Abstract
C14H19N2O2Cl, tetragonal, P41 (no. 76), a = 6.8614(8) Å, c = 29.820(5) Å, V = 1403.9(4) Å3, Z = 4, Rgt(F) = 0.0311, wRref(F2) = 0.0549, T = 150(2) K.
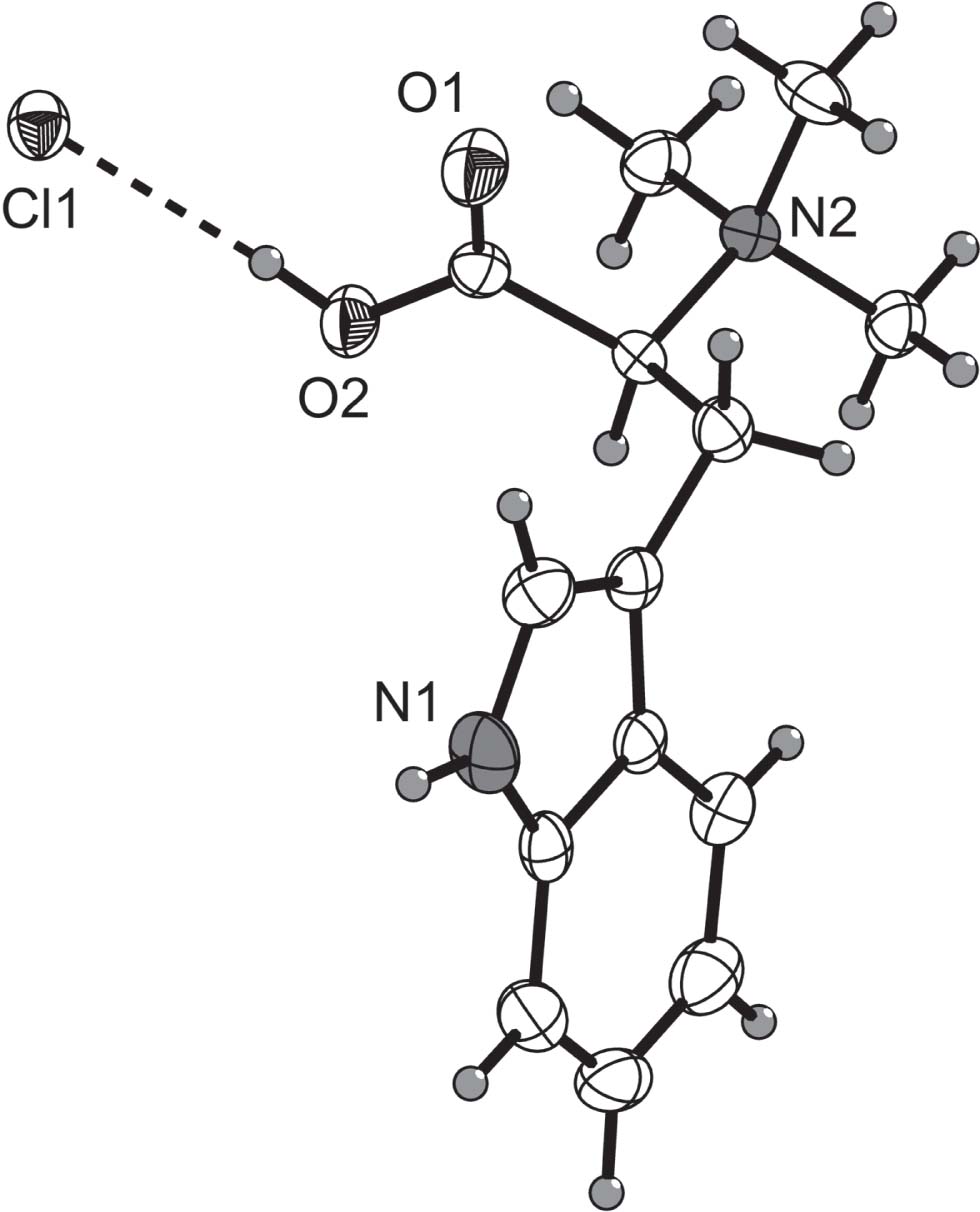
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | White polyhedron |
| Size: | 0.32 × 0.16 × 0.14 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.27 mm−1 |
| Diffractometer, scan mode: | Bruker CCD, ω and φ-scans |
| θmax, completeness: | 27.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11638, 3019, 0.038 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2637 |
| N(param)refined: | 183 |
| Programs: | Bruker [1], Olex2 [2], [3], SHELX [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cl1 | 0.04018(10) | 1.45430(9) | 0.47638(2) | 0.02804(16) |
| O1 | 0.3779(3) | 1.1704(3) | 0.55229(7) | 0.0357(5) |
| O2 | 0.1235(3) | 1.0590(3) | 0.51304(7) | 0.0313(5) |
| H2O | 0.109(6) | 1.182(5) | 0.5081(13) | 0.090(15)* |
| N1 | −0.0824(4) | 0.8822(4) | 0.66525(9) | 0.0366(6) |
| H1N | −0.159(4) | 0.953(4) | 0.6790(10) | 0.045(10)* |
| N2 | 0.5147(3) | 0.7691(3) | 0.51914(7) | 0.0240(5) |
| C2 | 0.0943(4) | 0.9371(4) | 0.64793(10) | 0.0325(7) |
| H2 | 0.156505 | 1.054591 | 0.654017 | 0.039* |
| C3 | 0.1661(4) | 0.7953(4) | 0.62053(9) | 0.0238(6) |
| C4 | 0.0255(4) | 0.6407(4) | 0.62101(8) | 0.0239(6) |
| C5 | 0.0184(4) | 0.4546(4) | 0.60159(9) | 0.0304(7) |
| H5 | 0.117737 | 0.411349 | 0.582846 | 0.036* |
| C6 | −0.1392(5) | 0.3380(5) | 0.61102(10) | 0.0390(8) |
| H6 | −0.146392 | 0.215273 | 0.597905 | 0.047* |
| C7 | −0.2897(5) | 0.3979(5) | 0.63978(10) | 0.0428(8) |
| H7 | −0.393192 | 0.314328 | 0.645715 | 0.051* |
| C8 | −0.2848(4) | 0.5798(5) | 0.65925(10) | 0.0366(8) |
| H8 | −0.383377 | 0.621048 | 0.678422 | 0.044* |
| C9 | −0.1275(4) | 0.6994(4) | 0.64927(9) | 0.0258(6) |
| C10 | 0.3581(4) | 0.7932(4) | 0.59680(8) | 0.0267(7) |
| H10A | 0.422333 | 0.669379 | 0.602056 | 0.032* |
| H10B | 0.440574 | 0.895523 | 0.608809 | 0.032* |
| C11 | 0.3319(4) | 0.8239(4) | 0.54620(9) | 0.0203(6) |
| H11 | 0.222864 | 0.743159 | 0.535912 | 0.024* |
| C12 | 0.2824(4) | 1.0382(4) | 0.53742(9) | 0.0236(6) |
| C13 | 0.5472(4) | 0.5536(4) | 0.52253(9) | 0.0286(7) |
| H13A | 0.433201 | 0.486034 | 0.512224 | 0.043* |
| H13B | 0.572480 | 0.519242 | 0.553191 | 0.043* |
| H13C | 0.656892 | 0.517503 | 0.504341 | 0.043* |
| C14 | 0.6971(4) | 0.8725(4) | 0.53410(11) | 0.0337(7) |
| H14A | 0.804213 | 0.833677 | 0.515414 | 0.051* |
| H14B | 0.724882 | 0.838965 | 0.564699 | 0.051* |
| H14C | 0.678526 | 1.010802 | 0.531735 | 0.051* |
| C15 | 0.4800(4) | 0.8139(4) | 0.47083(9) | 0.0305(7) |
| H15A | 0.588874 | 0.769285 | 0.453399 | 0.046* |
| H15B | 0.465300 | 0.952054 | 0.467063 | 0.046* |
| H15C | 0.363669 | 0.749153 | 0.460957 | 0.046* |
Source of material
