Search in Google Scholar
[6] Mattila-Sandholm T, Myllärinen P, Crittenden R, Mogensen G, Fondén R, Saarela M. Technological challenges for future probiotic foods. Int Dairy J. 2002;12:173–82.10.1016/S0958-6946(01)00099-1Search in Google Scholar
[7] Sarkar PK, Morrison E, Tinggi U, Somerset SM, Craven GS. B-group vitamin and mineral contents of soybeans during kinema production. J Sci Food and Agric. 1998;78:498–502.10.1002/(SICI)1097-0010(199812)78:4<498::AID-JSFA145>3.0.CO;2-CSearch in Google Scholar
[8] Boroojeni F, Svihus B, von Reichenbach HG, Zentek J. The effects of hydrothermal processing on feed hygiene, nutrient availability, intestinal microbiota and morphology in poultry - A review. Anim Feed Sci and Techn. 2016;220:187–215.10.1016/j.anifeedsci.2016.07.010Search in Google Scholar
[9] Dong Y, Li R, Liu Y, Ma L, Zha J, Qiao X, et al. Benefit of dietary supplementation with Bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probiot and Antimicrobial Prot. 2020;12:1385–97.10.1007/s12602-020-09643-wSearch in Google Scholar
[10] Stanley D, Hughes RJ, Moore RJ. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appld Microbiol and Biotech. 2014;98:4301–10.10.1007/s00253-014-5646-2Search in Google Scholar
[11] Mahmoud K, Obeidat B, Al-Sadi M, Hatahet SR. Effect of Bacillus subtilis supplementation and dietary crude protein level on growth performance and intestinal morphological changes of meat-type chicken. Livest Sci. 2017;195:99–104.10.1016/j.livsci.2016.11.015Search in Google Scholar
[12] Mingmongkolchai S, Panbangred W. Bacillus probiotics: an alternative to antibiotics for livestock production. J Appld Microbiol. 2018;124:1334–46.10.1111/jam.13690Search in Google Scholar PubMed
[13] Ramlucken U, Lalloo R, Roets Y, Moonsamy G, Jansen van Rensburg C, Thantsha MS. Advantages of Bacillus-based probiotics in poultry production. Livest Sci. 2020;241:104215.10.1016/j.livsci.2020.104215Search in Google Scholar
[14] Ogbuewu IP, Mabelebele M, Sebola NA, Mbajiorgu CA. Bacillus probiotics as alternatives to in-feed antibiotics and its influence on growth, serum chemistry, antioxidant status intestinal histomorphology, and lesion scores in disease-challenged broiler chickens. Front Vet Sci. 2022a;9:876725.10.3389/fvets.2022.876725Search in Google Scholar PubMed PubMed Central
[15] Simon O, Jadamus A, Vahjen W. Probiotic feed additives-effectiveness and expected modes of action. J Anim and Feed Sci. 2001;10:51–67.10.22358/jafs/70012/2001Search in Google Scholar
[16] Ahmed AT, Manirul M, Mun H, Sim H, Kim Y, Yang C. Effects of bacillus amyloliquefaciens as a probiotic strain on growth performance, caecal microflora, and faecal noxious gas emissions of broiler chickens. Poult Sci. 2014;93:1963–71.10.3382/ps.2013-03718Search in Google Scholar PubMed
[17] Boroojeni FG, Vahjen W, Manner K, Blanch A, Sandvang D, Zentek J. Bacillus subtilis in broiler diets with different levels of energy and protein. Poult Sci. 2018;97:3967–76.10.3382/ps/pey265Search in Google Scholar PubMed
[18] Krauze M, Abramowicz K, Ognik K. The effect of addition of probiotic bacteria (Bacillus subtilis or enterococcus faecium) or phytobiotic containing cinnamon oil to drinking water on the health and performance of broiler chickens. Annals Anim Sci. 2020;20:191–205.10.2478/aoas-2019-0059Search in Google Scholar
[19] Park JH, Kim IH. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult Sci. 2014;93:2054–9.10.3382/ps.2013-03818Search in Google Scholar PubMed
[20] Latorre CJD. Evaluation and selection of a Bacillus based direct-fed microbial candidate for in situ enzyme production to improve gut health integrity, bone quality and growth performance in poultry. Theses and Dissertations, University of Arkansas, Fayetteville. 2016;1–180.Search in Google Scholar
[21] Opalinski M, Maiorka A, Dahlke F, Cunha F, Vargas FSC, Cardozo E. On the use of a probiotic (Bacillus subtilis - strain DSM 17299) as growth promoter in broiler diets. Brazil J Poult Sci. 2007;9:99–103.10.1590/S1516-635X2007000200004Search in Google Scholar
[22] Ogbuewu IP, Mokolopi BG, Mbajiorgu CA. Meta-analysis of growth performance indices of broiler chickens in response to turmeric (Curcuma longa L.) supplementation. Anim Feed Sci and Techn. 2022b;283:115155.10.1016/j.anifeedsci.2021.115155Search in Google Scholar
[23] Ressing M, Blettner M, Klug SJ. Systematic literature reviews and meta-analyses: part 6 of a series on evaluation of scientific publications. Dtsch Arzteblatt Int. 2009;106:456–63.10.3238/arztebl.2009.0456Search in Google Scholar
[24] Sauvant DJ, Schmidely JJ, Daudin P, St-Pierre NR. Meta-analyses of experimental data in animal nutrition. Anim. 2008;2:1203–14.10.1017/S1751731108002280Search in Google Scholar PubMed
