Abstract
tHPA-ZSM-5 nanocomposites as a superior catalyst have been applied for the synthesis of indenopyrazolones through a three-component reaction of phenylhydrazine, benzaldehydes, and indan-1,2,3-trione at room temperature in acetonitrile. The zeolite catalyst has been characterized by X-ray diffraction, field emission scanning electronic microscopes, Fourier transform infrared, energy-dispersive spectroscopy, thermogravimetric analysis, and N2-adsorption analysis. The various aromatic aldehydes can be utilized in this method. These results showed that aromatic aldehydes with electron-withdrawing groups reacted faster than aldehydes with electron-releasing groups. Experimental simplicity, excellent yields in short reaction times, reusability of the catalyst, and low catalyst loading are some of the substantial features of this method.
Graphical abstract
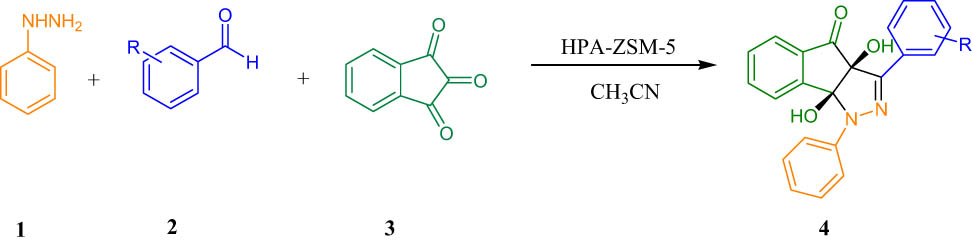
1 Introduction
Pyrazolones show anticancer (Saidachary et al., 2014), antimicrobial (Indrasena et al., 2014), antianalgesic (Khalil et al., 2014), antioxidant (Mazimba et al., 2014), antibacterial (Sivakumar et al., 2014), anti-diabetic (Mor and Sindhu, 2020), antifungal (Mor et al., 2017), antitumor (Rostom, 2006), and anticonvulsant (Ahsan et al., 2013) activities. Indenofused heterocycles have received considerable attention from synthetic chemists and medicinal professionals in recent years (Kaur et al., 2020; Singh et al., 2005, Singh, 2016). These properties make pyrazolones substantial objectives in organic synthesis. The past reports on the synthesis of pyrazolones have mentioned such catalysts as CH3COOH (Mor et al., 2019), [HMIM]HSO4 (Zang et al., 2011), 3-aminopropylated silica gel (Sobhani et al., 2012), sodium dodecyl sulfate (SDS) (Wang et al., 2005), silica-bonded S-sulfonic acid (Niknam et al., 2010), and Ce/SiO2 composites (Akondi et al., 2016). Each of these catalysts may have its own benefits but also suffer apparent disadvantages including high reaction times, low efficiency, unwanted reaction conditions, and the use of non-green catalysts. Therefore, to avoid these limitations, the discovery of an effective method for the synthesis of pyrazolones is still favored. The facility of accomplishment multicomponent reactions with a recyclable catalyst could advance the efficiency of organic reactions (Alirezvani et al., 2019; Davoodi et al., 2019). Heteropolyacids (HPAs) have polyoxometalate inorganic cages, which may adopt the Keggin structure with the common formula H3MX12O40, where X is the heteroatom and M is the central atom. Generally, M can be either Si or P, and X = Mo or W (Timofeeva, 2003). Immobilization of HPAs on silica structures as support results in more stability and increased catalytic activity (Molnár et al., 1999; Sofia et al., 2009). HPAs have been heterogenized using immobilization of HPAs on zirconium dioxide (Sunita et al., 2008), titanium dioxide (Waghmare et al., 2008), silica (Izumi et al., 1999; Safaei-Ghomi et al., 2020), zeolite (Mukai et al., 2003), and SBA-15 or MCM-41 (Bordoloi et al., 2007; Wang and Zhu, 2004). In this context, among different solid supports, nanocrystalline ZSM-5 zeolite is most preferred because of its many advantageous properties such as high surface area with different active sites, small pore sizes, short diffusion path, excellent chemical and thermal stability, and good accessibility (Liu et al., 2015; Su et al., 2020; Takmil et al., 2021; Xue et al., 2012). Ideally, utilizing environmental and green catalysts which can be easily recycled at the end of reactions has obtained great attention in recent years (Ghanbari et al., 2016; Gholamian et al., 2013). Heterogeneous catalysts are defined as solids or mixtures of solids that accelerate the chemical reaction without themselves undergoing changes (Dai et al., 2021; Karimi-Maleh et al., 2020; Keyikoglu et al., 2022; Orooji et al., 2021; Taherian et al., 2022). Nanostructures exhibit good catalytic activity due to their large surface area and active sites which are mainly responsible for their catalytic activity (Masoumi et al., 2016; Nabiyouni et al., 2015). In the current study, we investigated an easy way for the synthesis of indenopyrazolones through three-component reactions of phenylhydrazine, benzaldehydes, and indan-1,2,3-trione using HPA-ZSM-5 at room temperature in acetonitrile (Scheme 1).
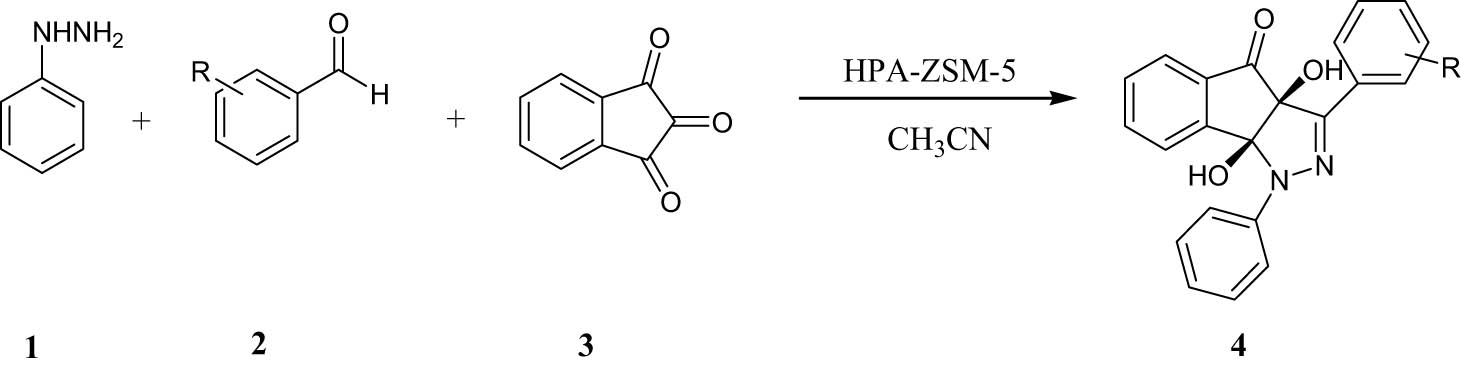
Synthesis of indenopyrazolones.
2 Results and discussion
The prepared catalyst was characterized by spectral techniques including X-ray diffraction (XRD), field emission scanning electronic microscopes (FE-SEM), Fourier transform infrared (FT-IR), energy-dispersive spectroscopy (EDX), thermogravimetric analysis (TGA), and N2-adsorption analysis, and BET analyses.
FT-IR studies on zeolite ZSM-5 and its immobilized catalysts were carried out (Figure 1). The unmodified product has bands at the following wavenumbers (cm−1): 548 (δSi–O–Si), 796 (νsSi–O–Si), 1,096 (νasSi–O–Si), 1,630 (adsorbed H2O), and 3,448 (νOH). The 796 band was shifted in the phosphomolybdic acids-modified sample to 787 cm−1 that is the result of overlapping with bands δ Mo–O–Mo (754 cm−1) (Javidi et al., 2014). The band of silanol groups shifted in the case of PMA-containing sample only. In addition, a new strong band at 962 cm−1 appeared in the spectra of modified ZSM-5. This band is typical for Keggin’s structure of HPAs and corresponds to νasMo–O–Mo or νasW–O–W vibrations (Amini et al., 2006).