All reagents were purchased from Sigma-Aldrich and Merck Company. Erythrina rubrinervia Kunth seeds were collected on the Star farm, municipality of Villahermosa-Tolima, central Colombia, 5°02′40′′ N, 75°7′38′′ W, 1,860 m above sea level, by Olimpo García in February 2014. Prof. Alfredo Torres Benitez identified the plant material, and a voucher specimen was deposited in the Herbarium of the Biology Department Tolima of Tolima University UT12960. Extraction and Isolation: Dried and ground seeds (1.25 g) of E. rubrinervia were defatted with n-hexane and then exhaustively extracted with ethanol at room temperature over a period of 15 days. After concentration under reduced pressure, a viscous brown liquid (35.9 g) was obtained. Finally, after usual work-up for alkaloids, L-hypaphorine precipitated as a white solid (3.2 g).
Experimental details
The structure was solved using OLEX2 [2] with the olex2.solve [3] and refined with the use of SHELX program package [4]. H-atoms attached to the N1 and O2 atoms were located in the difference Fourier maps and their positions and isotropic displacement parameters were refined freely. All other H atoms were then treated as riding atoms in geometrically idealized positions.
Comment
In this work, we report the isolation of L-hypaphorine from the seeds of E. rubrinervia and its crystallographic analysis. The genus Erythrina Mart. (Leguminoseae-Fabaceae) comprises around 115–118 known species [5], [6], [7] that grow in tropical-subtropical regions and in some temperate regions of the world in different ecosystems [7], [8], [9]. This alkaloid was first isolated from seeds of Erythrina hypaphorus Boerl [10]. However, it has been detected in several other genera and isolated from other species of Erythrina. The species of this genus are used traditionally to treat infections, such as malaria, inflammation, jaundice, anaemia, dysentery, female infertility, stomach pain, gonorrhea [11], [12] and for their anxiolitic effects. Moreover this compound has shown other interesting biological activities such as hypotensive, anticonvulsant, hypnotic, and analgesic ones [13], [14], [15].
The ORTEP diagram of the title structure with the atom-numbering scheme is shown in the figure. The indole group is essentially planar. The substituent group (at C3) is tilted out of the mean plane of the indole ring with a torsion angle C4—C3—C10—C11 of 76.1(3)°. The N1—C2 [1.371(4) Å] bond distance is similar to the average values reported for a Csp2-N in imidazole bond (1.370 Å) [16]. All the other relevant structural parameters (bond distances and angles) are as expected and in acceptable agreement with L-hypaphorine hydroiodide analogue [17]. In the crystal the molecules are linked via hydrogen bonds between chloride anions and organic molecules. The distance N1—H1N⋯Cl1 is 2.54(3) Å [angle of 157(3)°] and O2—H2O⋯Cl1 is 2.15(4) Å [angle of 162(4)°] and thus, the combination of both hydrogen bonds leads to the formation of chains running along the [001] direction.