[25] Koricheva J, Gurevitch J, Mengersen K. Handbook of Meta-analysis in Ecology and Evolution. Princeton Oxford, UK: Princeton University Press; 2013.10.1515/9781400846184Search in Google Scholar
[26] Ogbuewu IP, Mbajiorgu CA. Meta-analysis of probiotic-yeast (Saccharomyces cerevisiae) intervention on feed intake, feed efficiency and egg production indices in laying hens. Anim Prod Sci. 2020. 10.1071/AN20192.Search in Google Scholar
[27] Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; Chichester (UK): John Wiley and Sons; 2011. http://handbook.cochrane.org/. (Accessed on 27 th September 2020).Search in Google Scholar
[28] Jeong JS, Kim IH. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult Sci. 2014;93:3097–103.10.3382/ps.2014-04086Search in Google Scholar PubMed
[29] Zaghari M, Zahroojian N, Riahi M, Parhizkar S. Effect of Bacillus subtilis Spore (GalliPro®) Nutrients equivalency value on broiler chicken performance. Italian J Anim Sci. 2015;14:3555.10.4081/ijas.2015.3555Search in Google Scholar
[30] Li CI, Wang J, Zhang HJ, Wu SG, Hui QR, Yang CB, et al. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Frontiers in Physiol. 2019;9:1968.10.3389/fphys.2018.01968Search in Google Scholar PubMed PubMed Central
[31] Hmani H, Daoud L, Jlidi M, Jalleli K, Ali MB, Brahim AB, et al. A Bacillus subtilis strain as probiotic in poultry: selection based on in vitro functional properties and enzymatic potentialities. J Industr Microbiol and Biotechn. 2017;44:1157–66. 10.1007/s10295-017-1944-x.Search in Google Scholar PubMed
[32] Park JH, Yun HM, Kim IH. The effect of dietary Bacillus subtilis supplementation on the growth performance, blood profile, nutrient retention, and caecal microflora in broiler chickens. J Appld Anim Res. 2018;46:868–72.10.1080/09712119.2017.1411267Search in Google Scholar
[33] Nunes JO, Bertechini AG, de Brito JAG, Fassani EJ, Mesquita FR, Makiyama L, et al. Evaluation of the use of probiotic (Bacillus subtilis C-3102) as additive to improve performance in broiler chicken diets. Rev Brasileira de Zootec. 2012;41:2374–8.10.1590/S1516-35982012001100012Search in Google Scholar
[34] Hong Y, Cheng Y, Li Y, Li X, Zhou Z, Shi D, et al. Preliminary study on the effect of Bacillus amyloliquefaciens TL on caecal bacterial community structure of broiler chickens. BioMed Res Int. 2019;1–12. 10.1155/2019/5431354.Search in Google Scholar PubMed PubMed Central
[35] Trela J, Kieronczyk B, Hautekiet V, Józefiak D. Combination of Bacillus licheniformis and salinomycin: Effect on the growth performance and GIT microbial populations of broiler chickens. Anim. 2020;10:889. 10.3390/ani10050889.Search in Google Scholar PubMed PubMed Central
[36] Widiastuti E, Isroli I, Murwani R, Sartono TA, Wahyuni HI, Yudiarti T, et al. Dietary supplementation of butyric acid, probiotic Bacillus subtilis or their combination on weight gain, internal organ weight and carcass traits of the Indonesian indigenous crossbred chickens. Livest Res for Rural Dev. 2019;31(9), Article no. 134. http://www.lrrd.org/lrrd31/9/endwi31134.html.Search in Google Scholar
[37] Bajagai YS. Impact of Bacillus amyloliquefaciens probiotic strain H57 on the intestinal microbiota and broiler performance. PhD Thesis. The University of Queensland; 2017. p. 1–289.Search in Google Scholar
[38] Jacquier V, Nelson A, Jlali M, Rhayat L, Brinch KS, Devillard E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult Sci. 2019;98:2548–54.10.3382/ps/pey602Search in Google Scholar PubMed
[39] Bai K, Huang Q, Zhang J, He J, Zhang L, Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci. 2017;96:74–82.10.3382/ps/pew246Search in Google Scholar PubMed
[40] Harrington D, Sims M, Kehlet AB. Effect of Bacillus subtilis supplementation in low energy diets on broiler performance. J Appld Poult Res. 2016;25:29–39.10.3382/japr/pfv057Search in Google Scholar
[41] Fritil’s CA, Kersn JH, Motl MA, Kroger EC, Yan E, Si J, et al. Bacillus subtilis C-3102 (Calsporin) improves live performance and broiler chicken. J Appld Poult Res. 2000;9:149–55.10.1093/japr/9.2.149Search in Google Scholar
[42] Flores CA, Duong T, Augspurger N, Lee JT. Efficacy of Bacillus subtilis administered as a direct-fed microorganism in comparison to an antibiotic growth promoter and in diets with low and high DDGS inclusion levels in broiler chickens. J Appld Poult Res. 2019;28:902–11.10.3382/japr/pfz048Search in Google Scholar
[43] Zhang ZF, Zhou TX, Ao X, Kim IH. Effects of B-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize–soybean meal based diets. Livest Sci. 2012;150:419–24.10.1016/j.livsci.2012.10.003Search in Google Scholar
