Abstract
In this study, three types of ferrites nanoparticles including CoFe2O4, NiFe2O4, and ZnFe2O4 were synthesized by microwave-assisted hydrothermal method. The X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FTIR), and field emission scanning electron microscopy (FESEM) were employed to analyze synthesized nanoparticles and fabricated membranes. The morphology of membrane surface was investigated by surface images. The ability of ferrite nanoparticles was evaluated to the separation of sodium salt and heavy metals such as Cr2+, Pb2+, and Cu2+ from aqueous solutions. The modified membrane showed the enhancement of membrane surface hydrophilicity, porosity, and mean pore size. The results revealed a significant increase in pure water flux: 152.27, 178, and 172.68 L·m−2·h−1 for PES/0.001 wt% of CoFe2O4, PES/0.001 wt% NiFe2O4, and PES/0.001 wt% ZnFe2O4 NPs, respectively. Moreover, Na2SO4 rejection was reached 78% at 0.1 wt% of CoFe2O4 NPs. The highest Cr (II) rejection obtained 72% for PES/0.001 wt% of NiFe2O4 NPs while it was 46% for the neat PES membrane. The Pb(II) rejection reached above 75% at 0.1 wt% of CoFe2O4 NPs. The Cu(II) rejection was obtained 75% at 0.1 wt% of CoFe2O4 NPs. The ferrite NPs revealed the high potential of heavy metal removal in the filtration membranes.
1 Introduction
In recent years synthesis of ferrite nanoparticles and their usage in water treatment were described. Ferrites were obtained from various precursors with magnetic properties and attractive in many applications such as digital recording, microwave devices, sensors, catalysis, ferrofluids, and magnetic refrigeration systems. Spinel ferrites as the class of composite metal oxides with superior magnetic materials are containing ferric ions with general structure M2+Fe23+O4 (M = Co2+, Ni2+, Zn2+) because of low cost, high efficiency, resistivity, chemical, thermal strengths, tunable shape, magneto-crystalline anisotropy, electrical insulation, high surface area, surface active sites, and high potential in functionalization. The magnetic properties of ferrite depend on the dispersion of the transition metal ions among the cationic sites in the spinel structure (Ahmadian-Fard-Fini et al., 2018; Goodarzi et al., 2017; Hedayati, 2015; Hedayati et al., 2016a, 2016b; Heidary et al., 2017; Nabiyouni et al., 2012).
SF adsorbents are excellent choice for water treatment through the adsorption or degradation process (Ahmadian-Fard-Fini et al., 2019). The ferrite preparation by precursor method is including the blend of ions at the atomic level which its results after thermolysis is usually nanosized ferrites (Ahmadian-Fard-Fini et al., 2020; Hedayati et al., 2016a, 2016b; Kavousi et al., 2018; Kiani et al., 2019).
There are different methods for the production of SF NPs such as polymerized complex, micro-emulsions, and microwave hydrothermal flash method. These techniques have low rate of yield and need to a long time for the process complete. Moreover, some methods have expensive raw materials, high energy demands. But the chemical synthesis methods from aqueous solutions are simple and suitable for mass production. Also, the control of reaction parameters are easy such as pH, complexing agent, and concentration. Among SF NPs, previous studies have been used magnetic nanoparticles for water treatment. Most studies focused on the conventional iron oxide-based nanoparticles including magnetite (Fe3O4), hematite (α-Fe2O3), and maghemite (γ-Fe2O3). In recent years, the use of SFs has been widely applied as adsorbent to the capture of heavy metal ions. But there are fewer reports in the use of ferrite NPs especially MFe2O4 materials in the membrane filtration such as microfiltration, ultrafiltration, nanofiltration, and reverse osmosis. (BandehAli et al., 2019, 2021).
In this here was reported the synthesis of three different ferrite nanoparticles including CoFe3O4, NiFe2O4, and NiFe2O4 for the fabrication of PES-based NF membranes by microwave hydrothermal method. The synthesized nanoparticles and fabricated membranes were analyzed by XRD, FTIR, and FESEM. Furthermore, the surface morphology of the blend membranes was characterized by three-dimensional surface images. The ability of ferrite NPs were examined to separation of sodium salt and heavy metals such as Cr2+, Pb2+, and Cu2+ from aqueous solutions.