References
1. Bruker. Analytical X-ray Instruments Inc., Madison, WI, USA, (2000).Suche in Google Scholar
2. Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment – Olex2 dissected. Acta Crystallogr. A71 (2015) 59–75.10.1107/S2053273314022207Suche in Google Scholar
3. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
4. Sheldrick, G. M.: Program for the refinement of crystal structures, University of Göttingen. Acta Cryst. C71 (2015) 3–8.10.1107/S2053229614024218Suche in Google Scholar
5. Majinda, R. R. T.; Abegaz, B. M.; Bezabih, M.; Ngadjui, B. T.; Wanjala, C. C. W.; Mdee, L. K.; Bojase, G.; Silayo, A.; Masesane, I.; Yeboah, S. O.: Recent results from natural product research at the University of Botswana. Pure Appl. Chem. 73 (2001) 1197–1208.10.1351/pac200173071197Suche in Google Scholar
6. Guaratini, T.; Silva, D. B.; Bizaro, A. C.; Sartori, L. R.; Humpf, H. U.; Lopes, N. P.; Costa-Lotufo, L. V.; Lopes, J. L.: In vitro metabolism studies of erythraline, the major spiroalkaloid from Erythrina verna. BMC Complem. Altern. Med. 14 (2014) 61–65.10.1186/1472-6882-14-61Suche in Google Scholar
7. García-Beltrán, O.; Moreno-Palacios, M.: In alkaloids; biosynthesis, biological roles and health benefits. (Sobarzo-Sanchez, E. Ed.); Nova science publishers 5 (2015) 107–130.Suche in Google Scholar
8. García-Beltrán, O.; Areche, C.; Cassels, B. K.; Cuca-Suarez, L.: Coumarins isolated from Esenbeckia alata (Rutaceae). Biochem. Syst. Ecol. 52 (2014) 38–40.10.1016/j.bse.2013.12.011Suche in Google Scholar
9. Neill, D. A.: Experimental studies on species relationships in Erythrina (Leguminosae: Papilionoideae). Ann. Missouri Bot. Gard. 75 (1988) 886–969.10.2307/2399377Suche in Google Scholar
10. Hargreaves, R.; Jonson, D.; Millington, D.; Mondal, M.; Beavers, W.; Becker, L.; Young, C.; Rinehari, K. L.: Alkaloids of american species of erythrina. Lloydia 37 (1974) 569–580.Suche in Google Scholar
11. Saidu, K. J.; Onah, A.; Orisadipe, A.; Olusola, A.; Wambebe, C.; Gamaniel, K. J.: Antiplasmodial, analgesic, and anti-inflammatory activities of the aqueous extract of the stem bark of Erythrina senegalensis. J. Ethnopharmacol. 71 (2000) 275–280.10.1016/S0378-8741(00)00188-4Suche in Google Scholar
12. Lee, J.; Oh, W. K.; Ahn, J. S.; Kim, Y. H.; Mbafor, J. T.; Wandji, J.; Fomum, Z. T.: Prenylisoflavonoids from Erythrina senegalensis as novel HIV-1 protease inhibitors. Planta Med. 75 (2009) 268–270.10.1055/s-0028-1088395Suche in Google Scholar
13. Garín-Aguilar, M. E.; Ramírez, L. J. E.; Soto Hernández, M.; Valencia del Toro, G.; Martínez, V. M.: Effect of crude extracts of Erythrina americana Mill. on agressive behavior in rats. J. Ethnopharmacol. 69 (2000) 189–196.10.1016/S0378-8741(99)00121-XSuche in Google Scholar
14. Vasconcelos, S. M.; Lima, N. M.; Sales, G. T.; Cunha, G. M.; Aguiar, L. M.; Silveira, E. R.; Rodrigues, A. C.; Macedo, D. S.; Fonteles, M. M.; Sousa, F. C.; Viana, G. S.: Anticonvulsant activity of hydroalcoholic extracts from Erythrina velutina and Erythrina mulungu. J. Ethnopharmacol. 110 (2007) 271–274.10.1016/j.jep.2006.09.023Suche in Google Scholar PubMed
15. Ferreira de Lima, M. R.; de Souza Luna, J.; dos Santos, A. F.; Caño de Andrade, M. C.; Goulart SantAna, A. E.; Genet, J. P.; Marquez, B.; Neuville, L.; Moreau, N. J.: Anti-bacterial activity of some Brazilian medical plants. J. Ethnopharmacol. 105 (2006) 137–147.10.1016/j.jep.2005.10.026Suche in Google Scholar PubMed
16. Allen, F. H.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R.: Typical interatomic distances: organic compounds. International Tables for Crystallography C (2006) 790–811.10.1107/97809553602060000621Suche in Google Scholar
17. Jones, G. P.; Tiekink, E. R. T.: Crystal and molecular structure of l-hypaphorine hydroiodide. Z. Kristallogr. NCS 212 (1997) 881–883.10.1524/zkri.1997.212.12.881Suche in Google Scholar
©2020 Olimpo García-Beltrán et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of isopimara-7,15-dien-3-one, C20H30O
- Crystal structure of bis(6-aminopyridine-2-carboxylato-κ2O,N)-copper(II), C12H10O6N4Cu
