Abstract
C18H18N3PSe, monoclinic, P21/n (no. 14), a = 9.3064 (9) Å, b = 17.7311 (18) Å, c = 11.0556(11) Å, β = 105.317(1)°, V = 1759.5 Å3, Z = 4, Rgt(F) = 0.0365, wRref(F2) = 0.0919, T = 296(2) K.
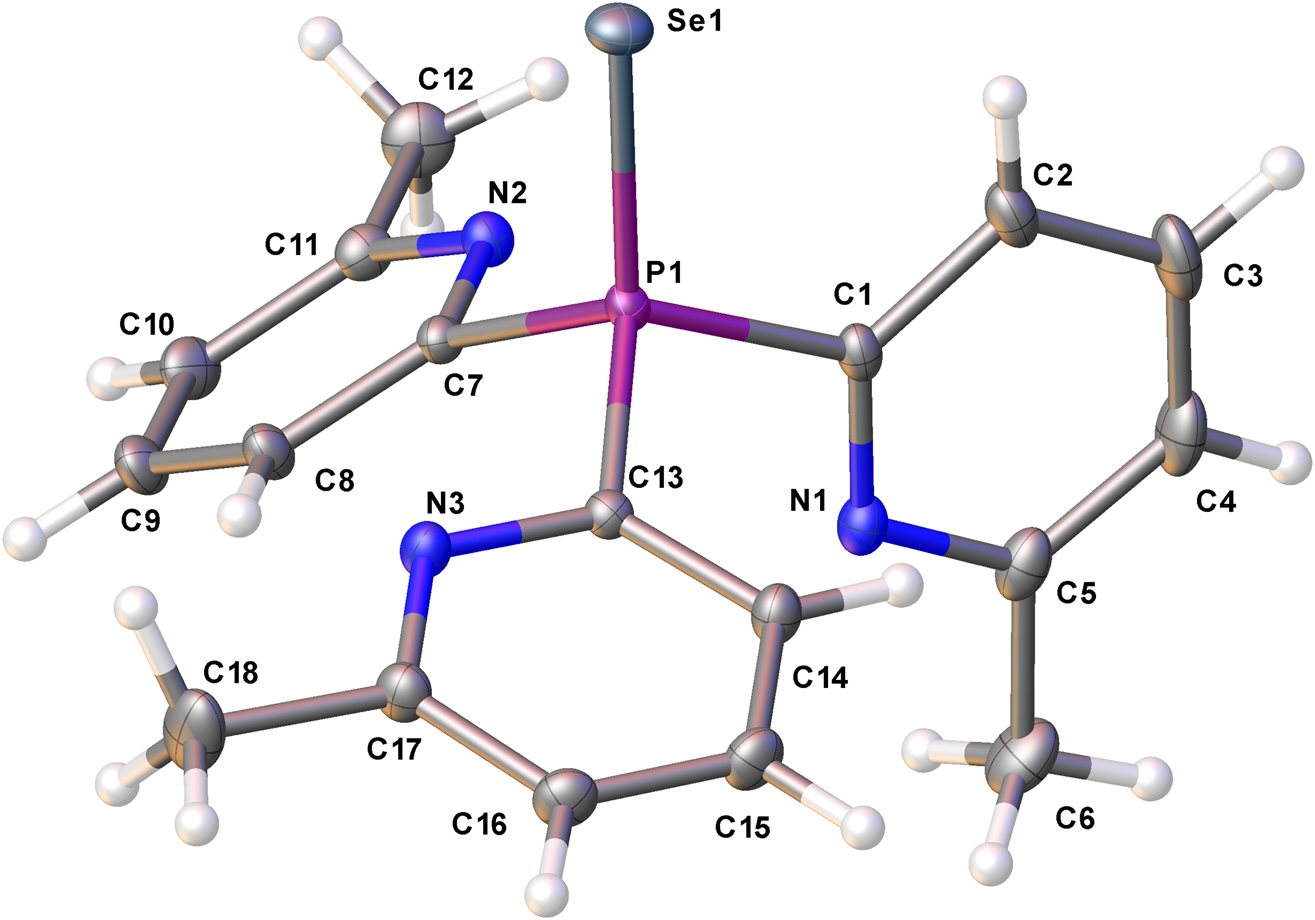
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.15 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.23 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 32.2°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 27,083, 5848, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5031 |
| N(param)refined: | 211 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.30290 (18) | 0.81857 (9) | 0.01628 (14) | 0.0313 (3) |
| C2 | 0.1945 (2) | 0.83306 (12) | −0.09355 (16) | 0.0439 (4) |
| H2 | 0.125608 | 0.871717 | −0.098819 | 0.053* |
| C3 | 0.1933 (3) | 0.78685 (15) | −0.19646 (18) | 0.0591 (6) |
| H3 | 0.121651 | 0.793598 | −0.272171 | 0.071* |
| C4 | 0.2988 (3) | 0.73171 (14) | −0.18430 (19) | 0.0560 (6) |
| H4 | 0.299159 | 0.700769 | −0.252133 | 0.067* |
| C5 | 0.4047 (2) | 0.72180 (11) | −0.07161 (18) | 0.0437 (4) |
| C6 | 0.5213 (3) | 0.66142 (13) | −0.0543 (2) | 0.0602 (6) |
| H6A | 0.617716 | 0.683062 | −0.018597 | 0.090* |
| H6B | 0.518845 | 0.639329 | −0.134092 | 0.090* |
| H6C | 0.501844 | 0.623220 | 0.000868 | 0.090* |
| C7 | 0.30510 (16) | 0.79401 (8) | 0.27103 (13) | 0.0276 (3) |
| C8 | 0.39940 (19) | 0.78926 (11) | 0.39083 (15) | 0.0367 (3) |
| H8 | 0.470297 | 0.826288 | 0.421962 | 0.044* |
| C9 | 0.3840 (2) | 0.72680 (12) | 0.46297 (17) | 0.0436 (4) |
| H9 | 0.445226 | 0.721146 | 0.543816 | 0.052* |
| C10 | 0.2779 (2) | 0.67407 (11) | 0.41359 (18) | 0.0436 (4) |
| H10 | 0.268310 | 0.631456 | 0.459869 | 0.052* |
| C11 | 0.18340 (19) | 0.68429 (9) | 0.29284 (17) | 0.0358 (3) |
| C12 | 0.0634 (3) | 0.62842 (11) | 0.2377 (2) | 0.0524 (5) |
| H12A | −0.009056 | 0.628384 | 0.285495 | 0.079* |
| H12B | 0.106214 | 0.579029 | 0.239832 | 0.079* |