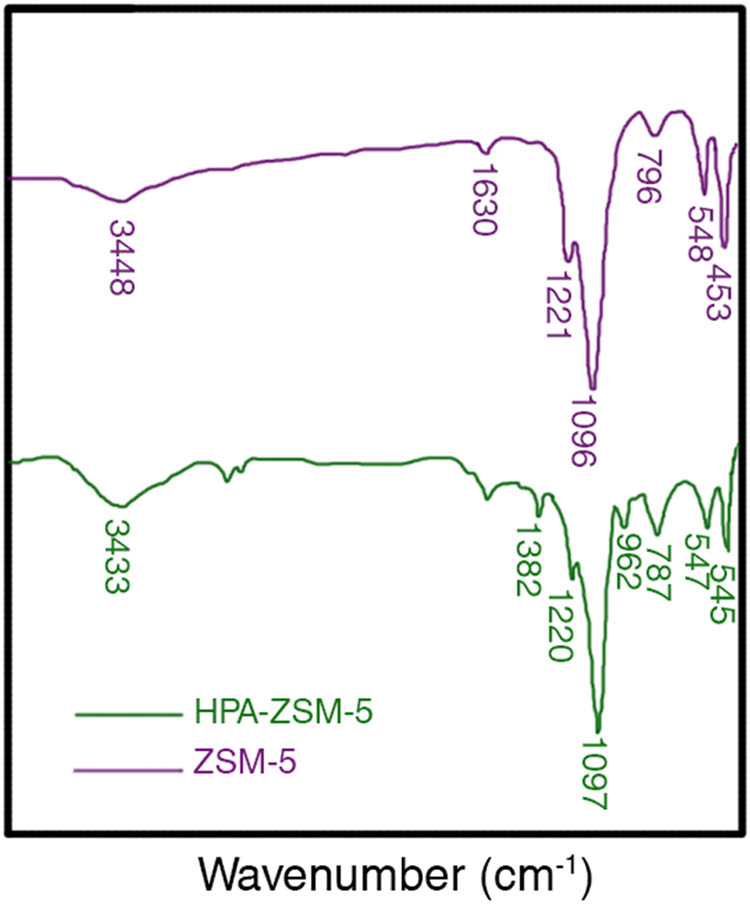
Fourier-transform infrared spectroscopy spectra of ZSM-5 and HPA-ZSM-5.
FE-SEM images of ZSM-5 and its immobilized catalyst are provided in Figure 2. After the immobilization, the surfaces of the catalyst were covered with a white translucent substance, and the surfaces became smoother. The particles became larger in size, and their profiles became clearer, indicating that HPA was immobilized on the surface of ZSM-5. The evaluation of the used catalyst structure by FE-SEM evidence that the morphology of the catalyst remained unchanged after the fifth cycle (Figure 2b and c).
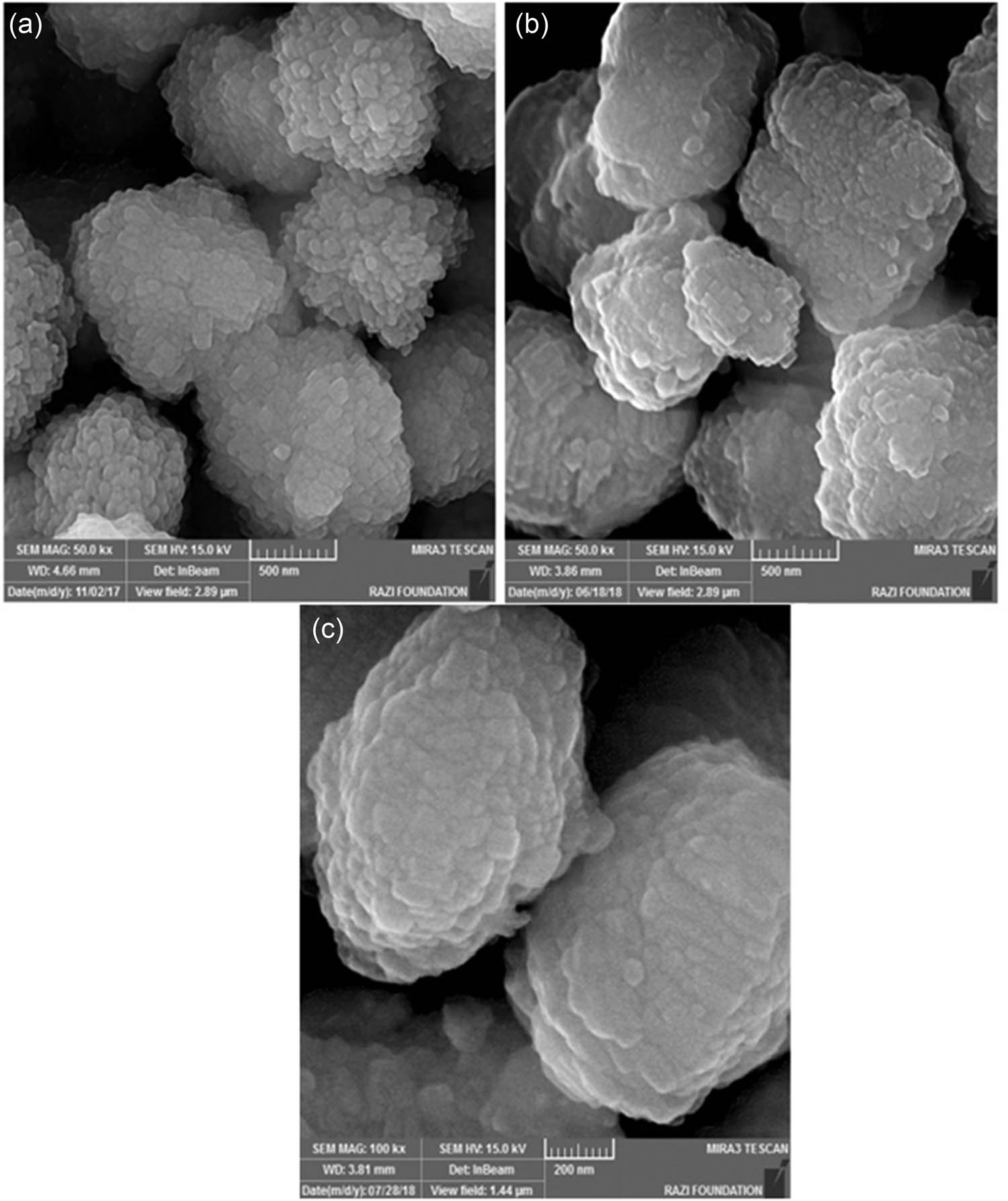
FE-SEM images of: (a) ZSM-5, (b) HPA-ZSM-5, and (c) the used HPA-ZSM-5.
EDX analysis (Figure 3) of the catalyst showed the presence of Al, P, O, Si, and Mo elements confirming the formation of the catalytic system as visualized. Elemental mapping images (Figure 3) of the catalyst showed uniform distribution of the elements P and Mo in the desired catalytic system.
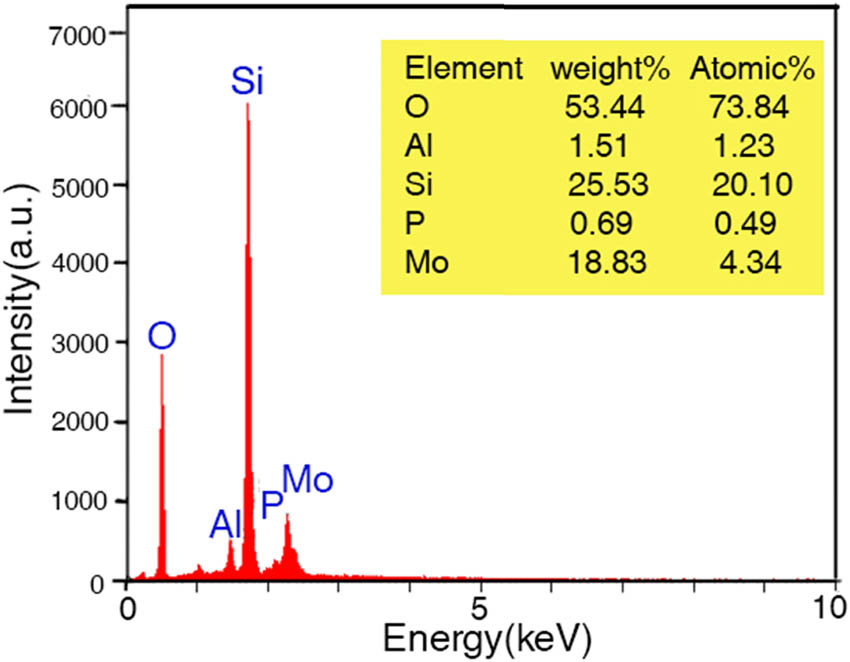
EDS of HPA-ZSM-5.