- Crystal structure of 5,6-diphenyldibenzo[c, g]chrysene, C38H24
- Poly[bis(dimethylformamide-κO)-(μ8-5,5′′-dicarboxy-[1,1′:4′,1′′-terphenyl]-2′,3,3′′,5′-tetracarboxylato-κ8O:O1:O2:O3:O4:O5:O6:O7)dizinc(II)] — dimethylformamide (1/2), C18H19N2O8Zn
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)(μ4- 2′,5,5′,5′′-tetracarboxy-[1,1′:4′,1′′-terphenyl]-3,3′′-dicarboxylato-κ4O:O′:O′′:O′′′)manganese(II)] — N,N-dimethylformamide (1/2), C36H40N4O16Mn
- Crystal structure of N,N-dimethyl-4-((7-nitrobenzo[c][1,2,5]thiadiazol-4-yl)ethynyl)aniline, C16H12N4O2S
- The crystal structure of 8a-methoxy 8a-methoxy-1,5,8a,9a-tetrahydro-4H-8,9-dioxa-3a1λ4-aza-8aλ4, C18H14BNO3
- Crystal structure of poly[diaqua-(μ2-5-isopropoxyisophthalato-κ2O:O′)-(μ2-(1,3-bis(3,5-di(1H-imidazol-1-yl)pyridine))-κ2N:N′)cobalt(II)] monohydrate, C22H25N5O8Co
- The crystal structure of acetoximium 1′-hydroxy-1H,1H′-5,5′-bitetrazole-1-olate monohydrate, C5H11N9O4
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-vinyl-1H-imidazol-3-ium hexafluoridophosphate(V), C9H13F6N2O2P
- The crystal structure of catena-poly[(μ2-4-(benzo[d]imidazol-2-yl)benzenecarboxylato-κ2N,O)-(μ2-4-(benzo[d]imidazol-2-yl)benzenecarboxylato-κ3N,O:O′)cadmium(II)]dihydrate, C28H22CdN4O6
- Enzyme-mediated synthesis and crystal structure of (2R,4S)-hydroxyketamine, C13H16ClNO2
- The crystal structure of bis(isothiocyanato-κ1N)-(methanol-κ1O)-[2-morpholine-4-yl-4,6-di(pyrazol-1-yl)-1,3,5-triazine-κ3N,N′,N′′] manganese(II), C16H18MnN10O2S2
- Crystal structure of bis{5-chloro-2-(((4-trifluoromethyl)imino)methyl)phenolato-κ2N,O}copper(II), C28H16Cl2CuF6N2O2
- Crystal structure of bis(1,3-phenylenedimethanaminium) bis(triiodide) tetraiodide – water (1/2) , C8H16I5N2O
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C25H18F3NO3S
- The crystal structure of bis{3-(diphenylphosphaneyl)propanoato-κ2O,P}platinum(II) dihydrate, C30H28O6P2Pt
- The crystal structure of (E)-2-(4-((4-fluorobenzyl)oxy)styryl)-4,6-dimethoxybenzaldehyde, C24H21FO4
- Crystal structure of bis[3-methoxy-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′]nickel(II), C28H26N8O4Ni
- Crystal structure of 1-(2-(pyridin-2-yl)-5-(pyridin-3-yl)-1,3,4-oxadiazol-3(2H)-yl)ethan-1-one, C14H12N4O2
- Synthesis and crystal structure of 3-N-acetyl-5-(pyridin-3-yl)-2-(quinolin-2-yl)-1,3,4-oxadiazoline, C18H14N4O2
- Crystal structure of 2-methyl-1H-perimidine, C12H10N2
- Crystal structure of (E)-2-(5,5-dimethyl-3-(4-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)oxy)styryl)cyclohex-2-en-1-ylidene)malononitrile, C25H19N5O4
- Structural elucidation of 1-(3-acetyl-2,6-dihydroxy-4-methoxyphenyl)-4,5-dihydroxy-2-methylanthracene-9,10-dione isolated from Bulbine latifolia (L.) Wild, C24H18O8
- Crystal structure of 3-cinnamoyl-4-hydroxybenzoic acid, C16H12O4
- The crystal structure of poly[bis(μ4-2,3-pyridinedicarboxylato)-(μ2-oxalyl dihydrazide)-dicadmium(II) dihydrate], C16H16O12N6Cd2
- Synthesis and crystal structure of 1-{4-[(3-bromo-2-hydroxy-benzylidene)amino]phenyl}ethanone, C15H12BrNO2
- Synthesis and crystal structure of 1-{4-[(2-bromo-6-hydroxy-benzylidene)amino]phenyl}ethanone, C15H12BrNO2
- Crystal structure of (4-aminobenzoato-κ2O,O′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′]nickel(II) perchlorate monohydrate, C23H44ClN5NiO7
- Crystal structure of 1-{4-[(4-fluoro-2-hydroxy-benzylidene)amino]phenyl}ethanone, C15H12FNO2
- Preparation and crystal structure of a non-symmetrical vanadium(II) dimer: tri-μ2-bromido-(hydrogen-tris(3-isopropyl-4-bromopyrazol-1-yl)borato-κ3N,N′,N′′)-tris(tetrahydrofuran-κO)divanadium(II) – tetrahydrofuran (1/1), C34H57BBr6N6O4V2
- Crystal structure of bis{2-(((4-(1-(hydroxyl-imino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C30H26CoN4O4
- Crystal structure of 3-((3-nitrophenyl)sulfonamido)propanoic acid — 4,4′-bipyridine (1/1), C19H18N4O6S
- Crystal structure of cyclo[diaqua-bis(μ2-3′,5-dicarboxy-[1,1′-biphenyl]-3,4′-dicarboxylato-κ4O,O′:O′′,O′′′)-bis(4,4′-bis(pyrid-4-yl)biphenyl-K1N)dicadmium(II)], C76H52Cd2N4O18
- Crystal structure of 1-(adamantan-1-yl)-3-aminothiourea, C11H19N3S