[44] Gao Z, Wu H, Shi L, Zhang X, Sheng R, Yin F, et al. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens. Anim Nutr. 2017;3:109–13.10.1016/j.aninu.2017.02.002Search in Google Scholar PubMed PubMed Central
[45] Aliakbarpour HR, Chamani M, Rahimi G, Sadeghi AA, Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian-Austral J Anim Sci. 2012;25:1285–93.10.5713/ajas.2012.12110Search in Google Scholar PubMed PubMed Central
[46] Al-Fataftah AR, Abdelqader A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim Feed Sci and Techn. 2014;198:279–85.10.1016/j.anifeedsci.2014.10.012Search in Google Scholar
[47] Cavazzoni V, Adami A, Castrovilli C. Performance of broiler chickens supplemented with Bacillus coagulans as probiotic. Brit Poult Sci. 1998;39:526–9.10.1080/00071669888719Search in Google Scholar PubMed
[48] Liu X, Yan H, Lv L, Xu Q, Yin C, Zhang K, et al. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Austral J Anim Sci. 2012;25:682–9.10.5713/ajas.2011.11334Search in Google Scholar PubMed PubMed Central
[49] Hung AT, Lin SY, Yang TY, Chou CK, Liu HC, Lu JJ, et al. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Anim Prod Sci. 2012;52:874–9.10.1071/AN11332Search in Google Scholar
[50] Sen S, Ingale SL, Kim YW, Kim JS, Kim KH, Lohakare JD, et al. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res in Vet Sci. 2012;93:264–8.10.1016/j.rvsc.2011.05.021Search in Google Scholar PubMed
[51] Santoso U. Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. Brit J Nutr. 1995;14:523–9.10.1079/BJN19950155Search in Google Scholar
[52] Jin LZ, Ho YW, Abdullah N, Jalaludin S. Influence of dried Bacillus subtilis and Lactobacilli cultures on intestinal microflora and performance in broilers. Asian J Appld Sci. 1996;9:397–403.10.5713/ajas.1996.397Search in Google Scholar
[53] Zhang L, Bai K, Zhang J, Xu W, Huang Q, Wang T. Dietary effects of Bacillus subtilis fmbj on the antioxidant capacity of broilers at an early age. Poult Sci. 2017;96:3564–73.10.3382/ps/pex172Search in Google Scholar PubMed
[54] Molnar AK, Podmaniczky B, Kürti P, Tenk I, Glávits R, Virág GY, et al. Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Brit Poult Sci. 2011;52:658–65.10.1080/00071668.2011.636029Search in Google Scholar PubMed
[55] Bai K, Feng C, Jiang L, Zhang L, Zhang J, Zhang L, et al. Dietary effects of Bacillus subtilis fmbj on growth performance, small intestinal morphology, and its antioxidant capacity of broilers. Poult Sci. 2018;97:2312–21.10.3382/ps/pey116Search in Google Scholar PubMed
[56] Upadhaya SD, Rudeaux F, Kim IH. Effects of inclusion of Bacillus subtilis (Gallipro) to energy- and protein-reduced diet on growth performance, nutrient digestibility, and meat quality and gas emission in broilers. Poult Sci. 2019;98:2169–78.10.3382/ps/pey573Search in Google Scholar PubMed
[57] Jayaraman S, Das PP, Saini PC, Roy B, Chatterjee PN. Use of Bacillus subtilis PB6 as a potential antibiotic growth promoter replacement in improving performance of broiler birds. Poult Sci. 2017;96:2614–22.10.3382/ps/pex079Search in Google Scholar
[58] Chen YC, Yu YH. Bacillus licheniformis–fermented products improve growth performance and the feacal microbiota community in broilers. Poult Sci. 2020;99:1432–43.10.1016/j.psj.2019.10.061Search in Google Scholar
[59] Reis MP, Fassani EJ, Garcia-Junior AAP, Rodrigues PB, Bertechini AG, Barrett N, et al. Effect of Bacillus subtilis (DSM 17299) on performance, digestibility, intestine morphology, and pH in broiler chickens. J Appld Poult Res. 2017;26:573–83.10.3382/japr/pfx032Search in Google Scholar
[60] Teo AY, Tan HM. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT)1. J Appld Poult Res. 2007;16:296–303.10.1093/japr/16.3.296Search in Google Scholar
[61] Li X, Qiang L, Liu K, Xu C. Effects of supplementation of fructooligosaccharide and/or Bacillus subtilis to diets on performance and on intestinal microflora in broilers. Arch Tierz Dummerstorf. 2008;51:64–70.10.5194/aab-51-64-2008Search in Google Scholar
[62] Lei X, Piao X, Ru Y, Zhang H, Péron A, Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and caecal microflora in broiler chickens. Asian Austral J Anim Sci. 2015;28:239–46.10.5713/ajas.14.0330Search in Google Scholar
[63] Gong L, Wang B, Mei X, Xu H, Qin Y, Li W, et al. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim Sci J. 2018;89:1561–71.10.1111/asj.13089Search in Google Scholar
[64] Borenstein M, Hedges LV, Higgins JPT, Rothstein HT. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111.10.1002/jrsm.12Search in Google Scholar