The XRD patterns of ZSM-5 and its immobilized catalyst are shown in Figure 4. In pattern (a), the peaks of high intensity at 23.4°, 24.1°, and 24.6° are the characteristic diffraction peaks of ZSM-5, indicating good crystallinity of our synthesized ZSM-5 (JCPDS = 44-0003). Compared with the ZSM-5 pattern, the HPA-ZSM-5 pattern exhibits all the diffraction peaks of ZSM-5, and the shape and intensity of the diffraction peaks have negligible changes, indicating that the prepared catalysts maintained the good crystallinity of ZSM-5 after the immobilization of the HPA onto ZSM-5.
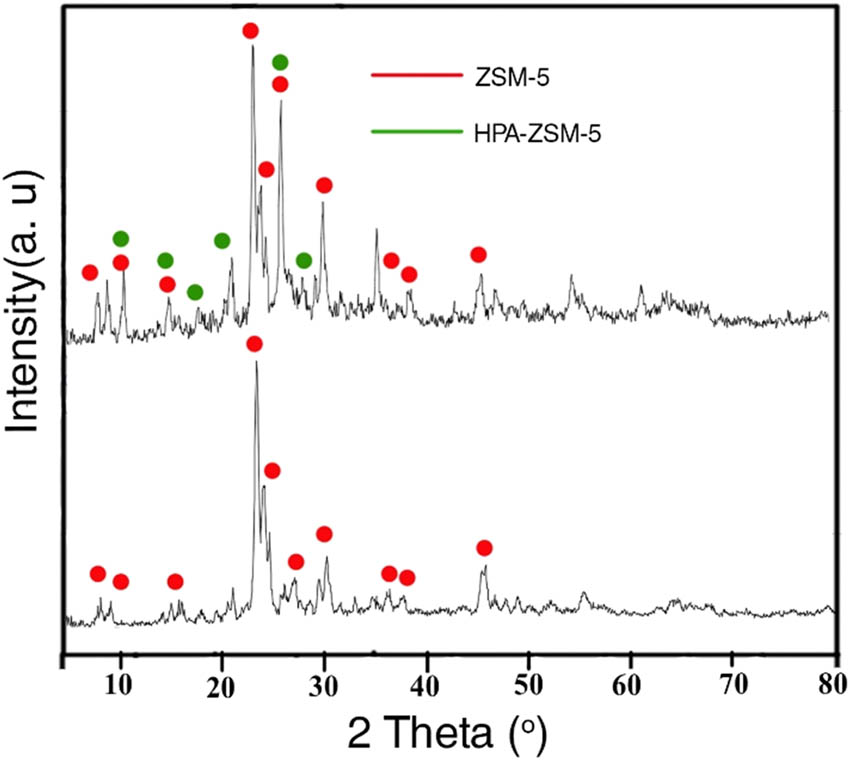
XRD of ZSM-5 and HPA-ZSM-5.
N2-sorption isotherms at 77 K of ZSM-5 and HPA-ZSM-5 are indicated in Figure 5. As shown in Figure 5, all the isotherms exhibited a typical type IV isotherm with an H1 hysteresis loop starting from P/P 0 = 0.5. The results presented that the BET-specific surface area of ZSM-5 increased from 170 to 240 m2‧g−1 after modification with HPA.
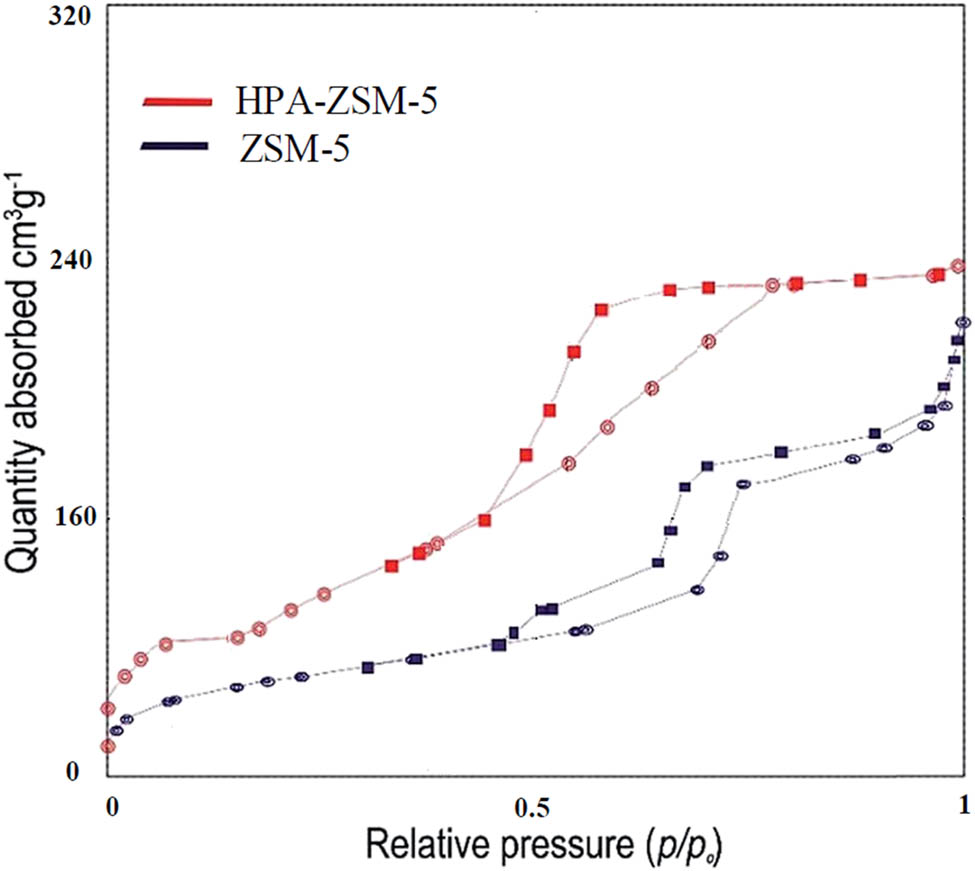
N2 adsorption–desorption isotherms of ZSM-5 and HPA-ZSM-5.
Thermogravimetric analysis (TGA) evaluates the thermal stability of HPA-ZSM-5 (Figure 6). A 3% decrease in weight between 100°C and 250°C is because of losing absorbed solvent on the external surface and molecules trapped among HPA-ZSM-5. The curve indicated a weight loss of about 4.5% from 350°C to 550°C due to the decomposition of the phosphomolybdic acid grafting to ZSM-5.