- Crystal structure of catena-poly[triaqua-(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(3′,5-dicarboxy-[1,1′-biphenyl]-3,4′-dicarboxylato-κO)nickel(II)], C32H26N2O11Ni
- Crystal structure of catena-poly[aqua-(μ4-4,4′-(pyridine-3,5-diyl)dibenzoato-κ4O,O′:O′′:O′′′)zinc(II)], C19H13NO5Zn
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium 2-carboxy-4-(3,5-dicarboxyphenoxy)benzoate hydrate, C38H28N2O10
- Crystal structure of 3-[(triisopropylsilanyl)-ethynyl]-6a,12a-dihydro-1H-1,4-diaza-benzo[α]anthracene-2,7,12-trione, C27H28N2O3Si
- Crystal structure of [(bis(1,10-phenanthroline-κ2N,N′)-(2-carboxy-4-(3-carboxy-5-carboxylatophenoxy)benzoato-κ2O:O′))nickel(II) monohydrate, (1,10-phenanthroline-κ2N:N′)-(μ2-(5-(3′,4′-dicarboxylphenoxy)-isophthalate-κ2O:O′))nickel(II)], C40H24N4O9Ni ⋅ H2O
- Crystal structure of 4-(3-(pyridin-3-yl)ureido)benzoic acid — adipic acid (2/1), C16H16N3O5
- Crystal structure of poly[bis{μ2-5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O:O′}-{μ2-4,4′-bipyridine-κ2N:N′}]cobalt(II), C40H30N2O8Co
- Crystal structure of aqua-(2,2′-bipyridine-κ2N,N′)(((3-nitrophenyl)sulfonyl)glycine-κ2N,O)copper(II) dihydrate, C18H20CuN4O9S
- Crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}copper(II), C32H28Br2CuN4O4
- Crystal structure of bis(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)zinc(II), C40H28Cl4N4O4Zn
- Crystal structure of 2-(3,6-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-oxoethyl acetate, C14H17NO4
- Crystal structure of poly[dibromido-bis(μ2-1,6-di(1H-imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C24H36Br2N8Cd
- Synthesis and crystal structure of ((6R,7S)-3-ethyl-6-phenyl-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanone hemihydrate, 2(C19H18N4OS) ⋅ H2O
- Crystal structure of 2-(5-(pyridin-3-yl)-4-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)pyridine, C17H12N6
- The crystal structure of N-((1E,2E)-1,3-bis(4-fluorophenyl)but-2-en-1-ylidene)-4-methylbenzenesulfonamide, C23H19F2NO2S
- Crystal structure of diacetato-κ1O-diethanol-κ1O-bis(μ2-2-(((2-hydroxyethyl)imino)methyl)-5-methoxyphenolato-κ4O,N,O′:O′′)dinickel(II), C28H42Ni2N2O12
- The crystal structure of catena-poly[chlorido-(μ2-1,4-bis(pyridin-3-yl-methoxy)benzene-κ2N:N′)copper(II)], C18H16ClN2O2Cu
- N′,N′′′-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(methaneylylidene))bis(2-hydroxybenzohydrazide)nickel(II), C30H24N4NiO6
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-(2-((quinolin-2-ylmethylene)amino)ethyl)spiro[isoindoline-1,9′-xanthen]-3-one, C38H37N5O2
- Crystal structure of 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-1-ium 5,3′,5′-tricarboxy-[1,10-biphenyl]-2-carboxylate, C25H17N2O8
- The crystal structure of 1-carboxy-2-(1H-indol-3-yl)-N,N,N-trimethylethan-1-ammonium chloride, C14H19N2O2Cl
- The crystal structure of 5-bromo-2-fluoronicotinic acid monohydrate, C6H5BrFNO3
- Crystal structure of ethyl 3-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C7H7F3N2O2
- Crystal structure of tetrakis(1H-benzo[d]imidazol-3-ium) bis(μ5-phenylphosphonato)-pentakis(μ2-oxido)-decaoxo-penta-molybdenum dihydrate, C40H42Mo5N8O23P2
- Structure of 7-(3,3,4,4,5,5-hexafluoro-2-(2-methylbenzo[b]thiophen-3-yl)cyclopent-1-en-1-yl)-8-methylquinoline, C24H15F6NS
- Crystal structure of monocarbonyl[2-((cyclopentylmethylene)amino)-5-methylphenolato-κ2N,O] (tricyclohexylphosphine)rhodium(I), C32H48NO2PRh
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(pyrazole-κN)rhenium(I)nitrate, C18H12O3N4Re
- Crystal structure of poly[diaqua-bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato-κ2N:O)nickel(II)], C28H22O6N8Ni
- Crystal structure of 4,4′-bis(pyridin-1-ium-4-yl)biphenyl poly[bis(μ2-4,4′-bis(pyrid-4-yl)biphenyl-K2N:N′)-tetrakis(μ4-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-K4O,O′:O′′:O′′′)-bis[[μ2-1,1′-biphenyl]-3-carboxyl-5-carboxylato-K2O:O′]tetracobalt(II)]— [1,1′-biphenyl]-3,5-dicarboxylic acid (1/2), C93H68N3O16Co2
- The crystal structure of 4a-formyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydro-1-2-6a,6b,9,9,12a-heptamethylpicen-10-yl acetate, C32H50O3
- Crystal structure of 3,3′-(1,2-phenylenebis(methylene))bis(1-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C16H20F12N4P2
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)zinc(II)], C4H8O8Zn
- The crystal structure of (6aR,6bS,8aS,8bR,9S,11aS,12aS,12bS)-10-(4-acetoxy-3-methylbutyl)-6a,8a,9-trimethyl-3,4,5,6,6a,6b,7,8,8a,8b,9,10,11a,12,12a,12b-hexadecahydro-1H-naphtho[2′,1′:4,5]indeno[2,1-b]furan-4-yl acetate, C31H48O5
- Crystal structure of 4,4′-(oxybis(methylene))bis(bromobenzene), C14H12Br2O
- Crystal structure of (N,N-dimethylsulphoxide)-[N-(3-ethoxy-2-(oxide)benzylidene)-3-methoxybenzenecarbohydrazonato-κ3N,O,O′]-dioxo-molybdenum(VI), C19H22MoN2O7S
- Crystal structure of dichlorido-bis(dimethyl sulphoxide-κO)-bis(4-methylbenzyl-κC1)tin(IV), C20H30Cl2O2S2Sn
- Crystal structure of (E)-2-amino-N′-(2-hydroxy-4-(2-(piperidin-1-yl)ethoxy)benzylidene)benzohydrazide monohydrate, C21H26N4O3 ⋅ H2O
- Crystal structure of chloridotris(4-chlorophenyl)(dimethyl sulfoxide-κO)tin(IV), C20H18Cl4OSSn
- Crystal structure of catena{di-aqua-sodium-[N-(hydroxyethyl), N-isopropyl-dithiocarbamato]}n, [C6H16NNaO2S2]n
- Crystal structure of 2,2,4,4,6,6-hexakis(4-chlorophenyl)-1,3,5,2,4,6-trithiatristanninane, C36H24Cl6S3Sn3
- Crystal structure of 6-methoxy-3-(5-(3-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-4H-chromen-4-one-methanol (1/1), C20H18N2O6
- Crystal structure of hexanedihydrazide, C6H14N4O2
- Crystal structure of tert-butyl 2-(hydroxymethyl)-5-{4-[(methoxycarbonyl)amino]phenyl}-2,5-dihydro-1H-pyrrole-1-carboxylate, C18H24N2O5
- Crystal structure of [(Z)-O-isopropyl N-(4-nitrophenyl)thiocarbamato-κS]-(triphenylphosphine-κP)-gold(I), C28H26AuN2O3PS
- Crystal structure of [O-ethyl N-(4-nitrophenyl)thiocarbamato-κS](tri-4-tolylphosphine-κP)gold(I) tetrahydrofuran solvate, C30H30AuN2O3PS, C4H8O
Artikel in diesem Heft
- Frontmatter
- Crystal structure of isopimara-7,15-dien-3-one, C20H30O
- Crystal structure of bis(6-aminopyridine-2-carboxylato-κ2O,N)-copper(II), C12H10O6N4Cu
- Crystal structure of 5,6-diphenyldibenzo[c, g]chrysene, C38H24
- Poly[bis(dimethylformamide-κO)-(μ8-5,5′′-dicarboxy-[1,1′:4′,1′′-terphenyl]-2′,3,3′′,5′-tetracarboxylato-κ8O:O1:O2:O3:O4:O5:O6:O7)dizinc(II)] — dimethylformamide (1/2), C18H19N2O8Zn
- The crystal structure of poly[bis(N,N-dimethylformamide-κ1O)(μ4- 2′,5,5′,5′′-tetracarboxy-[1,1′:4′,1′′-terphenyl]-3,3′′-dicarboxylato-κ4O:O′:O′′:O′′′)manganese(II)] — N,N-dimethylformamide (1/2), C36H40N4O16Mn
- Crystal structure of N,N-dimethyl-4-((7-nitrobenzo[c][1,2,5]thiadiazol-4-yl)ethynyl)aniline, C16H12N4O2S
- The crystal structure of 8a-methoxy 8a-methoxy-1,5,8a,9a-tetrahydro-4H-8,9-dioxa-3a1λ4-aza-8aλ4, C18H14BNO3
- Crystal structure of poly[diaqua-(μ2-5-isopropoxyisophthalato-κ2O:O′)-(μ2-(1,3-bis(3,5-di(1H-imidazol-1-yl)pyridine))-κ2N:N′)cobalt(II)] monohydrate, C22H25N5O8Co
- The crystal structure of acetoximium 1′-hydroxy-1H,1H′-5,5′-bitetrazole-1-olate monohydrate, C5H11N9O4
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-vinyl-1H-imidazol-3-ium hexafluoridophosphate(V), C9H13F6N2O2P
- The crystal structure of catena-poly[(μ2-4-(benzo[d]imidazol-2-yl)benzenecarboxylato-κ2N,O)-(μ2-4-(benzo[d]imidazol-2-yl)benzenecarboxylato-κ3N,O:O′)cadmium(II)]dihydrate, C28H22CdN4O6
- Enzyme-mediated synthesis and crystal structure of (2R,4S)-hydroxyketamine, C13H16ClNO2
- The crystal structure of bis(isothiocyanato-κ1N)-(methanol-κ1O)-[2-morpholine-4-yl-4,6-di(pyrazol-1-yl)-1,3,5-triazine-κ3N,N′,N′′] manganese(II), C16H18MnN10O2S2
- Crystal structure of bis{5-chloro-2-(((4-trifluoromethyl)imino)methyl)phenolato-κ2N,O}copper(II), C28H16Cl2CuF6N2O2
- Crystal structure of bis(1,3-phenylenedimethanaminium) bis(triiodide) tetraiodide – water (1/2) , C8H16I5N2O
- Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C25H18F3NO3S
- The crystal structure of bis{3-(diphenylphosphaneyl)propanoato-κ2O,P}platinum(II) dihydrate, C30H28O6P2Pt
- The crystal structure of (E)-2-(4-((4-fluorobenzyl)oxy)styryl)-4,6-dimethoxybenzaldehyde, C24H21FO4