[65] Wallace BC, Lajeunesse MJ, Dietz G, Dahabreh IJ, Trikalinos TA, Schmid CH, et al. OpenMEE: intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol and Evol. 2016;8:941–7.10.1111/2041-210X.12708Search in Google Scholar
[66] DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.10.1016/0197-2456(86)90046-2Search in Google Scholar
[67] Cohen J. Statistical power analysis for the behavioural sciences. New York: Academic Press; 1969.Search in Google Scholar
[68] Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; Chichester (UK): John Wiley and Sons; 2011. www.cochrane-handbook.org.Search in Google Scholar
[69] Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Med J. 2003;327:557–60.10.1136/bmj.327.7414.557Search in Google Scholar PubMed PubMed Central
[70] Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat in Med. 2002;21:1559–73.10.1002/sim.1187Search in Google Scholar PubMed
[71] Lean IJ, Rabiee AR, Duffield TF, Dohoo IR. Invited review: use of meta-analysis in animal health and reproduction: methods and applications. J Dairy Sci. 2009;92:3545–65.10.3168/jds.2009-2140Search in Google Scholar PubMed
[72] Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evol. 2005;59:464–8.10.1111/j.0014-3820.2005.tb01004.xSearch in Google Scholar
[73] Hu Y, Dun Y, Li S, Zhao S, Peng N, Liang Y. Effects of Bacillus subtilis KN-42 on growth performance, diarrhoea and faecal bacterial flora of weaned piglets. Asian Austral J Anim Sci. 2014;27:1131–40.10.5713/ajas.2013.13737Search in Google Scholar PubMed PubMed Central
[74] Gaggìa F, Mattarelli P, Biavati P. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141:SS15–SS28.10.1016/j.ijfoodmicro.2010.02.031Search in Google Scholar PubMed
[75] Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host microbial relationships in the intestine. Sci. 2001;291:881–4.10.1126/science.291.5505.881Search in Google Scholar PubMed
[76] Ahiwe EU, Omede AA, Abdallh MB, Iji PA. Managing dietary energy intake by broiler chickens to reduce production costs and improve product quality. In Yucel B, Taşkin T, editors, Animal husbandry and nutrition; 2018. p. 115–45. IntechOpen.10.5772/intechopen.76972Search in Google Scholar
[77] Stringhini JH, Laboissiére M, Muramatsu K, Leandro NSM, Café MB. Evaluation of performance and carcass yield of four lines of broilers raised in Goiás. Rev Brasileira de Zootec. 2003;32:183–90.10.1590/S1516-35982003000100023Search in Google Scholar
[78] Garcia Neto M, Campos EJ. Susceptibility of broiler lines to ascitic syndrome. Brazil Agric Res. 2004;39:803–8.10.1590/S0100-204X2004000800011Search in Google Scholar
[79] Baracho MS, Nääs IA, Lima NDS, Cordeiro AFS, Moura DJ. Factors affecting broiler production: A meta-analysis. Brazil J Poult Sci. 2019;1:1–9.10.1590/1806-9061-2019-1052Search in Google Scholar
[80] Aviagen 2011. http://en.aviagen.com/assets/Tech_Center/BB_Foreign_Language_Docs/Portuguese/Optimizing-the-Rate-of-Converting-Feed-of-the-Chicken-of-Cut.pdfSearch in Google Scholar
[81] Abudabos AM, Alyemni AH, Dafalla YM, Khan RU. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J Appld Anim Res. 2017;45:538–42.10.1080/09712119.2016.1219665Search in Google Scholar
[82] Adhikari B, Hernandez-Patlan D, Solis-Cruz B, Kwon YM, Arreguin MA, Latorre JD, et al. Evaluation of the antimicrobial and anti-inflammatory properties of Bacillus-DFM (NorumTM) in broiler chickens infected with Salmonella enteritidis. Front Vet Sci. 2019;6:282.10.3389/fvets.2019.00282Search in Google Scholar PubMed PubMed Central
[83] Abudabos AM, Aljumaah MR, Alkhulaifi MM, Alabdullatif A, Suliman GM, AL-Sulaiman AR. Comparative effects of Bacillus subtilis and Bacillus licheniformis on live performance, blood metabolites and intestinal features in broiler inoculated with Salmonella infection during the finisher phase. Microbial Pathogen. 2020;139:103870.10.1016/j.micpath.2019.103870Search in Google Scholar PubMed
[84] Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. John Wiley and Sons, Ltd; 2009.10.1002/9780470743386Search in Google Scholar
[85] Hopewell S, Loudon K, Clarke M, Oxman A, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews. 2009;(Issue 1). MR000006. 10.1002/14651858. MR000006.pub3.Search in Google Scholar
© 2022 Ifeanyichukwu Princewill Ogbuewu and Christain Anayo Mbajiorgu, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Foliar application of boron positively affects the growth, yield, and oil content of sesame (Sesamum indicum L.)
- Impacts of adopting specialized agricultural programs relying on “good practice” – Empirical evidence from fruit growers in Vietnam
- Evaluation of 11 potential trap crops for root-knot nematode (RKN) control under glasshouse conditions