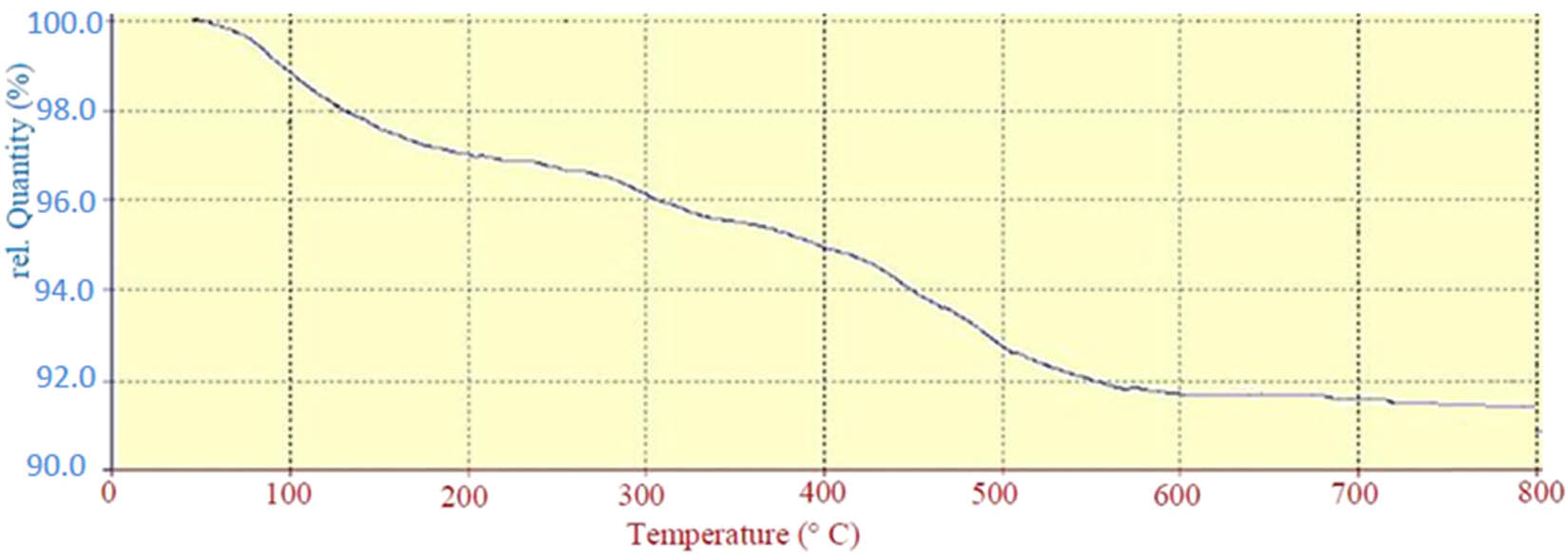
TGA curve of HPA-ZSM-5.
We investigated the reaction of phenylhydrazine, benzaldehyde, and indan-1,2,3-trione as a model reaction. To obtain the ideal reaction conditions for the preparation of compound 4a, we studied the diverse catalysts and solvents (Table 1). Screening of different catalysts containing Et3N, PTSA, nano-ZrO2, CAN, l-proline, nano-TiO2, HPA, ZSM-5, and HPA-ZSM-5 revealed HPA-ZSM-5 (6 mg) as the most effective catalyst to perform this reaction at room temperature (Table 1). Seeking of the reaction scope demonstrated that various aromatic aldehydes can be utilized in this method (Table 2). These results showed that aromatic aldehydes with electron-withdrawing groups reacted faster than aldehydes with electron-releasing groups as expected.
Optimization of reaction conditions using different catalysts under different conditionsa
| Entry | Catalyst (amount) | Solvent | Time (min) | Yield (%)b |
|---|---|---|---|---|
| 1 | — | CH3CN | 300 | 17 |
| 2 | Et3N (5 mol%) | CH3CN | 200 | 22 |
| 3 | l-Proline (5 mol%) | CH3CN | 150 | 25 |
| 4 | CAN (4 mol%) | CH3CN | 150 | 31 |
| 5 | p-TSA (4 mol%) | CH3CN | 150 | 40 |
| 6 | Nano-ZrO2(6 mg) | CH3CN | 140 | 48 |
| 7 | Nano-TiO2 (7 mg) | CH3CN | 140 | 42 |
| 8 | HPA (4 mol%) | CH3CN | 80 | 60 |
| 9 | ZSM-5 (8 mg) | CH3CN | 100 | 56 |
| 10 | HPA-ZSM-5 (6 mg) | H2O | 60 | 60 |
| 11 | HPA-ZSM-5 (6 mg) | DMF | 50 | 73 |
| 12 | HPA-ZSM-5 (6 mg) | EtOH | 30 | 79 |
| 13 | HPA-ZSM-5 (4 mg) | CH3CN | 30 | 84 |
| 14 | HPA-ZSM-5 (6 mg) | CH3CN | 30 | 92 |
| 15 | HPA-ZSM-5 (8 mg) | CH3CN | 30 | 92 |
aPhenylhydrazine (1 mmol), benzaldehyde (1 mmol), and indan-1,2,3-trione (1 mmol).
bIsolated yield.
Synthesis of indenopyrazolones
| Entry | R | Product | Time (min) | Yield (%)a | m.p. (°C) |
|---|---|---|---|---|---|
| 1 | H | 4a | 30 | 92 | 221–223 |
| 2 | 4-OMe | 4b | 50 | 84 | 211–214 |
| 3 | 4-Me | 4c | 50 | 86 | 249–251 |
| 4 | 4-Cl | 4d | 25 | 94 | 234–236 |
| 5 | 4-Br | 4e | 25 | 94 | 222–224 |
| 6 | 4-NO2 | 4f | 25 | 95 | 242–244 |
| 7 | 3-NO2 | 4g | 30 | 92 | 240–243 |
| 8 | 4-OH | 4h | 60 | 82 | 202–204 |
aIsolated yield.
The possibility of recycling the catalyst is an important process from different aspects such as environmental concerns and applicable commercial processes. The reusability of HPA-ZSM-5 was tested for the synthesis of 4a, and it was found that product yields reduced to a small extent on each reuse (run 1, 92%, run 2, 92%, run 3, 92%, run 4, 91%, run 5, 91%, run 6, 90%). After completion of the reaction, HPA-ZSM-5 was separated from the mixture using filtration. HPA-ZSM-5 was rinsed five times with ethanol and dried at room temperature for 15 h.
A mechanism for the preparation of indenopyrazolones using HPA-ZSM-5 is proposed (Scheme 2). First, the activated benzaldehyde by HPA-ZSM-5 is condensed with phenylhydrazine to give intermediate I, which attacks indan-1,2,3-trione to afford the zwitterionic intermediate II. Its tautomer III undergoes an intramolecular nucleophilic addition reaction, which affords product by H-atom-transfer reaction. A highly regiospecific synthesis and crystal structure of indenopyrazolone was reported by Yavari et al. (2012). The cis configuration of the hydroxy groups was proven by nuclear magnetic resonance (NMR) (both –OH groups are involved in intramolecular H-bond and X-ray crystal) (Lobo et al., 2011; Pilipecz et al., 2007).
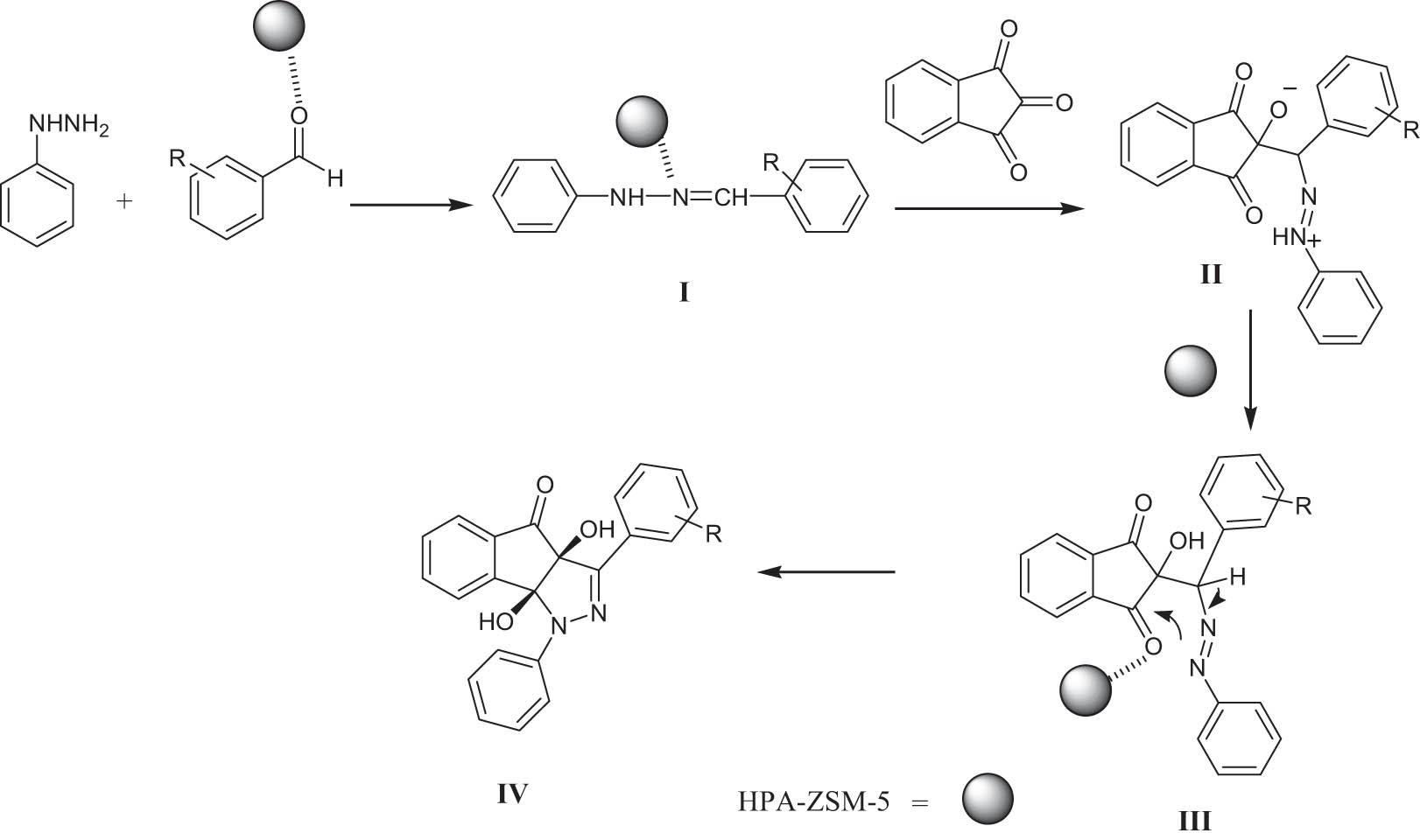
Possible mechanism for the preparation of indenopyrazolones using HPA-ZSM-5.