- Crystal structure of bis[3-methoxy-N-(1-(pyrazin-2-yl)ethylidene)benzohydrazonato-κ3O,N,N′]nickel(II), C28H26N8O4Ni
- Crystal structure of 1-(2-(pyridin-2-yl)-5-(pyridin-3-yl)-1,3,4-oxadiazol-3(2H)-yl)ethan-1-one, C14H12N4O2
- Synthesis and crystal structure of 3-N-acetyl-5-(pyridin-3-yl)-2-(quinolin-2-yl)-1,3,4-oxadiazoline, C18H14N4O2
- Crystal structure of 2-methyl-1H-perimidine, C12H10N2
- Crystal structure of (E)-2-(5,5-dimethyl-3-(4-((7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)oxy)styryl)cyclohex-2-en-1-ylidene)malononitrile, C25H19N5O4
- Structural elucidation of 1-(3-acetyl-2,6-dihydroxy-4-methoxyphenyl)-4,5-dihydroxy-2-methylanthracene-9,10-dione isolated from Bulbine latifolia (L.) Wild, C24H18O8
- Crystal structure of 3-cinnamoyl-4-hydroxybenzoic acid, C16H12O4
- The crystal structure of poly[bis(μ4-2,3-pyridinedicarboxylato)-(μ2-oxalyl dihydrazide)-dicadmium(II) dihydrate], C16H16O12N6Cd2
- Synthesis and crystal structure of 1-{4-[(3-bromo-2-hydroxy-benzylidene)amino]phenyl}ethanone, C15H12BrNO2
- Synthesis and crystal structure of 1-{4-[(2-bromo-6-hydroxy-benzylidene)amino]phenyl}ethanone, C15H12BrNO2
- Crystal structure of (4-aminobenzoato-κ2O,O′)-[5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N′′,N′′′]nickel(II) perchlorate monohydrate, C23H44ClN5NiO7
- Crystal structure of 1-{4-[(4-fluoro-2-hydroxy-benzylidene)amino]phenyl}ethanone, C15H12FNO2
- Preparation and crystal structure of a non-symmetrical vanadium(II) dimer: tri-μ2-bromido-(hydrogen-tris(3-isopropyl-4-bromopyrazol-1-yl)borato-κ3N,N′,N′′)-tris(tetrahydrofuran-κO)divanadium(II) – tetrahydrofuran (1/1), C34H57BBr6N6O4V2
- Crystal structure of bis{2-(((4-(1-(hydroxyl-imino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}cobalt(II), C30H26CoN4O4
- Crystal structure of 3-((3-nitrophenyl)sulfonamido)propanoic acid — 4,4′-bipyridine (1/1), C19H18N4O6S
- Crystal structure of cyclo[diaqua-bis(μ2-3′,5-dicarboxy-[1,1′-biphenyl]-3,4′-dicarboxylato-κ4O,O′:O′′,O′′′)-bis(4,4′-bis(pyrid-4-yl)biphenyl-K1N)dicadmium(II)], C76H52Cd2N4O18
- Crystal structure of 1-(adamantan-1-yl)-3-aminothiourea, C11H19N3S
- Crystal structure of catena-poly[triaqua-(μ2-1,4-di(pyridin-4-yl)benzene-κ2N:N′)-(3′,5-dicarboxy-[1,1′-biphenyl]-3,4′-dicarboxylato-κO)nickel(II)], C32H26N2O11Ni
- Crystal structure of catena-poly[aqua-(μ4-4,4′-(pyridine-3,5-diyl)dibenzoato-κ4O,O′:O′′:O′′′)zinc(II)], C19H13NO5Zn
- Crystal structure of 4-(4′-(pyridin-4-yl)-[1,1′-biphenyl]-4-yl)pyridin-1-ium 2-carboxy-4-(3,5-dicarboxyphenoxy)benzoate hydrate, C38H28N2O10
- Crystal structure of 3-[(triisopropylsilanyl)-ethynyl]-6a,12a-dihydro-1H-1,4-diaza-benzo[α]anthracene-2,7,12-trione, C27H28N2O3Si
- Crystal structure of [(bis(1,10-phenanthroline-κ2N,N′)-(2-carboxy-4-(3-carboxy-5-carboxylatophenoxy)benzoato-κ2O:O′))nickel(II) monohydrate, (1,10-phenanthroline-κ2N:N′)-(μ2-(5-(3′,4′-dicarboxylphenoxy)-isophthalate-κ2O:O′))nickel(II)], C40H24N4O9Ni ⋅ H2O
- Crystal structure of 4-(3-(pyridin-3-yl)ureido)benzoic acid — adipic acid (2/1), C16H16N3O5
- Crystal structure of poly[bis{μ2-5-carboxy-4′-methyl-[1,1′-biphenyl]-3-carboxylato-κ2O:O′}-{μ2-4,4′-bipyridine-κ2N:N′}]cobalt(II), C40H30N2O8Co
- Crystal structure of aqua-(2,2′-bipyridine-κ2N,N′)(((3-nitrophenyl)sulfonyl)glycine-κ2N,O)copper(II) dihydrate, C18H20CuN4O9S
- Crystal structure of bis{2-bromo-6-(((4-(1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}copper(II), C32H28Br2CuN4O4
- Crystal structure of bis(2-(2-((2,6-dichlorophenyl)amino)phenyl)acetato-κ2O,O′)-(1,10-phenanthroline-κ2N,N′)zinc(II), C40H28Cl4N4O4Zn
- Crystal structure of 2-(3,6-dimethyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)-2-oxoethyl acetate, C14H17NO4
- Crystal structure of poly[dibromido-bis(μ2-1,6-di(1H-imidazol-1-yl)hexane-κ2N:N′)cadmium(II)], C24H36Br2N8Cd
- Synthesis and crystal structure of ((6R,7S)-3-ethyl-6-phenyl-6,7-dihydro-5H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-7-yl)(phenyl)methanone hemihydrate, 2(C19H18N4OS) ⋅ H2O
- Crystal structure of 2-(5-(pyridin-3-yl)-4-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)pyridine, C17H12N6
- The crystal structure of N-((1E,2E)-1,3-bis(4-fluorophenyl)but-2-en-1-ylidene)-4-methylbenzenesulfonamide, C23H19F2NO2S
- Crystal structure of diacetato-κ1O-diethanol-κ1O-bis(μ2-2-(((2-hydroxyethyl)imino)methyl)-5-methoxyphenolato-κ4O,N,O′:O′′)dinickel(II), C28H42Ni2N2O12
- The crystal structure of catena-poly[chlorido-(μ2-1,4-bis(pyridin-3-yl-methoxy)benzene-κ2N:N′)copper(II)], C18H16ClN2O2Cu
- N′,N′′′-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis(methaneylylidene))bis(2-hydroxybenzohydrazide)nickel(II), C30H24N4NiO6
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2′,7′-dimethyl-2-(2-((quinolin-2-ylmethylene)amino)ethyl)spiro[isoindoline-1,9′-xanthen]-3-one, C38H37N5O2
- Crystal structure of 4,4′-di(1H-imidazol-1-yl)-1,1′-biphenyl-1-ium 5,3′,5′-tricarboxy-[1,10-biphenyl]-2-carboxylate, C25H17N2O8
- The crystal structure of 1-carboxy-2-(1H-indol-3-yl)-N,N,N-trimethylethan-1-ammonium chloride, C14H19N2O2Cl
- The crystal structure of 5-bromo-2-fluoronicotinic acid monohydrate, C6H5BrFNO3
- Crystal structure of ethyl 3-(trifluoromethyl)-1H-pyrazole-4-carboxylate, C7H7F3N2O2
- Crystal structure of tetrakis(1H-benzo[d]imidazol-3-ium) bis(μ5-phenylphosphonato)-pentakis(μ2-oxido)-decaoxo-penta-molybdenum dihydrate, C40H42Mo5N8O23P2
- Structure of 7-(3,3,4,4,5,5-hexafluoro-2-(2-methylbenzo[b]thiophen-3-yl)cyclopent-1-en-1-yl)-8-methylquinoline, C24H15F6NS
- Crystal structure of monocarbonyl[2-((cyclopentylmethylene)amino)-5-methylphenolato-κ2N,O] (tricyclohexylphosphine)rhodium(I), C32H48NO2PRh
- The crystal structure of fac-tricarbonyl(1,10-phenanthroline-κ2N,N′)-(pyrazole-κN)rhenium(I)nitrate, C18H12O3N4Re
- Crystal structure of poly[diaqua-bis(μ2-4-(3-(pyridin-3-yl)-1H-1,2,4-triazol-5-yl)benzoato-κ2N:O)nickel(II)], C28H22O6N8Ni
- Crystal structure of 4,4′-bis(pyridin-1-ium-4-yl)biphenyl poly[bis(μ2-4,4′-bis(pyrid-4-yl)biphenyl-K2N:N′)-tetrakis(μ4-4′-methyl-[1,1′-biphenyl]-3,5-dicarboxylato-K4O,O′:O′′:O′′′)-bis[[μ2-1,1′-biphenyl]-3-carboxyl-5-carboxylato-K2O:O′]tetracobalt(II)]— [1,1′-biphenyl]-3,5-dicarboxylic acid (1/2), C93H68N3O16Co2
- The crystal structure of 4a-formyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydro-1-2-6a,6b,9,9,12a-heptamethylpicen-10-yl acetate, C32H50O3
- Crystal structure of 3,3′-(1,2-phenylenebis(methylene))bis(1-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C16H20F12N4P2
- Crystal structure of catena-poly[diaqua-(μ2-tartrato-κ4O,O′:O′′,O′′′)zinc(II)], C4H8O8Zn
- The crystal structure of (6aR,6bS,8aS,8bR,9S,11aS,12aS,12bS)-10-(4-acetoxy-3-methylbutyl)-6a,8a,9-trimethyl-3,4,5,6,6a,6b,7,8,8a,8b,9,10,11a,12,12a,12b-hexadecahydro-1H-naphtho[2′,1′:4,5]indeno[2,1-b]furan-4-yl acetate, C31H48O5
- Crystal structure of 4,4′-(oxybis(methylene))bis(bromobenzene), C14H12Br2O
- Crystal structure of (N,N-dimethylsulphoxide)-[N-(3-ethoxy-2-(oxide)benzylidene)-3-methoxybenzenecarbohydrazonato-κ3N,O,O′]-dioxo-molybdenum(VI), C19H22MoN2O7S
- Crystal structure of dichlorido-bis(dimethyl sulphoxide-κO)-bis(4-methylbenzyl-κC1)tin(IV), C20H30Cl2O2S2Sn
- Crystal structure of (E)-2-amino-N′-(2-hydroxy-4-(2-(piperidin-1-yl)ethoxy)benzylidene)benzohydrazide monohydrate, C21H26N4O3 ⋅ H2O
- Crystal structure of chloridotris(4-chlorophenyl)(dimethyl sulfoxide-κO)tin(IV), C20H18Cl4OSSn
- Crystal structure of catena{di-aqua-sodium-[N-(hydroxyethyl), N-isopropyl-dithiocarbamato]}n, [C6H16NNaO2S2]n
- Crystal structure of 2,2,4,4,6,6-hexakis(4-chlorophenyl)-1,3,5,2,4,6-trithiatristanninane, C36H24Cl6S3Sn3
- Crystal structure of 6-methoxy-3-(5-(3-methoxyphenyl)-1,3,4-oxadiazol-2-yl)-4H-chromen-4-one-methanol (1/1), C20H18N2O6
- Crystal structure of hexanedihydrazide, C6H14N4O2
- Crystal structure of tert-butyl 2-(hydroxymethyl)-5-{4-[(methoxycarbonyl)amino]phenyl}-2,5-dihydro-1H-pyrrole-1-carboxylate, C18H24N2O5
- Crystal structure of [(Z)-O-isopropyl N-(4-nitrophenyl)thiocarbamato-κS]-(triphenylphosphine-κP)-gold(I), C28H26AuN2O3PS
- Crystal structure of [O-ethyl N-(4-nitrophenyl)thiocarbamato-κS](tri-4-tolylphosphine-κP)gold(I) tetrahydrofuran solvate, C30H30AuN2O3PS, C4H8O