| H12C | 0.015987 | 0.642028 | 0.152447 | 0.079* |
| C13 | 0.49402 (16) | 0.91372 (8) | 0.20445 (13) | 0.0274 (3) |
| C14 | 0.5695 (2) | 0.92949 (10) | 0.11526 (16) | 0.0355 (3) |
| H14 | 0.531612 | 0.914503 | 0.032324 | 0.043* |
| C15 | 0.7036 (2) | 0.96841 (11) | 0.15303 (18) | 0.0413 (4) |
| H15 | 0.756904 | 0.980683 | 0.095488 | 0.050* |
| C16 | 0.7559 (2) | 0.98838 (10) | 0.27697 (18) | 0.0409 (4) |
| H16 | 0.845887 | 1.013950 | 0.304219 | 0.049* |
| C17 | 0.6743 (2) | 0.97040 (10) | 0.36155 (16) | 0.0377 (3) |
| C18 | 0.7271 (3) | 0.99008 (18) | 0.4985 (2) | 0.0662 (7) |
| H18A | 0.650679 | 1.017716 | 0.523034 | 0.099* |
| H18B | 0.815408 | 1.020443 | 0.512744 | 0.099* |
| H18C | 0.748947 | 0.944638 | 0.547102 | 0.099* |
| N1 | 0.40678 (17) | 0.76489 (8) | 0.02889 (13) | 0.0355 (3) |
| N2 | 0.19774 (15) | 0.74375 (8) | 0.22234 (13) | 0.0319 (3) |
| N3 | 0.54352 (16) | 0.93426 (8) | 0.32509 (13) | 0.0330 (3) |
| P1 | 0.30913 (4) | 0.87031 (2) | 0.16087 (3) | 0.02617 (8) |
| Se1 | 0.13816 (2) | 0.95049 (2) | 0.14172 (2) | 0.04267 (7) |
Source of material
The 2-bromo-6-methylpyridine (30 mmol), red phosphorus (30 mmol) and powdered KOH (30 mmol) were dissolved in 20 mL DMSO and 1 mL H2O. The solution was stirred for 1 h at 120 °C under dry argon and cooled to room temperature. Then 40 mL H2O wad added and the mixture was extracted with CHCl3 (3 × 20 mL). The solvent was removed under reduced pressure and the residue washed with diethyl ether (10 mL) and dried in vacuo to afford the phosphine product as a microcrystalline powder. Powdered gray selenium (10 mmol) and degassed toluene (20 mL) were added to the above solids, and the suspension was refluxed for 3 h. After cooling to room temperature the excess selenium was filtered off. The filtrate was evaporated to dryness under vacuum and a beige-colored powder was obtained. Crystals were obtained by slow evaporation in CHCl3 and CH3OH within 7 days.