2 Result and discussion
The separation performance of the membranes was evaluated by a dead-end nano-filtration system in Figure 1 at pressure of 4.5 bar. Initially, the compaction of membranes was occurred at 5 bar for 30 min by deionized water to obtain a steady pure water flux before filtration test. The pure water flux (PWF) was obtained by:
where Jw,1 (L·m−2·h−1) is the permeate flux, V, A, and t are the volume of water permeate, effective area of membrane (11.94 cm2), and time (h), respectively.

Schematic diagram of the filtration system.
The rejection followed by Eq. 2:
where Cf is feed solution concentration (Na2SO4 (1,100 mg·L−1) and heavy metals (300 mg·L−1)) and Cp is the concentration of permeate solution.
Figure 2 shows the FTIR spectra of CoFe2O4, NiFe2O4, and ZnFe2O4 nanoparticles in the range of 500–4,000 cm−1. The presence of peaks below 600 cm−1 is assigned to tetrahedral and octahedral sites respectively in a spinel structure. Moreover, the binds near 600 cm−1 are attributed metal-oxygen intrinsic vibrations in tetrahedral complexes which assigned in the about of 565 cm−1 for Zn–O and Ni–O, 594 cm−1 for Co–O, and the peaks near the 500 cm−1 are related to the presence of Fe–O stretching bonds and it is clear in 410 and 414 cm−1 for ZnFe2O4 and CoFe2O4 NPs, respectively. The peaks around 3,400–3,500 cm−1 correspond to the presence of bending and stretching vibration of H–O–H bond in all FTIR spectra.

FTIR spectra of ferrite NPs: CoFe2O4, NiFe2O4, and ZnFe2O4.
XRD pattern of ferrite NPs is shown in Figure 3. The XRD analysis of ferrite nanoparticles presents the formation of crystalline structure of nanoparticles. The crystalline size of NPs was calculated by Scherrer equation about 16, 23, and 14 nm for CoFe2O4, NiFe2O4, and ZnFe2O4 NPs, respectively. Furthermore, the average size of ferrite nanoparticles by FESEM images in Figure 3 were obtained 23, 33, and 25 nm for CoFe2O4, NiFe2O4, and ZnFe2O4 nanoparticles, respectively.

FESEM images and XRD analysis of ferrite NPs: CoFe2O4, NiFe2O4, and ZnFe2O4.
The cross-sectional FESEM images of the fabricated membranes: pure PES and PES/CoFe2O4, PES/NiFe2O4, and PES/ZnFe2O4 are illustrated in Figures 4–6. The FESEM images show the asymmetric structure including a thin layer on the top of membrane and a finger-like structure as membrane support. This structure forms during an exchange between solvent (dimethylacetamide) and non-solvent (water) in the phase inversion process (Rana et al., 1993, 1996a, 1996b, 2000). Introducing ferrite NPs into the PES membrane change membrane morphology due to thermodynamic and kinetic effects of the system. The presence of nanoparticles disrupts the polymeric chains and reduces the interactions between polymer-polymers. Thus, more voids create into the membrane structure. The introducing nanoparticles into the PES membrane led to the formation of larger and longer pores due to increasing the exchange between solvent and water. As shown in Table 1 and Figure 4, the mean pore size of membrane enhanced to 3.24 nm for 0.01 wt% CoFe2O4 NPs and then reduced to 1.09 nm at 1 wt% CoFe2O4 NPs. Moreover, membrane porosity decreased from 70.9% at 0.1 wt% to 65.3% at 1 wt% CoFe2O4 NPs. These reductions can be attributed to pore filling, increasing solution viscosity. By introducing NiFe2O4 nanoparticles, did not observe a significant change in the membrane porosity, but increase mean pore size from 1.3 nm for pure PES membrane to 8.67 nm at 0.1 wt% NiFe2O4 nanoparticles (Table 1 and Figure 5). The decline of mean pore size at 0.01 wt% NiFe2O4 NPs (M2) can be explained to the bad dispersion of nanoparticles and nanoparticles aggregation into the membrane (Cui et al., 1998; Ghanbari et al., 2016; Khodabakhshi et al., 2018). Furthermore, the man pore size increased from 1.3 nm for the pure PES membrane to 4.56 nm at 0.01 wt% ZnFe2O4 NPs, then it reduced to 1.6 nm at 1 wt% ZnFe2O4 NPs due to pore filling with nanoparticles (Table 1 and Figure 6). As shown in FESEM images and Table 1, the introducing NiFe2O4 nanoparticles create the larger pore compared with another ferrite nanoparticles and the highest membrane porosity obtained for PES/CoFe2O4 membranes.
