Abstract
C32H32N2O2, orthorhombic,
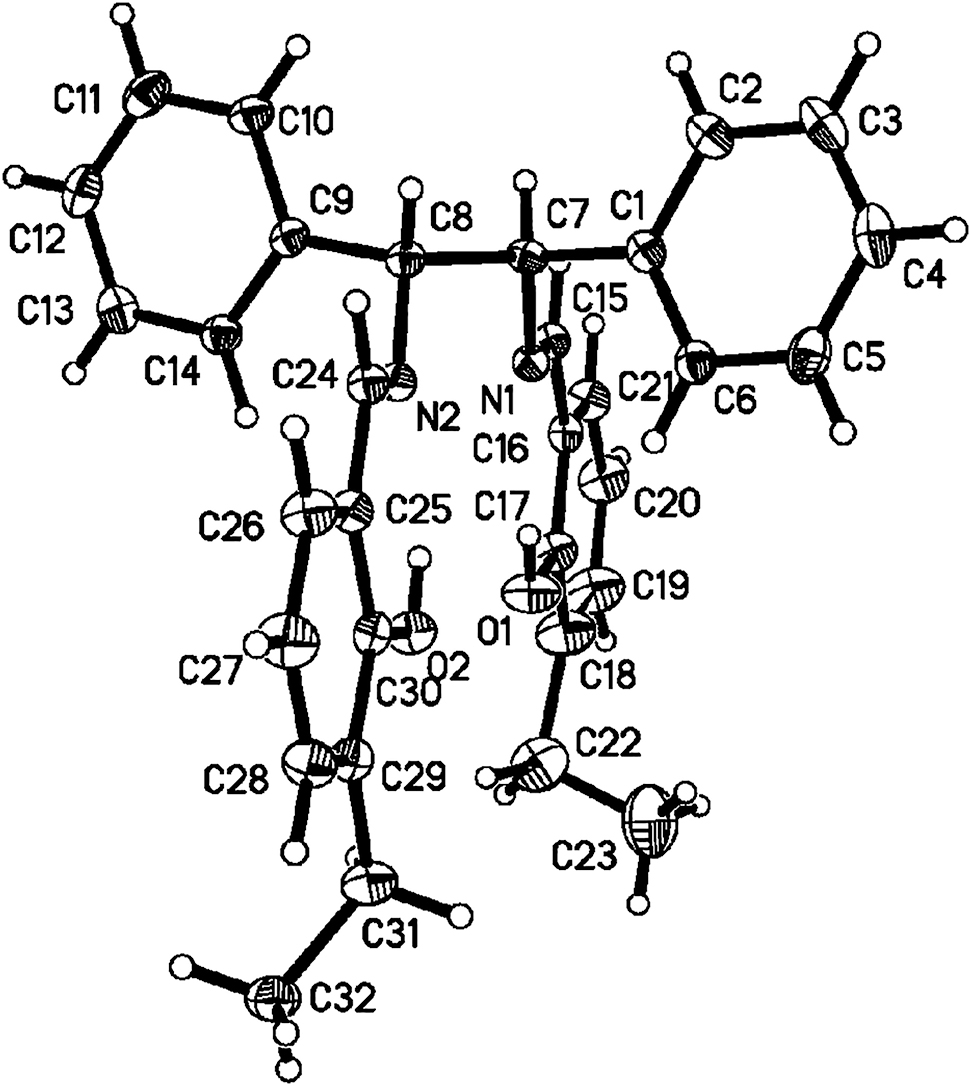
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.13 × 0.12 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.59 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 73.8°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12,561, 5133, 0.024 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5044 |
| N(param)refined: | 329 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], Olex2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.6789 (3) | 0.32760 (18) | 0.83208 (10) | 0.0439 (6) |
| H1 | 0.639420 | 0.351668 | 0.800779 | 0.066* |
| O2 | 0.7564 (2) | 0.24223 (15) | 0.68175 (9) | 0.0322 (4) |
| H2 | 0.713378 | 0.297399 | 0.684240 | 0.048* |
| N1 | 0.5336 (2) | 0.46340 (18) | 0.76594 (10) | 0.0246 (5) |
| N2 | 0.6306 (2) | 0.40912 (18) | 0.64151 (10) | 0.0256 (5) |
| C1 | 0.3444 (3) | 0.4322 (2) | 0.69217 (12) | 0.0273 (6) |
| C2 | 0.2207 (3) | 0.4771 (3) | 0.67516 (14) | 0.0353 (6) |
| H2A | 0.211018 | 0.549854 | 0.675520 | 0.042* |
| C3 | 0.1104 (3) | 0.4138 (3) | 0.65749 (16) | 0.0445 (8) |
| H3 | 0.027836 | 0.444509 | 0.645943 | 0.053* |
| C4 | 0.1236 (3) | 0.3059 (3) | 0.65709 (16) | 0.0441 (8) |
| H4 | 0.049815 | 0.264019 | 0.645477 | 0.053* |
| C5 | 0.2459 (3) | 0.2599 (3) | 0.67386 (15) | 0.0404 (7) |
| H5 | 0.254620 | 0.187130 | 0.673122 | 0.048* |
| C6 | 0.3562 (3) | 0.3220 (2) | 0.69188 (13) | 0.0316 (6) |
| H6 | 0.438045 | 0.290510 | 0.703796 | 0.038* |
| C7 | 0.4638 (3) | 0.5024 (2) | 0.70887 (12) | 0.0260 (5) |
| H7 | 0.428554 | 0.572972 | 0.718036 | 0.031* |
| C8 | 0.5647 (3) | 0.5104 (2) | 0.65175 (12) | 0.0250 (5) |
| H8 | 0.511601 | 0.527226 | 0.613415 | 0.030* |
| C9 | 0.6702 (3) | 0.5964 (2) | 0.66101 (12) | 0.0258 (5) |
| C10 | 0.6379 (3) | 0.6985 (2) | 0.64190 (14) | 0.0338 (6) |