- Technical efficiency of resource-poor maize farmers in northern Ghana
- Bulk density: An index for measuring critical soil compaction levels for groundnut cultivation
- Efficiency of the European Union farm types: Scenarios with and without the 2013 CAP measures
- Participatory validation and optimization of the Triple S method for sweetpotato planting material conservation in southern Ethiopia
- Selection of high-yield maize hybrid under different cropping systems based on stability and adaptability parameters
- Soil test-based phosphorus fertilizer recommendation for malting barley production on Nitisols
- Effects of domestication and temperature on the growth and survival of the giant freshwater prawn (Macrobrachium rosenbergii) postlarvae
- Influence of irrigation regime on gas exchange, growth, and oil quality of field grown, Texas (USA) olive trees
- Present status and prospects of value addition industry for agricultural produce – A review
- Competitiveness and impact of government policy on chili in Indonesia
- Growth of Rucola on Mars soil simulant under the influence of pig slurry and earthworms
- Effect of potassium fertilizer application in teff yield and nutrient uptake on Vertisols in the central highlands of Ethiopia
- Dissection of social interaction and community engagement of smallholder oil palm in reducing conflict using soft system methodology
- Farmers’ perception, awareness, and constraints of organic rice farming in Indonesia
- Improving the capacity of local food network through local food hubs’ development
- Quality evaluation of gluten-free biscuits prepared with algarrobo flour as a partial sugar replacer
- Effect of pre-slaughter weight on morphological composition of pig carcasses
- Study of the impact of increasing the highest retail price of subsidized fertilizer on rice production in Indonesia
- Agrobiodiversity and perceived climatic change effect on family farming systems in semiarid tropics of Kenya
- Influences of inter- and intra-row spacing on the growth and head yield of cabbage (Brassica oleracea var. capitata) in western Amhara, Ethiopia
- The supply chain and its development concept of fresh mulberry fruit in Thailand: Observations in Nan Province, the largest production area
- Toward achieving sustainable development agenda: Nexus between agriculture, trade openness, and oil rents in Nigeria
- Phenotyping cowpea accessions at the seedling stage for drought tolerance in controlled environments
- Apparent nutrient utilization and metabolic growth rate of Nile tilapia, Oreochromis niloticus, cultured in recirculating aquaculture and biofloc systems
- Influence of season and rangeland-type on serum biochemistry of indigenous Zulu sheep
- Meta-analysis of responses of broiler chickens to Bacillus supplementation: Intestinal histomorphometry and blood immunoglobulin
- Weed composition and maize yield in a former tin-mining area: A case study in Malim Nawar, Malaysia
- Strategies for overcoming farmers’ lives in volcano-prone areas: A case study in Mount Semeru, Indonesia
- Principal component and cluster analyses based characterization of maize fields in southern central Rift Valley of Ethiopia
- Profitability and financial performance of European Union farms: An analysis at both regional and national levels
- Analysis of trends and variability of climatic parameters in Teff growing belts of Ethiopia
- Farmers’ food security in the volcanic area: A case in Mount Merapi, Indonesia
- Strategy to improve the sustainability of “porang” (Amorphophallus muelleri Blume) farming in support of the triple export movement policy in Indonesia
- Agrarian contracts, relations between agents, and perception on energy crops in the sugarcane supply chain: The Peruvian case
- Factors influencing the adoption of conservation agriculture by smallholder farmers in KwaZulu-Natal, South Africa
- Meta-analysis of zinc feed additive on enhancement of semen quality, fertility and hatchability performance in breeder chickens
- Meta-analysis of the potential of dietary Bacillus spp. in improving growth performance traits in broiler chickens
- Biocomposites from agricultural wastes and mycelia of a local mushroom, Lentinus squarrosulus (Mont.) Singer
- Cross transferability of barley nuclear SSRs to pearl millet genome provides new molecular tools for genetic analyses and marker assisted selection
- Detection of encapsulant addition in butterfly-pea (Clitoria ternatea L.) extract powder using visible–near-infrared spectroscopy and chemometrics analysis
- The willingness of farmers to preserve sustainable food agricultural land in Yogyakarta, Indonesia