Porosity and mean pore size of the pure PES membrane and the modified membranes
| Mem. | PES/CoFe2O4 | PES/NiFe2O4 | PES/ZnFe2O4 | |||
|---|---|---|---|---|---|---|
| Porosity (%) | Mean pore size (nm) | Porosity (%) | Mean pore size (nm) | Porosity (%) | Mean pore size (nm) | |
| M0 | 50 | 1.3 | 50 | 1.3 | 50 | 1.3 |
| M1 | 51.8 | 5.55 | 58.9 | 5.46 | 50.9 | 1.29 |
| M2 | 65.6 | 3.24 | 55.5 | 1.79 | 35.39 | 4.56 |
| M3 | 70.9 | 1.29 | 54.5 | 8.67 | 48.9 | 1.75 |
| M4 | 65.3 | 1.09 | 62.3 | 8.26 | 79 | 1.61 |
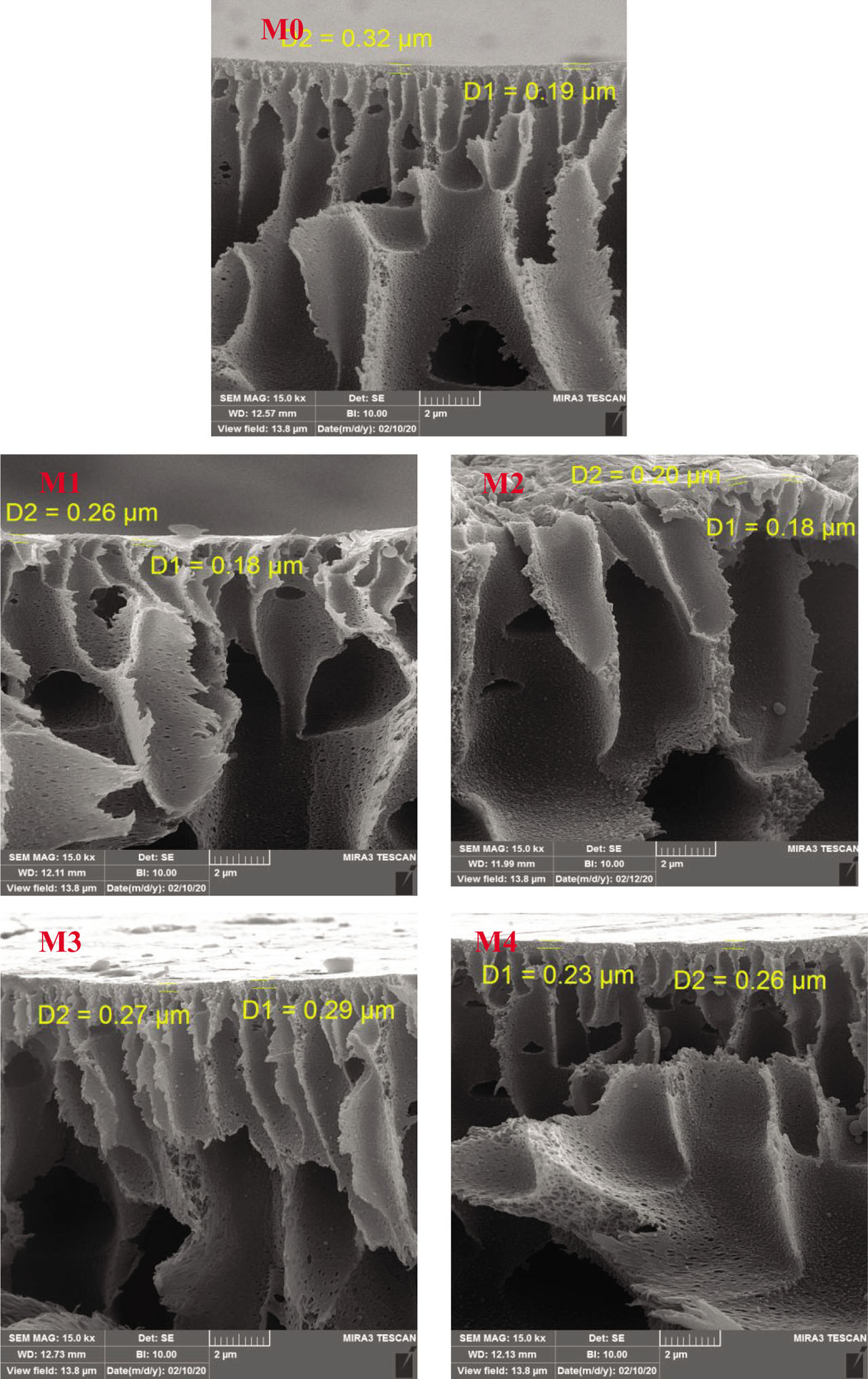
FESEM images of unmodified membrane (M0) and PES/CoFe2O4 membranes (M1–M4)..