3 Conclusion
In conclusion, we demonstrated an effective method for the preparation of indenopyrazolones using HPA-ZSM-5 (6 mg) through a three-component reaction of phenylhydrazine, benzaldehydes and indan-1,2,3-trione at room temperature in acetonitrile. The zeolite catalyst has been characterized by XRD, FE-SEM, FT-IR, EDS, and N2-adsorption analysis. Seeking of the reaction scope demonstrated that various aromatic aldehydes can be utilized in this method. These results showed that aromatic aldehydes with electron-withdrawing groups reacted faster than aldehydes with electron-releasing groups as expected. The advantages of this method include its great yields in concise times, the retrievable of the catalyst, low catalyst loading, and an easy work-up method.
Experimental
Chemicals and apparatus
NMR spectra were recorded on Bruker Avance-400 MHz spectrometers in the presence of tetramethylsilane as the internal standard. The infra red spectra were recorded on the FT-IR Magna 550 apparatus using KBr discs. Melting points were determined on Electrothermal 9200 and were not corrected. The elemental analyses (C, H, N) were obtained using a Carlo ERBA Model EA 1108 analyzer. The XRD patterns were recorded on an X-ray diffractometer (PHILIPS, PW 1510, Netherlands) using Cu-Kα radiation (λ = 0.154056 nm) in the range 2θ = 0.8–10°. FE-SEM of nanocatalyst was visualized by SEM (MIRA3). EDS measurement was carried out with the SAMX analyser. The N2 adsorption/desorption analysis (BET) was performed using an automated gas adsorption analyser (BEL SORP mini II).
Preparation of ZSM-5
The zeolite precursor was prepared by adding tetrapropylammonium hydroxide, tetraethyl orthosilicate to a mixed aqueous solution of aluminium isopropoxide [Al(ίPro)3], and NaOH with stirring. The mixture was converted to gel. The gel was stirred for 20 h. The mole composition of the gel was 1Al2O3:46SiO2:4TPA:5Na2O:2,500H2O. The resulting gel was sealed in Teflon-lined autoclaves and heated at 165°C for 72 h. The solid product was recovered by filtration, washed with deionized water several times, and dried in an oven at 100°C overnight. The as-synthesized material was then calcined at 550°C for 8 h to remove the templates.
Preparation of HPA-ZSM-5
ZSM-5 zeolites (1 g) were added to the solution of 0.3 g of phosphomolybdic acid (HPA) in ethanol (25 mL), and the reaction mixture was stirred for 24 h. The mixture was filtered, washed with deionized water several times, and dried in an oven at 100°C overnight. The as-synthesized material was subjected to product HPA-ZSM-5 at 400°C for 2 h.
General procedure for the synthesis of indenopyrazolones
A mixture of phenylhydrazine (1.0 mmol), benzaldehydes (1.0 mmol), ninhydrin (1.0 mmol) and 6 mg of HPA-ZSM-5 in acetonitrile (10 mL) was stirred for the appropriate times. After completion of the reaction thin layer chromatography , the catalyst was separated from the mixture using filtration. The solvent was evaporated, and the residue was washed with cold diethyl ether to get a pure product.
Spectra data
cis-3a,8b-Dihydro-3a,8b-dihydroxy-1,3-diphenylindeno[1,2-c]pyrazol-4(1H)-one (4a)
Yellow solid, m.p. 221–223°C, IR (KBr): ν max = 3,433, 3,055, 1,736, 1,458 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.14 (s, OH), 6.19 (s, OH), 7.15 (d, J = 8.0 Hz, 1H, ArH), 7.19–7.31 (m, 3H, ArH), 7.42 (t, J = 7.6 Hz, 1H, ArH), 7.49 (t, J = 7.6 Hz, 1H, ArH), 7.53–7.67 (m, 5H, ArH), 8.23 (d, J = 7.6 Hz, 2H, ArH), 8.49 (d, J = 7.6 Hz, 1H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 89.3 (C), 96.8 (C), 118.3 (2 CH), 121.5 (CH), 122.7 (CH), 123.3 (CH), 124.1 (CH), 126.3 (2 CH), 129.6 (2 CH), 130.8 (CH), 130.9 (C), 132.9 (2 CH), 133.6 (CH), 137.3 (C), 139.9 (C), 142.4 (C), 147.8 (C), 196.8 (C═O). Anal. calcd for C22H16N2O3: C, 74.15, H, 4.53, N, 7.86%, Found C, 74.12, H, 4.58, N, 7.93%.
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-methoxyphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4b)
Yellow solid, m.p. 211–214°C, IR (KBr): ν max = 3,423, 3,284, 1,705, 1,593 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.66 (s, OCH3), 6.14 (s, OH), 6.24 (s, OH), 6.89 (d, J = 8.2 Hz, 2H, ArH), 7.06 (t, J = 7.2 Hz, 1H, ArH), 7.19 (t, J = 7.4 Hz, 2H, ArH), 7.28 (t, J = 7.2 Hz, 1H, ArH), 7.39 (t, J = 7.4 Hz, 1H, ArH), 7.51 (d, J = 7.8 Hz, 1H, ArH), 7.56 (d, J = 7.4 Hz, 1H, ArH), 7.62 (d, J = 7.8 Hz, 2H, ArH), 8.03 (d, J = 8.4 Hz, 2H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 55.5 (OCH3), 90.2 (C), 96.5 (C), 113.6 (2 CH), 117.8 (2 CH), 122.4 (CH), 123.8 (CH), 124.4 (C), 125.9 (CH), 128.6 (2 CH), 129.4 (2 CH), 130.4 (CH), 135.4 (C), 136.8 (CH), 142.9 (C), 143.4 (C), 147.7 (C), 160.5 (C), 197.6 (C═O). Anal. calcd for C23H18N2O4: C, 71.49, H, 4.70, N, 7.25%, Found C, 71.39, H, 4.63, N, 7.19%.