- Transparent conductive far-infrared radiative film based on polyvinyl alcohol with carbon fiber apply in agriculture greenhouse
- Grain yield stability of black soybean lines across three agroecosystems in West Java, Indonesia
- Forms of land access in the sugarcane agroindustry: A comparison of Brazilian and Peruvian cases
- Assessment of the factors contributing to the lack of agricultural mechanization in Jiroft, Iran
- Do poor farmers have entrepreneurship skill, intention, and competence? Lessons from transmigration program in rural Gorontalo Province, Indonesia
- Communication networks used by smallholder livestock farmers during disease outbreaks: Case study in the Free State, South Africa
- Sustainability of Arabica coffee business in West Java, Indonesia: A multidimensional scaling approach
- Farmers’ perspectives on the adoption of smart farming technology to support food farming in Aceh Province, Indonesia
- Rice yield grown in different fertilizer combination and planting methods: Case study in Buru Island, Indonesia
- Paclobutrazol and benzylaminopurine improve potato yield grown under high temperatures in lowland and medium land
- Agricultural sciences publication activity in Russia and the impact of the national project “Science.” A bibliometric analysis
- Storage conditions and postharvest practices lead to aflatoxin contamination in maize in two counties (Makueni and Baringo) in Kenya
- Relationship of potato yield and factors of influence on the background of herbological protection
- Biology and life cycle Of Diatraea busckella (Lepidoptera: Crambidae) under simulated altitudinal profile in controlled conditions
- Evaluation of combustion characteristics performances and emissions of a diesel engine using diesel and biodiesel fuel blends containing graphene oxide nanoparticles
- Effect of various varieties and dosage of potassium fertilizer on growth, yield, and quality of red chili (Capsicum annuum L.)
- Review Articles
- Germination ecology of three Asteraceae annuals Arctotis hirsuta, Oncosiphon suffruticosum, and Cotula duckittiae in the winter-rainfall region of South Africa: A review
- Animal waste antibiotic residues and resistance genes: A review
- A brief and comprehensive history of the development and use of feed analysis: A review
- The evolving state of food security in Nigeria amidst the COVID-19 pandemic – A review
- Short Communication
- Response of cannabidiol hemp (Cannabis sativa L.) varieties grown in the southeastern United States to nitrogen fertilization
- Special Issue on the International Conference on Multidisciplinary Research – Agrarian Sciences
- Special issue on the International Conference on Multidisciplinary Research – Agrarian Sciences: Message from the editor
- Maritime pine land use environmental impact evolution in the context of life cycle assessment
- Influence of different parameters on the characteristics of hazelnut (var. Grada de Viseu) grown in Portugal
- Organic food consumption and eating habit in Morocco, Algeria, and Tunisia during the COVID-19 pandemic lockdown
- Customer knowledge and behavior on the use of food refrigerated display cabinets: A Portuguese case
- Perceptions and knowledge regarding quality and safety of plastic materials used for food packaging
- Understanding the role of media and food labels to disseminate food related information in Lebanon
- Liquefaction and chemical composition of walnut shells
- Validation of an analytical methodology to determine humic substances using low-volume toxic reagents
- Special Issue on the International Conference on Agribusiness and Rural Development – IConARD 2020
- Behavioral response of breeder toward development program of Ongole crossbred cattle in Yogyakarta Special Region, Indonesia
- Special Issue on the 2nd ICSARD 2020
- Perceived attributes driving the adoption of system of rice intensification: The Indonesian farmers’ view
- Value-added analysis of Lactobacillus acidophilus cell encapsulation using Eucheuma cottonii by freeze-drying and spray-drying
- Investigating the elicited emotion of single-origin chocolate towards sustainable chocolate production in Indonesia
- Temperature and duration of vernalization effect on the vegetative growth of garlic (Allium sativum L.) clones in Indonesia
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Prediction model for agro-tourism development using adaptive neuro-fuzzy inference system method
- Special Issue of International Web Conference on Food Choice and Eating Motivation
- Can ingredients and information interventions affect the hedonic level and (emo-sensory) perceptions of the milk chocolate and cocoa drink’s consumers?