| H10 | 0.551544 | 0.713166 | 0.625636 | 0.041* |
| C11 | 0.7340 (4) | 0.7783 (2) | 0.64705 (16) | 0.0426 (8) |
| H11 | 0.712019 | 0.846188 | 0.634143 | 0.051* |
| C12 | 0.8614 (4) | 0.7572 (3) | 0.67116 (15) | 0.0421 (7) |
| H12 | 0.925849 | 0.810646 | 0.674337 | 0.051* |
| C13 | 0.8941 (3) | 0.6565 (3) | 0.69071 (15) | 0.0392 (7) |
| H13 | 0.980550 | 0.642483 | 0.707092 | 0.047* |
| C14 | 0.7985 (3) | 0.5762 (2) | 0.68602 (13) | 0.0314 (6) |
| H14 | 0.820708 | 0.508760 | 0.699676 | 0.038* |
| C15 | 0.5159 (3) | 0.5141 (2) | 0.81800 (13) | 0.0262 (5) |
| H15 | 0.465318 | 0.575987 | 0.816969 | 0.031* |
| C16 | 0.5716 (3) | 0.4789 (2) | 0.87885 (12) | 0.0266 (5) |
| C17 | 0.6504 (3) | 0.3873 (2) | 0.88375 (13) | 0.0344 (6) |
| C18 | 0.7057 (5) | 0.3569 (3) | 0.94287 (17) | 0.0556 (9) |
| C19 | 0.6700 (5) | 0.4170 (3) | 0.99639 (16) | 0.0583 (11) |
| H19 | 0.699968 | 0.395120 | 1.036385 | 0.070* |
| C20 | 0.5925 (4) | 0.5070 (3) | 0.99227 (15) | 0.0464 (8) |
| H20 | 0.572640 | 0.545837 | 1.028721 | 0.056* |
| C21 | 0.5447 (3) | 0.5388 (3) | 0.93344 (14) | 0.0356 (6) |
| H21 | 0.494133 | 0.600447 | 0.930036 | 0.043* |
| C22 | 0.8091 (5) | 0.2626 (4) | 0.9480 (2) | 0.0645 (10) |
| H22A | 0.855318 | 0.251761 | 0.907563 | 0.077* |
| H22B | 0.877170 | 0.276721 | 0.980547 | 0.077* |
| C23 | 0.7290 (6) | 0.1703 (5) | 0.9649 (3) | 0.0855 (16) |
| H23A | 0.663891 | 0.156148 | 0.931702 | 0.128* |
| H23B | 0.681683 | 0.182998 | 1.004286 | 0.128* |
| H23C | 0.788601 | 0.110925 | 0.969793 | 0.128* |
| C24 | 0.6364 (3) | 0.3722 (2) | 0.58495 (12) | 0.0270 (5) |
| H24 | 0.595413 | 0.409393 | 0.551853 | 0.032* |
| C25 | 0.7054 (3) | 0.2731 (2) | 0.57068 (12) | 0.0268 (5) |
| C26 | 0.7155 (4) | 0.2385 (2) | 0.50772 (13) | 0.0360 (7) |
| H26 | 0.675961 | 0.277889 | 0.475200 | 0.043* |
| C27 | 0.7833 (4) | 0.1468 (3) | 0.49300 (15) | 0.0422 (8) |