FESEM images of PES/NiFe2O4 membranes (M1–M4).

FESEM images of PES/ZnFe2O4 membranes (M1–M4).
The morphology of the membrane surface and membrane surface roughness was determined by SPM software (version 6.4, Femtoscan). Three-dimensional surface images of the pure PES membrane and modified membranes at 1 wt% of nanoparticle are shown in Figure 7 at scanning area 6 × 6 μm. Moreover, the membrane roughness parameter including the average roughness (Ra) is presented in Figure 7. According to the results, the modified membranes showed a smoother surface compared with pure PES membrane. By increasing the nanoparticles reduced surface roughness due to pore filling and valleys by the nanoparticles on the membrane surface and then reduction of pore sizes.

3D surface images of the pure PES membrane and PES/CoFe2O4, PES/NiFe2O4, and PES/ZnFe2O4 at 1 wt% NPs.
The surface hydrophilicty of the fabricated membranes was determined by contact angle and water content measuring (Table 2). All modified membranes showed lower contact angle compared with the pure PES membrane (65°). It is clear that the use of ferrite NPs enhance membrane hydrophilicity and leads to easy transport of the water molecules. Moreover, the highest water content (79%) obtained to PES/1 wt%-ZnFe2O4 due to its high porosity (79%) (refer to Table 1). The results of the water content showed the increment of water content and membrane hydrophilicity by using ferrite nanoparticles as additive into the PES as the membrane matrix.
Contact angle and water content of all fabricated membranes
| Mem. | PES/CoFe2O4 | PES/NiFe2O4 | PES/ZnFe2O4 | |||
|---|---|---|---|---|---|---|
| Contact angle (°) | Water content (%) | Contact angle (°) | Water content (%) | Contact angle (°) | Water content (%) | |
| M0 | 63 | 66 | 63 | 66 | 63 | 66 |
| M1 | 50 | 74.6 | 48 | 77 | 51 | 76 |
| M2 | 38 | 78.8 | 39 | 72 | 53 | 63.7 |
| M3 | 30 | 77.2 | 47 | 69.9 | 36 | 66 |
| M4 | 39 | 75.5 | 28 | 72.3 | 44 | 78.1 |
Figure 8 indicates PWF of the prepared membranes. It is clear PWF increased significantly compared to the pure PES membrane and the highest PWF was obtained 152, 178, and 172 L·m−2·h−1 for PES/0.001 wt% CoFe2O4, PES/0.001 wt% NiFe2O4, and PES/0.01 wt% ZnFe2O4, respectively. This trend is agreement with the increase of mean pore size of the membrane in Table 1. Bigger pores increase water transport and lead to more PWF (Etminan et al., 2018; Ghanbari et al., 2015; Moradi et al., 2018). The decrease of PWF related to decreasing mean pores size and pore filling at high concentration of NPs. Moreover, the formation of interconnect structure of the fabricated membranes is another reason to decline PWF.

Pure water flux of the pure PES membrane and PES/CoFe2O4, PES/NiFe2O4, and PES/ZnFe2O4 membranes at 4.5 bar pressure.
Figure 9a shows the Na2SO4 rejection for pure PES and three types of modified membranes. Introducing ferrite NPs enhanced Na2SO4 rejection to 78%, 72%, and 62% at 0.1 wt% CoFe2O4, NiFe2O4, and ZnFe2O4 NPs. The increase of Na2SO4 removal describes with Donnan exclusion effects. Therefore, negative charges on the membrane surface repulse SO42− ions. Furthermore, the hydrophilic surface of the modified membranes decreases foulant deposition and concentration polarization which leads to high separation performance. Then Na2SO4 rejection reduces in the high concentration of nanoparticles (1 wt%) due to nanoparticle accumulation on the membrane surface. It seems that CoFe2O4 and ZnFe2O4 NPs with smaller sizes have better dispersion into the polymeric solution and their membranes have better salt separation than PES/NiFe2O4 membranes.