Comment
Pyridyl-substituted phosphines have been extensively used in metal ion coordination chemistry due to their fundamental importance and prospective applications [5], [6], [7]. Tris(2-pyridyl)phosphine exhibits rich coordination capabilities owing to its structure. On the other hand, there are many studies on the substitution of pyridine-4-yl substituents in the substituents of pyridine [8, 9]. However, the pyridine-6-yl substituents are less investigated.
The asymmetric unit contains one independent molecule. The nitrogen atoms (N2, N3) are almost form a plane with P1 and C1, while the remaining nitrogen atom (N1) forms a plane with C1, P1 and Se1 (see the figure). This type of arrangement is different from previously seen ones in the structure of tris(2-pyridyl)phosphine oxide and tris(2-pyridyl)phosphine sulphide. However, in the reported tris(2-pyridyl)phosphine selenide structure [10], all nitrogen atoms point in the same direction (towards Se), which can be related to the steric requirement bearing a methyl group at the 6-position of the pyridyl ring. The P=Se bond distance of 2.1022(4) Å is comparable with similar structures found in the literature showing typical double bond character [8], [9], [10]. The pyridine ring (N1/C1–C5) makes dihedral angles of 70.97(11)° and 72.42(11)° with the other two pyridine rings (N2/C7–C11 and N3/C13–C17), respectively. In addition, the crystal structure exhibits weak C–H⃛π interactions. [C(6)–H(6A)⃛Cg2′ = 2.94 (4)°Å; Cg2 is the centroid of pyridine ring (N2, C6, C7, C8, C9, C10); ′ = x−1/2, −y+1/2, z+1/2]. The stability of crystal structure can be accounted by this interaction. Furthermore, there are no other obvious π⃛π stacking interactions, as the centroid to centroid distance between the two nearest rings is greater than 4.5 Å.
Funding source: Qiannan Normal University for Nationalities
Award Identifier / Grant number: qnsy2017002
Funding source: Scientific and Technological Project for Agriculture of Qiannan
Award Identifier / Grant number: [2018]12
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The authors is grateful to the projects of Qiannan Normal University for Nationalities (qnsy2017002) and the scientific and technological project for agriculture of Qiannan (Qiannankehe[2018]12) for financial support.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT—Plus, XPREP; Bruker AXS Inc.: Madison, Wisconsin, USA, 2008.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341.10.1107/S0021889808042726Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Search in Google Scholar
5. Newkome, G. R. Pyridylphosphines. Chem. Rev. 1993, 93, 2067–2089.10.1021/cr00022a006Search in Google Scholar
6. Zhang, Z. Z., Cheng, H. Chemistry of 2-(diphenylphosphino)pyridine. Coord. Chem. Rev. 1996, 147, 1–39.10.1016/0010-8545(94)01112-5Search in Google Scholar
7. Nippe, M., Khnayzer, R. S., Panetier, J. A., Zee, D. Z., Olaiya, B. S., Head-Gordon, M., Chang, C. J., Castellano, F. N., Long, J. R. Catalytic proton reduction with transition metal complexes of the redox-active ligand bpy2PYMe. Chem. Sci. 2013, 4, 3934–3945.10.1039/c3sc51660aSearch in Google Scholar