| H27 | 0.789439 | 0.124098 | 0.450882 | 0.051* |
| C28 | 0.8424 (3) | 0.0887 (2) | 0.54176 (15) | 0.0364 (7) |
| H28 | 0.888045 | 0.026869 | 0.531477 | 0.044* |
| C29 | 0.8360 (3) | 0.1193 (2) | 0.60534 (13) | 0.0290 (6) |
| C30 | 0.7651 (3) | 0.2123 (2) | 0.61966 (12) | 0.0256 (5) |
| C31 | 0.9035 (4) | 0.0582 (3) | 0.65828 (16) | 0.0396 (7) |
| H31A | 0.840309 | 0.004905 | 0.673357 | 0.048* |
| H31B | 0.921679 | 0.105529 | 0.693570 | 0.048* |
| C32 | 1.0362 (4) | 0.0051 (3) | 0.6391 (2) | 0.0479 (8) |
| H32A | 1.018357 | −0.045495 | 0.606058 | 0.072* |
| H32B | 1.074920 | −0.029840 | 0.675442 | 0.072* |
| H32C | 1.099145 | 0.056967 | 0.623522 | 0.072* |
Source of material
A solution of 2-ethylphenol (2.4 g, 20 mmol), MgCl2 (5.7 g, 60 mmol), paraformaldehyde (9.0 g, 0.1 mol), triethylamine (15 g 149 mmol) in acetonitrile (50 mL) was refluxed until full consumption of 2-ethylphenol (as indicated by TLC). The mixture was quenched with HCl (1 M) and extracted with ethyl acetate. The combined organic layers were washed with brine, dried with MgSO4 and evaporated under reduced pressure. The crude residue was purified by column chromatography to obtain 3-ethyl-2-hydroxybenzaldehyde (2.2 g, 73% yield) as a yellow oil.
Synthesis of the title compound [5]: 3-ethyl-2- hydroxybenzaldehyde (1 g, 6.7 mmol) was dissolved in 30 mL of ethanol and (1R, 2R)-(−)-1,2-diphenylethane-1,2-diamine (0.71 g, 3.35 mmol) was added to the solution. The solution was heated to reflux for 4 h. The solvent was removed in vacuo and 1.48 g of the product was obtained as yellow solid (95% yield). Crystals were obtained by slow evaporation of an ethanol solution at room temperature over a period of seven days, yield: 0.72 g (93%). M.p.: 105–107 °C. Elemental analysis – found: C, 80.68%; H, 6.72%; N, 5.84%; calculated for C32H32N2O2: C, 80.64%; H, 6.77%; N, 5.88%.