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-methylphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4c)
Yellow solid, m.p. 249–251°C, IR (KBr): ν max = 3,435, 3,266, 1,719, 1,588 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 2.34 (s, CH3), 6.13 (s, OH), 6.16 (s, OH), 7.08 (t, J = 7.8 Hz, 1H, ArH), 7.21 (d, J = 7.9 Hz, 2H, ArH), 7.35 (t, J = 7.8 Hz, 2H, ArH), 7.52 (t, J = 7.8 Hz, 1H, ArH), 7.56 (t, J = 8.0 Hz, 1H, ArH), 7.72 (d, J = 7.9 Hz, 1H, ArH), 7.93 (d, J = 7.8 Hz, 2H, ArH), 8.08 (d, J = 7.7 Hz, 2H, ArH), 8.48 (d, J = 7.8 Hz, 1H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 21.3 (CH3), 89.5 (C), 95.6 (C), 126.1 (CH), 126.3 (2 CH), 126.7 (2 CH), 129.2 (CH), 129.5 (CH), 129.7 (2 CH), 129.8 (CH), 129.9 (2 CH), 130.8 (C), 134.6 (CH), 136.9 (C), 138.4 (C), 140.4 (C), 142.2 (C), 146.8 (C), 197.4 (C═O). Anal. calcd for C23H18N2O3: C, 74.58, H, 4.90, N, 7.56%, Found C, 74.48, H, 4.83, N, 7.53%.
cis-3-(4-Chlorophenyl)-3a,8b-dihydro-3a,8b-dihydroxy-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4d)
Yellow solid, m.p. 234–236°C, IR (KBr): ν max = 3,452, 3,260, 1,699, 1,593 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.08 (s, OH), 6.12 (s, OH), 7.03 (t, J = 7.8 Hz, 1H, ArH), 7.33 (t, J = 7.8 Hz, 2H, ArH), 7.41–7.51 (m, 3H, ArH), 7.53 (t, J = 7.8 Hz, 1H, ArH), 7.63 (d, J = 7.3 Hz, 1H, ArH), 7.74 (d, J = 7.0 Hz, 1H, ArH), 7.86 (d, J = 7.1 Hz, 2H, ArH), 8.15 (d, J = 7.7 Hz, 2H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 89.7 (C), 96.7 (C), 118.2 (2 CH), 122.6 (CH), 124.5 (CH), 126.3 (CH), 128.6 (2 CH), 128.9 (2 CH), 129.4 (2 CH), 130.3 (C), 130.9 (CH), 134.9 (C), 135.6 (C), 136.8 (CH), 142.7 (C), 142.9 (C), 147.6 (C), 197.3 (C═O). Anal. calcd for C22H15ClN2O3: C, 67.61, H, 3.87, N, 7.17%, Found C, 67.54, H, 3.84, N, 7.13%.
cis-3-(4-Bromophenyl)-3a,8b-dihydro-3a,8b-dihydroxy-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4e)
Yellow solid, m.p. 222–224°C, IR (KBr): ν max = 3,440, 3,255, 1,694, 1,585 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.03 (s, OH), 6.15 (s, OH), 7.07 (t, J = 7.8 Hz, 1H, ArH), 7.39 (t, J = 7.3 Hz, 2H, ArH), 7.51 (t, J = 7.9 Hz, 1H, ArH), 7.62 (d, J = 7.9 Hz, 2H, ArH), 7.72 (t, J = 7.1 Hz, 1H, ArH), 7.82 (d, J = 7.6 Hz, 1H, ArH), 7.91–7.99 (m, 3H, ArH), 8.06 (d, J = 7.9 Hz, 2H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 89.6 (C), 96.4 (C), 118.4 (2 CH), 122.9 (CH), 124.4 (CH), 126.3 (CH), 128.7 (2 CH), 129.3 (2 CH), 130.5 (C), 130.6 (CH), 131.7 (2 CH), 135.5 (C), 136.8 (CH), 137.2 (C), 142.3 (C),142.7 (C), 147.6 (C), 196.4 (C═O). Anal. calcd for C22H15BrN2O3: C, 60.71, H, 3.47, N, 6.44%, Found C, 60.64, H, 3.42, N, 6.33%.
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-nitrophenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4f)
Yellow solid, m.p. 242–244°C, IR (KBr): ν max = 3,401, 3,042, 1,718, 1,562, 1,353 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.09 (s, OH), 6.17 (s, OH), 7.15–7.26 (m, 5H, ArH), 7.29–7.61 (m, 4H, ArH), 8.26 (d, J = 7.1 Hz, 2H, ArH), 8.38 (d, J = 7.2 Hz, 2H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 89.6 (C), 97.8 (C), 118.4 (2 CH), 122.6 (2 CH), 123.3 (CH), 123.4 (CH), 125.5 (CH), 126.8 (2 CH), 128.9 (2 CH), 130.5 (CH), 134.6 (C), 136.5 (CH), 137.9 (C), 140.7 (C), 142.3 (C), 146.9 (C), 147.5 (C), 197.3 (C═O). Anal. calcd for C22H15N3O5: C, 65.83, H, 3.77, N, 10.47%, Found C, 65.74, H, 3.72, N, 10.43%.
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(3-nitrophenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4g)
Yellow solid, m.p. 240–242°C, IR (KBr): ν max = 3,463, 1,725, 1,591, 1,504 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 6.26 (s, OH), 6.37 (s, OH), 7.51–7.81 (m, 10H, ArH), 8.08 (d, J = 8.1 Hz, 1H, ArH), 8.52 (d, J = 7.8 Hz, 1H, ArH), 8.62 (s, 1H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 89.4 (C), 96.8 (C), 118.3 (2 CH), 121.6 (CH), 122.8 (CH), 123.3 (CH), 124.1 (CH), 126.3 (CH), 129.6 (2 CH), 129.7 (CH), 130.8 (CH), 132.9 (CH), 133.4 (C), 135.3 (C), 137.2 (CH), 140.9 (C), 142.8 (C), 147.7 (C), 147.4 (C), 196.8 (C═O). Anal. calcd for C22H15N3O5: C, 65.83, H, 3.77, N, 10.47%, Found C, 65.73, H, 3.69, N, 10.36%.
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-hydroxyphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4h)
Yellow solid, m.p. 202–204°C, IR (KBr): ν max = 3,421, 1,756, 1,535, 1,457 cm−1. 1H NMR (400 MHz, CDCl3): δ (ppm) = 4.59 (s, OH), 6.14 (s, OH), 6.18 (s, OH), 6.96 (t, J = 7.4 Hz, 2H, ArH), 7.16–7.23 (m, 2H, ArH), 7.27 (m, 1H, ArH), 7.41–7.51 (m, 2H, ArH), 7.56–7.62 (m, 5H, ArH), 7.94 (d, J = 7.8 Hz, 1H, ArH). 13C NMR (100 MHz, CDCl3): δ (ppm) = 89.3 (C), 94.1 (C), 116.4 (CH), 118.5 (2 CH), 119.3 (CH), 123.9 (CH), 124.5 (CH), 125.6 (CH), 127.8 (CH), 129.4 (2 CH), 134.6 (C), 134.8 (C), 137.3 (CH), 141.2 (C), 144.9 (C), 146.3 (C), 157.5 (C), 197.3 (C═O). Anal. calcd for C22H16N2O4: C, 70.96, H, 4.33, N, 7.52%, Found C, 70.83, H, 4.25, N, 7.43%.
Acknowledgments
The authors acknowledge a reviewer who provided helpful insights. The authors are grateful to University of Kashan for supporting this work by Grant no: 159196/XXI.
-
Funding information: The researchers are thankful to the University of Kashan for supporting this work through Grant No. 363010/III.
-
Author contributions: Seyyed Mohammad Ebrahimi: experimental data collection, writing; Javad Safaei-Ghomi: project resources and supervision; Mohammaed Abdulridha Mutashar: project administration, writing.
-
Conflict of interest: Authors state no conflict of interest.
Appendix
![Figure A1
cis-3a,8b-Dihydro-3a,8b-dihydroxy-1,3-diphenylindeno[1,2-c]pyrazol-4(1H)-one (4a).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_007.jpg)
cis-3a,8b-Dihydro-3a,8b-dihydroxy-1,3-diphenylindeno[1,2-c]pyrazol-4(1H)-one (4a).
![Figure A2
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-methoxyphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4b).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_008.jpg)
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-methoxyphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4b).
![Figure A3
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-methylphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4c).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_009.jpg)
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-methylphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4c).
![Figure A4
cis-3-(4-Chlorophenyl)-3a,8b-dihydro-3a,8b-dihydroxy-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4d).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_010.jpg)
cis-3-(4-Chlorophenyl)-3a,8b-dihydro-3a,8b-dihydroxy-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4d).