Articles in the same Issue
- Regular Articles
- Foliar application of boron positively affects the growth, yield, and oil content of sesame (Sesamum indicum L.)
- Impacts of adopting specialized agricultural programs relying on “good practice” – Empirical evidence from fruit growers in Vietnam
- Evaluation of 11 potential trap crops for root-knot nematode (RKN) control under glasshouse conditions
- Technical efficiency of resource-poor maize farmers in northern Ghana
- Bulk density: An index for measuring critical soil compaction levels for groundnut cultivation
- Efficiency of the European Union farm types: Scenarios with and without the 2013 CAP measures
- Participatory validation and optimization of the Triple S method for sweetpotato planting material conservation in southern Ethiopia
- Selection of high-yield maize hybrid under different cropping systems based on stability and adaptability parameters
- Soil test-based phosphorus fertilizer recommendation for malting barley production on Nitisols
- Effects of domestication and temperature on the growth and survival of the giant freshwater prawn (Macrobrachium rosenbergii) postlarvae
- Influence of irrigation regime on gas exchange, growth, and oil quality of field grown, Texas (USA) olive trees
- Present status and prospects of value addition industry for agricultural produce – A review
- Competitiveness and impact of government policy on chili in Indonesia
- Growth of Rucola on Mars soil simulant under the influence of pig slurry and earthworms
- Effect of potassium fertilizer application in teff yield and nutrient uptake on Vertisols in the central highlands of Ethiopia
- Dissection of social interaction and community engagement of smallholder oil palm in reducing conflict using soft system methodology
- Farmers’ perception, awareness, and constraints of organic rice farming in Indonesia
- Improving the capacity of local food network through local food hubs’ development
- Quality evaluation of gluten-free biscuits prepared with algarrobo flour as a partial sugar replacer
- Effect of pre-slaughter weight on morphological composition of pig carcasses
- Study of the impact of increasing the highest retail price of subsidized fertilizer on rice production in Indonesia
- Agrobiodiversity and perceived climatic change effect on family farming systems in semiarid tropics of Kenya
- Influences of inter- and intra-row spacing on the growth and head yield of cabbage (Brassica oleracea var. capitata) in western Amhara, Ethiopia
- The supply chain and its development concept of fresh mulberry fruit in Thailand: Observations in Nan Province, the largest production area
- Toward achieving sustainable development agenda: Nexus between agriculture, trade openness, and oil rents in Nigeria
- Phenotyping cowpea accessions at the seedling stage for drought tolerance in controlled environments
- Apparent nutrient utilization and metabolic growth rate of Nile tilapia, Oreochromis niloticus, cultured in recirculating aquaculture and biofloc systems
- Influence of season and rangeland-type on serum biochemistry of indigenous Zulu sheep
- Meta-analysis of responses of broiler chickens to Bacillus supplementation: Intestinal histomorphometry and blood immunoglobulin
- Weed composition and maize yield in a former tin-mining area: A case study in Malim Nawar, Malaysia
- Strategies for overcoming farmers’ lives in volcano-prone areas: A case study in Mount Semeru, Indonesia
- Principal component and cluster analyses based characterization of maize fields in southern central Rift Valley of Ethiopia
- Profitability and financial performance of European Union farms: An analysis at both regional and national levels
- Analysis of trends and variability of climatic parameters in Teff growing belts of Ethiopia
- Farmers’ food security in the volcanic area: A case in Mount Merapi, Indonesia
- Strategy to improve the sustainability of “porang” (Amorphophallus muelleri Blume) farming in support of the triple export movement policy in Indonesia
- Agrarian contracts, relations between agents, and perception on energy crops in the sugarcane supply chain: The Peruvian case
- Factors influencing the adoption of conservation agriculture by smallholder farmers in KwaZulu-Natal, South Africa
- Meta-analysis of zinc feed additive on enhancement of semen quality, fertility and hatchability performance in breeder chickens
- Meta-analysis of the potential of dietary Bacillus spp. in improving growth performance traits in broiler chickens
- Biocomposites from agricultural wastes and mycelia of a local mushroom, Lentinus squarrosulus (Mont.) Singer
- Cross transferability of barley nuclear SSRs to pearl millet genome provides new molecular tools for genetic analyses and marker assisted selection
- Detection of encapsulant addition in butterfly-pea (Clitoria ternatea L.) extract powder using visible–near-infrared spectroscopy and chemometrics analysis
- The willingness of farmers to preserve sustainable food agricultural land in Yogyakarta, Indonesia
- Transparent conductive far-infrared radiative film based on polyvinyl alcohol with carbon fiber apply in agriculture greenhouse
- Grain yield stability of black soybean lines across three agroecosystems in West Java, Indonesia
- Forms of land access in the sugarcane agroindustry: A comparison of Brazilian and Peruvian cases
- Assessment of the factors contributing to the lack of agricultural mechanization in Jiroft, Iran
- Do poor farmers have entrepreneurship skill, intention, and competence? Lessons from transmigration program in rural Gorontalo Province, Indonesia
- Communication networks used by smallholder livestock farmers during disease outbreaks: Case study in the Free State, South Africa
- Sustainability of Arabica coffee business in West Java, Indonesia: A multidimensional scaling approach
- Farmers’ perspectives on the adoption of smart farming technology to support food farming in Aceh Province, Indonesia
- Rice yield grown in different fertilizer combination and planting methods: Case study in Buru Island, Indonesia
- Paclobutrazol and benzylaminopurine improve potato yield grown under high temperatures in lowland and medium land
- Agricultural sciences publication activity in Russia and the impact of the national project “Science.” A bibliometric analysis
- Storage conditions and postharvest practices lead to aflatoxin contamination in maize in two counties (Makueni and Baringo) in Kenya
- Relationship of potato yield and factors of influence on the background of herbological protection
- Biology and life cycle Of Diatraea busckella (Lepidoptera: Crambidae) under simulated altitudinal profile in controlled conditions
- Evaluation of combustion characteristics performances and emissions of a diesel engine using diesel and biodiesel fuel blends containing graphene oxide nanoparticles
- Effect of various varieties and dosage of potassium fertilizer on growth, yield, and quality of red chili (Capsicum annuum L.)
- Review Articles
- Germination ecology of three Asteraceae annuals Arctotis hirsuta, Oncosiphon suffruticosum, and Cotula duckittiae in the winter-rainfall region of South Africa: A review
- Animal waste antibiotic residues and resistance genes: A review
- A brief and comprehensive history of the development and use of feed analysis: A review
- The evolving state of food security in Nigeria amidst the COVID-19 pandemic – A review
- Short Communication
- Response of cannabidiol hemp (Cannabis sativa L.) varieties grown in the southeastern United States to nitrogen fertilization
- Special Issue on the International Conference on Multidisciplinary Research – Agrarian Sciences
- Special issue on the International Conference on Multidisciplinary Research – Agrarian Sciences: Message from the editor
- Maritime pine land use environmental impact evolution in the context of life cycle assessment
- Influence of different parameters on the characteristics of hazelnut (var. Grada de Viseu) grown in Portugal
- Organic food consumption and eating habit in Morocco, Algeria, and Tunisia during the COVID-19 pandemic lockdown
- Customer knowledge and behavior on the use of food refrigerated display cabinets: A Portuguese case
- Perceptions and knowledge regarding quality and safety of plastic materials used for food packaging
- Understanding the role of media and food labels to disseminate food related information in Lebanon
- Liquefaction and chemical composition of walnut shells
- Validation of an analytical methodology to determine humic substances using low-volume toxic reagents
- Special Issue on the International Conference on Agribusiness and Rural Development – IConARD 2020
- Behavioral response of breeder toward development program of Ongole crossbred cattle in Yogyakarta Special Region, Indonesia
- Special Issue on the 2nd ICSARD 2020
- Perceived attributes driving the adoption of system of rice intensification: The Indonesian farmers’ view
- Value-added analysis of Lactobacillus acidophilus cell encapsulation using Eucheuma cottonii by freeze-drying and spray-drying
- Investigating the elicited emotion of single-origin chocolate towards sustainable chocolate production in Indonesia
- Temperature and duration of vernalization effect on the vegetative growth of garlic (Allium sativum L.) clones in Indonesia
- Special Issue on Agriculture, Climate Change, Information Technology, Food and Animal (ACIFAS 2020)
- Prediction model for agro-tourism development using adaptive neuro-fuzzy inference system method
- Special Issue of International Web Conference on Food Choice and Eating Motivation
- Can ingredients and information interventions affect the hedonic level and (emo-sensory) perceptions of the milk chocolate and cocoa drink’s consumers?