Experimental details
Data were collected via CrysAlisPRO 1.171.39.7e [1], the structure of crystal was determined by SHELXT [2] and refined by OLEX2 [4] and SHELXL [3].
The absolute structure determination succeeded as the derived Flack parameter is found to be near zero with a low standard uncertainty [−0.02(9) from 2090 selected quotients] using Parsons’ method [6]. There is a small disorder of one ethyl group (C22, C23) which was not included in the refinement.
Comment
Hugo Schiff described the condensation between an aldehyde and an amine leading to a Schiff base in 1864 [7]. Schiff base ligands are privileged ligands, as they are easily prepared by the condensation between aldehydes and imines. Stereogenic centres can be introduced in the synthetic design. Schiff base ligands are able to coordinate many different metals, and to stabilize them in various oxidation states, enabling the use of Schiff base metal complexes for a large variety of useful catalytic transformations [8], [9], [10]. When two equivalents of salicylaldehyde are combined with a diamine, a particular chelating Schiff base is obtained – known as salen – with four coordinating sites and two axial sites open to ancillary ligands. Here, we report a new chiral salen compound.
The asymmetric unit of the title structure contains one 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol). In the crystal structure, bond lengths and bond angles within the molecule are in agreement with the values reported [11]. The bond lengths of C15–N1 and C24–N2 are 1.278(3) and 1.275(3) Å, respectively. As a result of the conjugation of the benzene moiety and adjacent carbon-nitrogen double bond, the bond lengths of C15–C16 and C24–C25 are 1.457(4) and 1.461(4) Å respectively, which is shorter than that of typical C–C bond. Furthermore, the bond lengths of C17–O1 and C30–O2 are 1.351(3) and 1.358(3) Å, respectively. The bond angles (C15–N1–C7) and (C24–N2–C8) are 117.5(2)° and 118.8(2)°, respectively. There are two intramolecular hydrogen bonds (d D···A 2.63 Å; H···A 1.904 Å) between H1 and N1 atom and (d D···A 2.594 Å; H···A 1.864 Å) between H2 and N2 atom, which stabilize the crystal structure [11], [12]. In the crystal packing, dipole-dipole and van der Waals interactions are effective besides an intermolecular hydrogen bond in the molecular packing.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21861045
Funding source: Zunyi Medical University
Award Identifier / Grant number: ZYDC2020038
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: National Natural Science Foundation of China (grant no. 21861045) and Technology University Students Science and Technology Innovation and Entrepreneurship Training Program Project in Zunyi Medical University (No. ZYDC2020038).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Corporation. CrysAlisPRO; Yarnton, Oxfordshire, England, 2015.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Reiss, H., Shalit, H., Vershinin, V., More, N. Y., Forckosh, H., Pappo, D. Cobalt [salen]-catalyzed selective aerobic oxidative cross-coupling between electron-rich phenols and 2-naphthols. J. Org. Chem. 2019, 84, 7950–7960; https://doi.org/10.1021/acs.joc.9b00822.Search in Google Scholar
6. Parsons, S., Flack, H. D., Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, B69, 249–259; https://doi.org/10.1107/s2052519213010014.Search in Google Scholar
7. Schiff, H. The syntheses and characterization of Schiff base. Ann. Chem. Pharm. Suppl. 1864, 3, 343–349.Search in Google Scholar
8. Belokon, Y. N., North, M., Parsons, T. Vanadium-catalyzed asymmetric cyanohydrin synthesis. Org. Lett. 2000, 2, 1617–1619; https://doi.org/10.1021/ol005893e.Search in Google Scholar
9. Zeng, X. P., Cao, Z. Y., Wang, X., Chen, L., Zhou, F., Zhu, F., Wang, C. H., Zhou, J. Activation of chiral (salen)AlCl complex by phosphorane for highly enantioselective cyanosilylation of ketones and enones. J. Am. Chem. Soc. 2016, 138, 416–425; https://doi.org/10.1021/jacs.5b11476.Search in Google Scholar
10. Kunisu, T., Oguma, T., Katsuki, T. Aerobic oxidative kinetic resolution of secondary alcohols with naphthoxide-bound iron(salan) complex. J. Am. Chem. Soc. 2011, 133, 12937–12939; https://doi.org/10.1021/ja204426s.Search in Google Scholar
11. Jiao, L., Tan, H., Sun, J., Zhang, L., Wu, Q. Crystal structure of rac-trans-N,N′-bis(3,5-dibromosalicylidene)-1,2-cyclohexanediamine, C20H18Br4N2O2. Z. Kristallogr. NCS 2020, 235, 847–848; https://doi.org/10.1515/ncrs-2020-0052.Search in Google Scholar
12. Wu, Q., Tang, Y., Li, J., Li, Y., Fang, Y. Crystal structure of rac-trans-N,N′-bis(3,5-diiodosalicylidene)-1,2-cyclohexanediamine, C20H18I4N2O2. Z. Kristallogr. NCS 2019, 234, 69–70; https://doi.org/10.1515/ncrs-2018-0173.Search in Google Scholar
© 2021 Zeng-Bing Xu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of poly[(μ2-aqua-tetraaqua-(μ3-glutarato-κ4O,O′:O′:O′′)-(μ5-glutarato-κ6O:O,O′:O′:O′′:O′′′)distrontium(II)], C10H22O13Sr2
- The crystal structure of acetato-κ1O-{(2-(2-(2-aminophenoxy)ethoxy)phenyl)(4-oxo-4-phenylbut-2-en-2-yl)amido-κ2N,N′,O}copper(II), C26H26CuN2O5
- Crystal structure of dimethanolato-k2O:O-bis(1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κN)-bis(thiocyanato-κN)dicopper(II), C34H32Cu2N12O2S2
- Crystal structure of poly[diaqua-bis(μ2-3-(pyrimidin-5-yl)benzoato-κ2N:O)cobalt(II)] dihydrate, [Co(C11H11O2N2)2(H2O)2]
- Crystal structure of bis(3,3-dimethyl-1-phenylbut-1-en-2-yl)(trimethylsilyl)amido-k1N)zinc(II), Zn(C15H24NSi)2
- Crystal structure of catena-poly[(μ2-methanolato-κ2O:O)-(μ2-1-((2-methyl-1H-benzo[d]imidazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-κ2N:N′)-(thiocyanato-κ1N)copper(II)] 0.25 hydrate, C17H16CuN6OS ⋅ 0.5H2O
- The crystal structure of 2-amino-5-nitroanilinium iodide monohydrate, C6H8IN3O2
- The crystal structure of 3-amino-5-carboxypyridin-1-ium perchlorate monohydrate, C6H9ClN2O7
- Crystal structure of 7-hydroxy-2,4-dimethoxy-9,10-dihydrophenanthrene from Arundina graminifolia, C16H16O3
- Crystal structure of 6,6′-((1E, 1′E)-(((1R, 2R)-1,2-diphenylethane-1,2-diyl) bis(azanylylidene))bis(methanylylidene))bis(2-ethylphenol), C32H32N2O2
- The crystal structure of 2-amino-5-carboxypyridin-1-ium iodide monohydrate, C6H9IN2O3
- The crystal structure of 2-(3,5-difluorophenyl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C16H11BF2N2
- Crystal structure of bis{(2-pyridinyl)-1-phenyl-1-isopropylmethanolato-κ2N,O}nickel, C30H32N2NiO2
- Crystal structure of poly[(m3-3-carboxyadamantane-1-carboxylato-κ3O:O′:O″)-(phenanthroline-κ2N,N′)sodium(II)], C24H23N2NaO4
- Crystal structure of 2-phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C22H20B2F4N4
- Crystal structure of 4-tert-butyl-2-N-(2-pyridylmethyl)aminophenol, C16H20N2O
- The crystal structure of (3Z,3′Z)-4,4′-((1,4-phenylenebis(methylene))bis(azanediyl))bis(pent-3-en-2-one), C18H24N2O2
- Crystal structure of (morpholine-1-carbodithioato-κ2-S,S′)bis(triphenylphosphine-κ-P)gold(I), C41H38AuNOP2S2
- Crystal structure of 1,4-bis(4-bromobenzyl)-4-(4-chlorophenyl)-1,4-dihydropyridine-3-carbonitrile, C26H19Br2ClN2
- The crystal structure of fac-tricarbonyl (N′-benzoyl-N,N-diphenylcarbamimidothioato-κ2S,O)-(pyrazole-κN)rhenium(I) — methanol (1/1) C26H23O4N4SRe
- The crystal structure of Ba2Mn(SeO3)2Cl2 containing 1∞[Mn(SeO3)2Cl2]4− chains
- Crystal structure of 3,3′,3″-((1E,1′E,1″E)-((nitrilotris(ethane-2,1-diyl))tris(azaneylylidene)) tris(methaneylylidene))tris(4-hydroxy-1-naphthaldehyde) monohydrate, C42H36N4O6·H2O
- The crystal structure of 4-(6-acetyl-5-methyl-4,7-dihydrotetrazolo[1,5-a]pyrimidin-7-yl)benzonitrile, C14H12N6O
- Crystal structure of benzo[d][1,3]dioxol-5-yl-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18O5
- The crystal structure of ethyl 5-methyl-7-(4-(phenylthio)phenyl)-4,7-dihydrotetrazolo[1,5-a]pyrimidine-6-carboxylate, C20H19N5O2S
- Crystal structure of N′,N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis(methaneylylidene))-di(isonicotinohydrazide)– water – dimethylformamide (1/4/2), C25H24N8O2·4H2O·2C3H7NO
- Synthesis and crystal structure of 4-(2,4-dinitrophenoxy)benzaldehyde, C13H8N2O6
- The crystal structure of 1-dodecylpyridin-1-ium bromide monohydrate, C17H32BrNO
- Crystal structure of (E)-amino(2-(4-(dimethylamino)benzylidene)hydrazineyl)methaniminium nitrate, C10H16N6O3
- Crystal structure of (E)-(2-((1H-pyrrol-2-yl)methylene)hydrazineyl)(amino)methaniminium nitrate monohydrate, C6H12N6O4
- The crystal structure of hexakis(1-propylimidazole-κ1N)copper(II) dichloride, C36H60Cl2CuN12
- The crystal structure of bis{(μ2-3,3-dimethyl-1-phenylbut-1-en-2-yl)((dimethylamino)dimethylsilyl)amido-κ3N,N′:N′}dilithium, C32H54Li2N4Si2
- The crystal structure of methyl 4-(1H-naphtho[1,8-de][1,3,2]diazaborinin-2(3H)-yl)benzoate, C18H15BN2O2
- Crystal structure of (E)-N-(1-((2-chlorothiazol-5-yl)methyl)pyridin-2(1H)-ylidene)-2,2,2-trifluoroacetamide, C11H7ClF3N3OS
- Crystal structure of N′, N‴-((propane-2,2-diylbis(1H-pyrrole-5,2-diyl))bis (methaneylylidene))di(picolinohydrazide) – water – methanol (1/1/1), C25H24N8O2·H2O·CH3OH
- Crystal structure of 3-(2-chloro-benzyl)-7-[4-(2-chloro-benzyl)-piperazin-1-yl]-5,6,8-trifluoro-3H-quinazolin-4-one, C26H21Cl2F3N4O
- Crystal structure of N1,N2-bis(2-fluorobenzyl)benzene-1,2-diamine,C20H18F2N2
- The crystal structure of 2-(benzo[d][1,3]dioxol-5-yl)-2,3-dihydro-1H-naphtho[1,8-de][1,3,2]diazaborinine, C17H13BN2O2
- The crystal structure of 6,6′-((1E,1′E)-hydrazine-1,2-diylidenebis(methaneylylidene)) bis(2-bromo-4-nitrophenol) — dimethylsulfoxide (1/2), C14H8Br2N4O6⋅2(C2H6OS)
- Selective biocatalytic synthesis and crystal structure of (2R,6R)-hydroxyketaminium chloride, C13H17Cl2NO2
- Crystal structure of bis{tetraaqua-[μ3-1-(4-carboxylatophenyl)-5-methyl-1H-pyrazole-3-carboxylate-κ4N,O,O′,O″] [μ2-1-methyl-1H-pyrazole-3,5-dicarboxylate-κ3N,O:O]dicobalt(II)} dihydrate, C36H44Co4N8O26
- Crystal structure of diethyl-2,2′-naphthalene-2,3-diylbis(oxy)diacetate, C18H20O6
- Synthesis and crystal structure of poly[(μ3-2-(2-carboxylatophenyl)-1H-benzo[d]imidazole-5-carboxylato-κO,O′:O′;:O″, O″′)-(μ2-1-(4-(1Himidazol-1-yl)phenyl)-1H-imidazole-κ2N:N′)cadmium(II)], C27H18CdN6O4
- The crystal structure of catena-poly[diaqua-bis(μ2-2-((2-(2-phenylacetyl)hydrazineylidene)methyl)benzoato-κ2O:O')zinc(II)], C32H30N4O8Zn
- The crystal structure of 2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-naphtho [1,8-de][1,3,2]diazaborinine, C18H17BN2O2
- The crystal structure of hexakis(1-ethylimidazole-κ1N)nickel(II) dichloride – 1-ethylimidazole (1/2), C40H64Cl2NiN16
- Crystal structure of diaqua-bis(2,4-dinitrophenolato-κ2O,O′)copper(II) 1.5 hydrate, C12H13CuN4O13.5
- Crystal structure of N′,N‴-((1E,1′E)-((decane-1,10-diylbis(oxy))bis(2,1-phenylene)) bis(methaneylylidene))di(isonicotinohydrazide), C36H40N6O4
- The crystal structure of 2-[(R)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a-tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Synthesis and crystal structure of (1E,2E)-3-(anthracen-9-yl)-1-(4-methoxyphenyl)prop-2-en-1-one oxime, C24H19NO2
- Synthesis and crystal structure of (2E,2′E)-3,3′-(1,3-phenylene)bis(1-(3-bromophenyl)prop-2-en-1-one), C24H16Br2O2
- The crystal structure of catena-poly[bis(µ2-1,2-bis((1H-imidazol-1-yl)methyl)benzene- κ2N:N′)-bis(nitrato-κO)copper(II)], C28H28N10O6Cu
- Synthesis and crystal structure of the novel chiral acetyl-3-thiophene-5-(9-anthryl)-2-pyrazoline, C23H18N2OS
- Crystal structure of (E)-3-(dimethylamino)-1-(thiophen-3-yl)prop-2-en-1-one, C9H11NOS
- Crystal structure of catena-poly[aqua-(4-iodopyridine-2,6-dicarboxylato-κ3N,O,O′)-(μ2-4-amino-4H-1,2,4-triazole-κ2N:N′) copper(II)], C9H8N5O5CuI
- Crystal structure of cyclopropane-1,2,3-triyltris(phenylmethanone), C24H18O3
- Crystal structure of bis(amino(thioureido)methaniminium) terephthalate, C12H18N8O4S2
- A three-dimensional Eu(III) framework in the crystal structure of dimethylaminium poly[dimethylformamide-κ1N)bis(μ4-terephthalato-κ4O:O′:O′′:O′′′)europium(III)] monohydrate, C21H25EuN2O10
- Crystal structure of 2-methoxyphenyl 2-(6-methoxynaphthalen-2-yl)propanoate, C21H20O4
- The crystal structure of Hexakis(diethylamido)dimolybdenum, Mo2(NEt2)6