![Figure A5
cis-3-(4-Bromophenyl)-3a,8b-dihydro-3a,8b-dihydroxy-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4e).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_011.jpg)
cis-3-(4-Bromophenyl)-3a,8b-dihydro-3a,8b-dihydroxy-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4e).
![Figure A6
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-nitrophenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4f).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_012.jpg)
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-nitrophenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4f).
![Figure A7
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(3-nitrophenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4g).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_013.jpg)
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(3-nitrophenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4g).
![Figure A8
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-hydroxyphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4h).](/document/doi/10.1515/mgmc-2022-0003/asset/graphic/j_mgmc-2022-0003_fig_014.jpg)
cis-3a,8b-Dihydro-3a,8b-dihydroxy-3-(4-hydroxyphenyl)-1-phenylindeno[1,2-c]pyrazol-4(1H)-one (4h).
References
Ahsan M.J., Khalilullah H., Stables J.P., Govindasamy J., Synthesis and anticonvulsant activity of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues. J. Enzyme Inhib. Med Chem., 2013, 28, 644–650.10.3109/14756366.2012.663364Suche in Google Scholar PubMed
Akondi A.M., Kantam M.L., Trivedi R., Bharatam J., Vemulapalli S.P.B., Bhargava S.K., et al., Ce/SiO2 composite as an efficient catalyst for the multicomponent one-pot synthesis of substituted pyrazolones in aqueous media and their antimicrobial activities. J. Mol. Catal. A. Chem., 2016, 411, 325–336.10.1016/j.molcata.2015.11.004Suche in Google Scholar
Alirezvani, Z., Dekamin M.G., Valiey E., Cu(II) and magnetite nanoparticles decorated melamine-functionalized chitosan: A synergistic multifunctional catalyst for sustainable cascade oxidation of benzyl alcohols/Knoevenagel condensation. Sci. Rep. 2019, 9, 17758–17769.10.1038/s41598-019-53765-3Suche in Google Scholar PubMed PubMed Central
Amini M.M., Shaabani A., Bazgir A., Tangstophosphoric acid (H3PW12O40): An efficient and eco-friendly catalyst for the one-pot synthesis of dihydropyrimidin-2 (1H)-ones. Catal. Commun., 2006, 7, 843–847.10.1016/j.catcom.2006.02.027Suche in Google Scholar
Bordoloi A., Lefebvre F., Halligudi S.B., Selective oxidation of anthracene using inorganic–organic hybrid materials based on molybdovanadophosphoric acids. J. Catal. 2007, 247, 166–175.10.1016/j.jcat.2007.01.020Suche in Google Scholar
Davoodi F., Dekamin M.G., Alirezvani Z., A practical and highly efficient synthesis of densely functionalized nicotinonitrile derivatives catalyzed by zinc oxide‐decorated superparamagnetic silica attached to graphene oxide nanocomposite. Appl. Organometal. Chem. 2019, 33, 4735-4747.10.1002/aoc.4735Suche in Google Scholar
Dai F., Luo J., Zhou S., Qin X., Liu D., Qi H., Porous hafnium-containing acid/base bifunctional catalysts for efficient upgrading of bio-derived aldehydes. J. Bioresour. Bioprod. 2021, 6, 243–253.10.1016/j.jobab.2021.04.006Suche in Google Scholar
Ghanbari D., Sharifi S., Naraghi A., Nabiyouni G., Photo-degradation of azo-dyes by applicable magnetic zeolite Y–Silver–CoFe2O4 nanocomposites. J. Mater. Sci.: Mater. Electron., 2016, 27, 5315–5323.10.1007/s10854-016-4430-8Suche in Google Scholar
Gholamian F., Salavati-Niasari M., Ghanbari D., Sabet M., The effect of flower-like magnesium hydroxide nanostructure on the thermal stability of cellulose acetate and acrylonitrile–butadiene–styrene. J. Clust. Sci., 2013, 24, 73–84.10.1007/s10876-012-0518-3Suche in Google Scholar
Indrasena A., Riyaz S., Mallipeddi P.L., Padmaja P., Sridhar B., Dubey P.K., Design, synthesis, and biological evaluation of indolylidinepyrazolones as potential anti-bacterial agents. Tetrahedron Lett., 2014, 55, 5014–5018.10.1016/j.tetlet.2014.05.131Suche in Google Scholar
Izumi Y., Hisano K., Hida T., Acid catalysis of silica-included heteropolyacid in polar reaction media. Appl. Catal. A: Gen., 1999, 181, 277–282.10.1016/S0926-860X(98)00399-8Suche in Google Scholar
Javidi J., Esmaeilpour M., Rahiminezhad Z., Dodeji F.N., Synthesis and characterization of H3PW12O40 and H3PMo12O40 nanoparticles by a simple method. J. Clust. Sci., 2014, 25, 1511–1524.10.1007/s10876-014-0745-xSuche in Google Scholar
Karimi-Maleh H., Karimi F., Orooji Y., Mansouri G., Razmjou A., Aygun A., et al., A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep., 2020, 10, 11699–11712.10.1038/s41598-020-68663-2Suche in Google Scholar
Kaur N., Kumar A., Singh K., Synthesis of Novel Indenopyrimidine Sulfonamides from Indenopyrimidine-2-Amines via S–N Bond Formation. Polycycl. Aromat. Compd., 2020. 10.1080/10406638.2020.1809470.Suche in Google Scholar
Keyikoglu R., Khataee A., Lin H., Orooji Y., Vanadium (V)-doped ZnFe layered double hydroxide for enhanced sonocatalytic degradation of pymetrozine. Biochem. Eng. J., 2022, 434, 134730–134739.10.1016/j.cej.2022.134730Suche in Google Scholar
Khalil N.A., Ahmed E.M., Mohamed K.O., Nissan Y.M., Zaitone S.A.B., Synthesis and biological evaluation of new pyrazolone–pyridazine conjugates as anti-inflammatory and analgesic agents. Bioorg. Med. Chem., 2014, 22, 2080–2089.10.1016/j.bmc.2014.02.042Suche in Google Scholar
Liu P., Zhang Z., Jia M., Gao X., Yu J., ZSM-5 zeolites with different SiO2/Al2O3 ratios as fluid catalytic cracking catalyst additives for residue cracking. Chin. J. Catal., 2015, 36, 806–812.10.1016/S1872-2067(14)60311-9Suche in Google Scholar
Lobo G., Zuleta E., Charris K., Capparelli M.V., Briceno A., Angel J., et al., Synthesis and crystal structure of (4bRS,9bRS)-5-(2,4-dimethoxyphenyl)-4b,9b-7,7-dimethyldihydroxy-4b,5,6,7,8,9b-hexahydroindeno[1,2-b]indole-9,10-dione. J. Chem. Res., 2011, 35, 222–224.10.3184/174751911X13015834294266Suche in Google Scholar
Masoumi S., Nabiyouni G., Ghanbari D., Photo-degradation of Congored, acid brown and acid violet: photo catalyst and magnetic investigation of CuFe2O4–TiO2–Ag nanocomposites. J. Mater. Sci.: Mater. Electron., 2016, 27, 11017–11033.10.1007/s10854-016-5218-6Suche in Google Scholar
Mazimba O., Wale K., Loeto D., Kwape T., Antioxidant and antimicrobial studies on fused-ring pyrazolones and isoxazolones. Bioorg. Med. Chem., 2014, 22, 6564–6569.10.1016/j.bmc.2014.10.015Suche in Google Scholar PubMed
Molnár A., Keresszegi C., Török B., Heteropoly acids immobilized into a silica matrix: characterization and catalytic applications. Appl. Catal. A: Gen., 1999, 189, 217–224.10.1016/S0926-860X(99)00278-1Suche in Google Scholar