8. Hu, X. C., Sun, T. Q., Zheng, C. Y. Synthesis, crystal structures and magnetic properties of two iron (II) tris(pyridyl)phosphine selenides complexes. Phosphorus, Sulfur Silicon Relat. Elem 2018, 193, 300–305.10.1080/10426507.2017.1417295Search in Google Scholar
9. Wang, H. Q., Wu, N., Zheng, J., Zheng, C. Y., Wang, D. J. Synthesis and structures of two mononuclear iron(ii) complexes derived from polypyridine ligands. Mendeleev Commun. 2020, 30, 100–102.10.1016/j.mencom.2020.01.033Search in Google Scholar
10. Kharat, A. N., Bakhoda, A., Hajiashrafi, T., Abbasi, A. Synthesis, characterization, and crystal structures of tris(2-pyridyl)phosphine sulfide and selenide. Phosphorus, Sulfur Silicon Relat. Elem. 2010, 185, 2341–2347.10.1080/10426501003636778Search in Google Scholar
© 2022 Gui-Ling Wu, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of {2,2′-{cyclohexane-1,2-diylbis[(azanylylidene)methylylidene]}bis(2,4-dibromophenolato)-κ4 N,N′,O,O′}copper(II) ─ diethylformamide (1/1), C23H23Br4CuN3O3
- The crystal structure of 2-(2-methyl-6-phenyl-4H-pyran-4-ylidene)-1H-indene-1,3(2H)-dione, C21H14O3
- Crystal structure of bis((1-methylbenzimidazol-2-yl)methyl)amine, C18H19N5
- Crystal structure of (E)-N′-(1-(2-hydroxy-4-methoxyphenyl)ethylidene) isonicotinohydrazide, C15H15N3O3
- Crystal structure of 2-((4-phenyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)acetonitrile, C15H11N5S
- The crystal structure of 2,2′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene))bis(4-chlorophenol), C14H10Cl2N2O2
- Dichlorido-{2,6-bis(4,5-dihydro-1H-pyrazol-3-yl)pyridine-κ3 N,N′,N″}zinc(II), C11H9C12N5Zn
- The crystal structure of dichlorido-(2-((4-phenyl-2H-1,2,3-triazol-2-yl)methyl)pyridine-κ2N,N′)palladium(II), C14H12Cl2N4Pd
- The crystal structure of 1-(N1-benzyl-2-methyl-4-nitro-imidazol-5-yl)-4-(prop-2-yn-1-yl) piperazine, C18H21N5O2
- Crystal structure of (μ4-(1,2,4,5-tetra(1,2,4-triazol-1-ylmethyl)-benzene-κ4N:N1:N2:N3)disilver(I) diperchlorate
- The crystal structure of 1-(2-bromoethane)-4-amine-3,5-dinitropyrazole, C5H6Br1N5O4
- Crystal structure of (E)-1-(4-benzyl-3,5-dioxomorpholin-2-ylidene)ethyl acetate, C15H15N1O5
- The crystal structure of poly[diaqua-(μ2-1,2,4,5-tetrakis(1,2,4-triazol-1-ylmethyl)-benzene-κ2N:N′)-bis(μ3-terephthalato-κ3O:O′:O′′)dicadmium(II)], C17H15N6O5Cd
- Crystal structure of (E)-N′-(1-(5-chloro-2-hydroxyphenyl) ethylidene)thiophene-2-carbohydrazide, C13H11ClN2O2S
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylato-k2 N,O)cobalt(II)]-monohydrate, C36H26N4O5Co
- Crystal structure of (E)-N′-(2-chloro-6-hydroxybenzylidene)-3-hydroxybenzo-hydrazide monohydrate, C14H13ClN2O4
- Crystal structure of 1,1′-(methylene)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C42H30N14Ni2S8
- Crystal structure of 1,1′-(1,2-ethanediyl)bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S)nickel(II), C20H14N6NiS4
- The crystal structure of 1-methyl-1H-pyrazol-2-ium nitrate, C4H7O3N3
- The crystal structure of 4,4′-diselanediylbis(8-(hexyloxy)-3,6-dimethyl-1-(piperidin-1-yl)isoquinoline-7-carbonitrile), C46H60N6O2Se2
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine selenide, C18H18N3PSe
- The crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane ─ acetone (1/1), C11H12N8O9
- Crystal structure of [diaqua[2,2′-(1,2-phenylene)bis(1H-imidazole-4-carboxylato-5-carboxy)-κ4N,N′,O,O′]nickel(II)] tetrahydrate, C16H12N4NiO10·4H2O
- The crystal structure of tris(4-methyl-1H-pyrazol-1-yl)methane, C13H16N6
- The crystal structure of 5,6-dichloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H8Cl2N2O2
- Crystal structure of (E)-(2-methoxy-benzylidene)-(4-[1,2,4]triazol-1-yl-phenyl)-amine, C16H14N4O
- The crystal structure of (Z)-2-(4-(4-bromophenyl)thiazol-2-yl)-4-(3-hydroxybut-2-enoyl)-5-methyl -1,2-dihydro-3H-pyrazol-3-one – methanol (1/1), C18H18N3O4S
- Crystal structure of tetraaqua-tris(nitrato-κ2 O,O′) erbium(III) monohydrate, Er(NO3)3·5H2O, H10ErN3O14
- The crystal structure of 1-methyl-2-nitro-1H-imidazole 3-oxide, C4H5N3O3
- The crystal structure of 1-methyl-2-nitroimidazole, C4H5N3O2
- The crystal structure of 2-carboxyl-4-nitroimidazole monohydrate, C4H5N3O5
- Crystal structure of bis[hydrido-hexaphenylcarbodiphosphoran][tetra-trifluoromethyl-(μ-diiodo)-diplatinat]
- The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3 O:S:S)sodium(I)], C10H14N3O5SNa
- The twinned crystal structure of [4,4′-bipyridine]-1,1′-diium hexachloridostannate(IV), C10H10N2SnCl6
- The crystal structure of [(2,2′-bipyridine-k2 N,N)-bis(6-phenylpyridine-2-carboxylate-k2 N,O)copper(II)], C34H24N4O4Cu
- Crystal structure of trans-1,2-bis(pyridinium-4-yl) ethylene bis(2-carboxy-4-bromobenzoate) – water (1/4), C14H14BrNO6
- Crystal structure of poly[diaqua-(μ3-fumarato)-(μ3-maleato)-(μ4-1,2,4,5-tetrakis((1H-1,2,4-triazol-1-yl)methyl)benzene)tetracadmium(II)] dihydrate, C34H32N12O9Cd4
- Crystal structure of a second modification of Pachypodol, C18H16O7
- Crystal structure of methyl 2-(4-(2-(cyclopentyl-amino)-1-(N-(4-methoxyphenyl)-1-methyl-5-phenyl-1-H-pyrazole-3-carboxamido)-2-oxoethyl)phenyl)acetate, C34H36N4O5
- The crystal structure of catena-poly[(m2-4,4′-bipyridine-κ2 N:N)-bis(6-phenylpyridine-2-carboxylato-κ2 N,O) zinc(II)], C34H24N4O4Zn
- The crystal structure of hexaquamagnesium(II) (2,4-bis(nitroimino)-6-oxo-1,3,5-triazinane-1,3-diide), C3H15MgN7O12
- The crystal structure of 7-Bromo-2-(4-chloro-phenyl)-quinoxaline, C14H9BrClN2
- Crystal structure of methyl 4-{[4-(4-cyanobenzamido)phenyl]amino}benzofuro[2,3-d]pyrimidine-6-carboxylate, C26H17N5O4
- The crystal structure of (4SR)-7-(3,4-dichlorobenzyl)-4,8,8-trimethyl-7,8-dihydroimidazo[5,1c][1,2,4]triazine-3,6(2H,4H)-dione, C15H16Cl2N4O2
- Crystal structure of catena-poly[{μ2-3-carboxy-2,3-bis((4-methylbenzoyl)oxy)propanoato-κ2 O:O′}tris(methanol-κ1 O)lanthanum(III)], C63H63LaO27