Mor S., Mohil R., Nagoria S., Kumar A., Lal K., Kumar D., et al., Regioselective Synthesis, Antimicrobial Evaluation and QSAR Studies of Some 3-Aryl-1-heteroarylindeno[1,2-c]pyrazol-4(1H)-ones. J. Heterocycl. Chem., 2017, 54, 1327–1341.10.1002/jhet.2710Suche in Google Scholar
Mor S., Sindhu S., Synthesis, type II diabetes inhibitory activity, antimicrobial evaluation and docking studies of indeno[1,2-c]pyrazol-4(1H)-ones. Med. Chem. Res., 2020, 29, 46–62.10.1007/s00044-019-02457-8Suche in Google Scholar
Mor S., Sindhu S., Nagoria S., Khatri M., Garg P., Sandhu H., et al., Synthesis, biological evaluation, and molecular docking studies of some N‐thiazolyl hydrazones and indenopyrazolones. J. Heterocycl. Chem., 2019, 56, 1622–1633.10.1002/jhet.3548Suche in Google Scholar
Mukai S.R., Shimoda M., Lin L., Tamon H., Masuda T., Improvement of the preparation method of “ship-in-the-bottle” type 12-molybdophosphoric acid encaged Y-type zeolite catalysts. Appl. Catal. A: Gen., 2003, 256, 107–113.10.1016/S0926-860X(03)00392-2Suche in Google Scholar
Nabiyouni G., Ghanbari D., Ghasemi J., Yousofnejad A., Microwave-assisted synthesis of MgFe2O4-ZnO nanocomposite and its photo-catalyst investigation in methyl orange degradation. J. Nanostruct., 2015, 5, 289–295.Suche in Google Scholar
Niknam K., Saberi D., Sadegheyan M., Deris A., Silica-bonded S-sulfonic acid: an efficient and recyclable solid acid catalyst for the synthesis of 4, 4′-(arylmethylene) bis (1H-pyrazol-5-ols). Tetrahedron Lett., 2010, 51, 692–694.10.1016/j.tetlet.2009.11.114Suche in Google Scholar
Orooji Y., Tanhaei B., Ayati A., Tabrizi S.H., Alizadeh M., Bamoharram F.F., et al., Heterogeneous UV-Switchable Au nanoparticles decorated tungstophosphoric acid/TiO2 for efficient photocatalytic degradation process. Chemosphere., 2021, 281, 130795–130804.10.1016/j.chemosphere.2021.130795Suche in Google Scholar PubMed
Pilipecz M.V., Mucsi Z., Nemes P., Scheiber P., Chemistry of nitroenamines. synthesis of pyrrolizine derivatives. Heterocycles 2007, 71, 1919–1928.10.3987/COM-07-11073Suche in Google Scholar
Rostom S.A.F., Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]-pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea)pharmacophores, and some derived thiazole ring systems. Bioorg. Med. Chem., 2006, 14, 6475–6485.10.1016/j.bmc.2006.06.020Suche in Google Scholar PubMed
Safaei-Ghomi J., Abbas A.K., Shahpiri M., Synthesis of imidazoles promoted by H3PW12O40-amino-functionalized CdFe12O19@SiO2 nanocomposite. Nanocomposites, 2020, 6, 149–157.10.1080/20550324.2020.1858246Suche in Google Scholar
Saidachary G., Veera Prasad K., Divya D., Singh A., Ramesh U., Sridhar B., et al., Convenient one-pot synthesis, anti-mycobacterial and anticancer activities of novel benzoxepinoisoxazolones and pyrazolones. Eur. J. Med. Chem., 2014, 76, 460–469.10.1016/j.ejmech.2014.02.042Suche in Google Scholar PubMed
Sivakumar K.K., Rajasekaran A., Senthilkumar P., Wattamwar P.P., Conventional and microwave assisted synthesis of pyrazolone Mannich bases possessing anti-inflammatory, analgesic, ulcerogenic effect and antimicrobial properties. Bioorg. Med. Chem. Lett., 2014, 24, 2940–2944.10.1016/j.bmcl.2014.04.067Suche in Google Scholar PubMed
Singh K., Sharma P.K., Dhawan S.N., Singh S.P., Synthesis and characterisation of some novel indeno[1,2-c]pyrazoles. J. Chem. Research., 2005, 2005, 526–529.10.3184/030823405774663345Suche in Google Scholar
Singh K., Applications of indan-1,3-dione in heterocyclic synthesis. Curr. Org. Synth., 2016, 13, 385–407.10.2174/1570179412666150817222851Suche in Google Scholar
Sobhani S., Hasaninejad A.R., Maleki M.F., Parizi Z.P., Tandem Knoevenagel–Michael reaction of 1-Phenyl-3-methyl-5-pyrazolone with Aldehydes using 3-aminopropylated silica gel as an efficient and reusable heterogeneous catalyst. Synth. Commun., 2012, 42, 2245–2255.10.1080/00397911.2011.555589Suche in Google Scholar
Sofia L.T.A., Krishnan A., Sankar M., Raj N.K.K., Manikandan P., Rajamohanan P.R., et al., Immobilization of phosphotungstic acid (pta) on imidazole functionalized silica: evidence for the nature of pta binding by solid state nmr and reaction studies. J. Phys. Chem. C., 2009, 113, 21114–21122.10.1021/jp906108eSuche in Google Scholar
Su M., Li W., Ma Q., Zhu B., Production of jet fuel intermediates from biomass platform compounds via aldol condensation reaction over iron-modified MCM-41 lewis acid zeolite. J. Bioresour.Bioprod., 2020, 5, 256–265.10.1016/j.jobab.2020.10.004Suche in Google Scholar
Sunita G., Devassy B.M., Vinu A., Sawant D.P., Balasubramanian V.V., Halligudi S.B., Synthesis of biodiesel over zirconia-supported isopoly and heteropoly tungstate catalysts. Catal. Commun., 2008, 9, 696–702.10.1016/j.catcom.2007.08.007Suche in Google Scholar
Taherian Z., Gharahshiran V.S., Khataee A., Orooji Y., Synergistic effect of freeze-drying and promoters on the catalytic performance of Ni/MgAl layered double hydroxide. Fuel., 2022, 311, 122620–122629.10.1016/j.fuel.2021.122620Suche in Google Scholar
Takmil N.F., Jaleh B., Mohazzab B.F., Khazalpour S., Rostami-Vartooni A., Nguyen T.H.C., et al., Hydrogen production by Electrochemical reaction using waste zeolite boosted with Titania and Au nanoparticles. Inorg. Chem. Commun., 2021, 133, 108891–108896.10.1016/j.inoche.2021.108891Suche in Google Scholar
Timofeeva M.N., Acid catalysis by heteropoly acids. Appl. Catal. A: Gen. 2003, 256, 19–35.10.1016/S0926-860X(03)00386-7Suche in Google Scholar
Waghmare N.G., Kasinathan P., Amrute A., Lucas N., Halligudi S.B., Titania supported silicotungstic acid: An efficient solid acid catalyst for veratrole acylation. Catal. Commun. 2008, 9, 2026–2029.10.1016/j.catcom.2008.03.043Suche in Google Scholar
Wang W., Wang S.X., Qin X.Y., Li J.T., Reaction of aldehydes and pyrazolones in the presence of sodium dodecyl sulfate in aqueous media. Synth. Commun., 2005, 35, 1263–1269.10.1081/SCC-200054854Suche in Google Scholar
Wang J., Zhu H., Alkylation of 1-dodecene with benzene over H3PW12O40 supported on mesoporous silica SBA-15. Catal. Lett., 2004, 93, 209–212.10.1023/B:CATL.0000017078.26948.b4Suche in Google Scholar
Xue Z., Ma J., Zhang T., Miao H., Li R., Synthesis of nanosized ZSM-5 zeolite with intracrystalline mesopores. Mater. Lett., 2012, 68, 1–3.10.1016/j.matlet.2011.10.019Suche in Google Scholar
Yavari I., Seyfi S., Skoulika S., A convenient synthesis of functionalized indenopyrazolones from indan‐1, 2, 3‐trione, benzaldehydes, and phenylhydrazine. Helvetica Chimica Acta., 2012, 95, 1581–1585.10.1002/hlca.201200053Suche in Google Scholar
Zang H., Su Q., Mo Y., Cheng B., Ionic liquid under ultrasonic irradiation towards a facile synthesis of pyrazolone derivatives. Ultrason. Sonochem., 2011, 18, 68–72.10.1016/j.ultsonch.2010.08.001Suche in Google Scholar PubMed
© 2022 Seyyed Mohammad Ebrahimi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure
Artikel in diesem Heft
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure