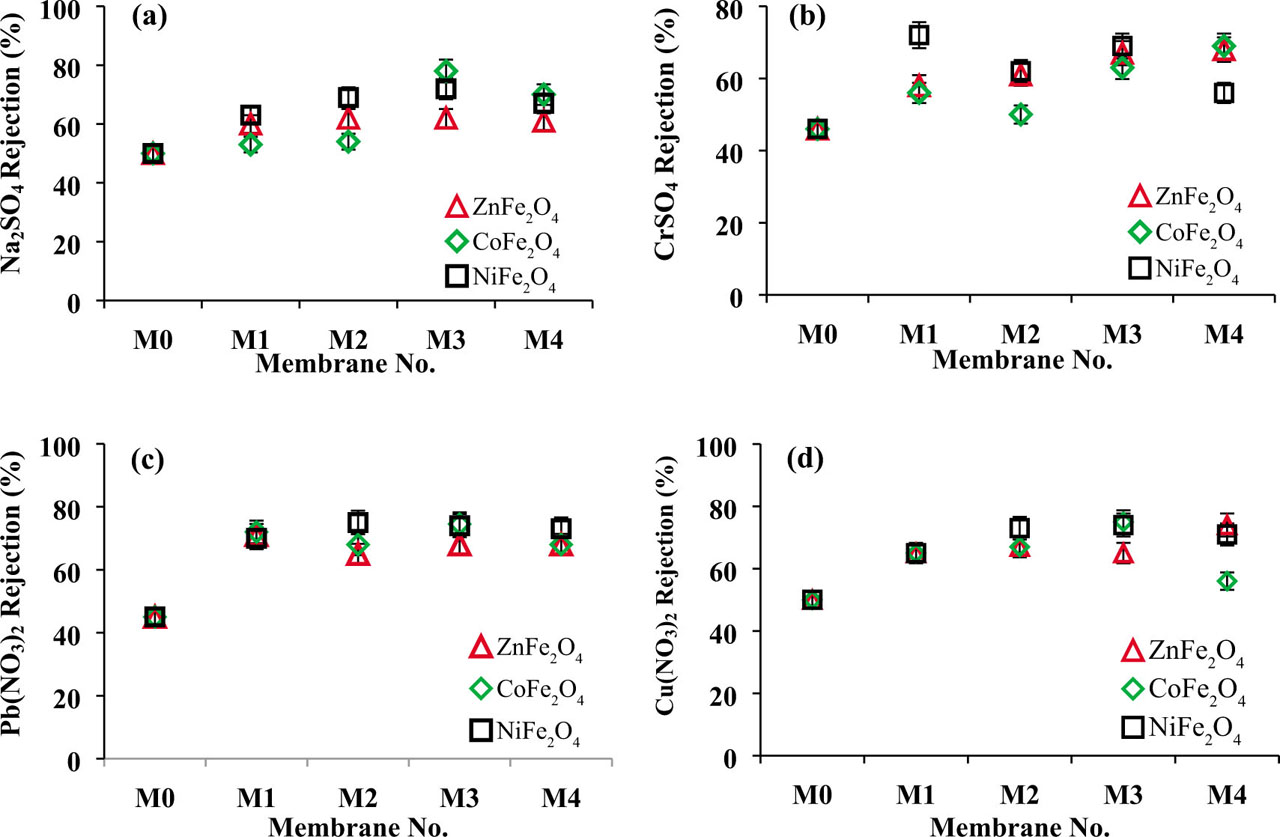
The rejection of Na2SO4, CrSO4, Pb(NO3)2, and Cu(NO3)2 by the pure PES membrane and PES/CoFe2O4, PES/NiFe2O4, and PES/ZnFe2O4 membranes.
Figures 9b,c show the change of Cr(II), Pb(II), and Cu(II) rejection at different concentration of ferrite nanoparticles. The heavy metals removal has an increasing trend. However, the reduction of heavy rejection in high concentration (1 wt%) is due to accumulation of NPs and decline of active sites to heavy metal adsorption. Cr(II) rejection reached 69% and 68% at 1 wt% Co Fe2O4 and ZnFe2O4 NPs. Moreover, the highest Cr2+ rejection was 72% for PES/0.001 wt% NiFe2O4 while it was 46% for the neat PES membrane. The Pb(II) rejection reach above 75% at 0.1 wt% CoFe2O4, 0.01 wt% NiFe2O4, and 0.001 wt% ZnFe2O4 NPs while it was 45% for the pure PES membrane. Cu(II) rejection obtained 75% and 74% at 0.1 wt% of CoFe2O4 and NiFe2O4 NPs. The highest Cu(II) rejection was 74% for PES/1 wt% ZnFe2O4 membrane while Cu2+ rejection was 50% for the pure PES membrane. Mechanisms for the heavy metal rejection are due to the ion electrostatic exclusion via negative charges on the membrane surface and the ion adsorption by NPs. Of course, good dispersion of NPs at suitable values promotes metal ion rejection. It was noticed that ions with a high hydration radius have lower the diffusion coefficient value for the cations in the aqueous solution Pb2+ > Cr2+ > Cu2+. Therefore Pb2+ ions transport across the membrane is easier than other metal ions which increase the adsorption capacity of modified membranes due to their spinel structure. Moreover, Cu2+ ion removal is higher than Cr2+ ions due to the high hydration radius.
3 Conclusion
In this research, CoFe2O4, NiFe2O4, and ZnFe2O4 nanoparticles were synthesized by microwave-assisted hydrothermal method. The various concentrations of synthesized nanoparticles were used to fabricate PES nano-filtration membranes by phase inversion method. FTIR, FESEM, XRD analysis, and three-dimensional surface images were used to analysis membrane and nanoparticles. The separation performance of modified membrane was investigated by sodium salt separation and heavy metals removal. PWF enhanced significantly as well as membrane hydrophilicity and mean pore size of membrane. Modified membrane showed outstanding sodium salt and heavy metal removal and Na2SO4, Cr(II), Pb(II), and Cu(II) rejection enhanced 36%, 36%, 40%, and 33% compared with the neat PES membrane.
Experimental
Synthesis of ferrite nanoparticles
Polyethersulfone (PES, Mw = 58,000 g·mol−1) was provided by BASF, Germany, polyvinylpyrrolidone (PVP, Mw = 25,000 g·mol−1) as pores creating agent were provided from Merck, Germany. N, N-dimethylacetamide (DMAc, Mw = 87.12 g·mol−1) as solvent was prepared from DAEJUNG, Korea. Co(NO3)2, Ni(NO3)2, Zn(NO3)2, Fe(NO3)3·9H2O, and NaOH were prepared from Merck, Germany. Moreover, the aqueous solutions of Na2SO4 and CrSO4, Pb(NO3)2 and Cu(NO3)2 were provided from Merck, Germany for the membrane separation performance analysis.
0.01 mol of Co(NO3)2, Ni(NO3)2, and Zn(NO3)2 were dissolved in the de-ionized water, separately. Then 0.02 mol of Fe(NO3)3·9H2O were added to metal solutions in the prior step. Then NaOH solution (1 M) was added to solution (for reaching pH = 10) under microwaves (600 W) for 30 min. After that the solution was heated at autoclave reactor at 180°C for 5 h. The precipitated nanoparticles were centrifuged and washed with distillate water and were placed in oven for drying.
Fabrication of NF membranes
Nanofiltration (NF) membranes were produced through phase inversion method with immersion into the deionized water bath. The polymeric solutions were prepared from PVP (1 wt%) and PES (18%) in DMAc as solvent with different amounts of ferrite nanoparticles. Then casting solutions were stirred for 5 h at room temperature. Polymeric solutions were placed for 40 min in an ultrasonic bath for better dispersion of nanoparticles in solution. The homogeneous solutions conserved for 12 h without stirring in the same temperature due to removing air bubble. Polymeric solutions were cast on a clean glass plates by an applicator and then they were instantly drenched into the distillate water bath. The blend membranes were placed in deionized water for 24 h to remove any soluble material in water and were kept between two filter papers for drying at room temperature. Table 3 indicates the details of the prepared membrane compositions.
The composition of materials in the membrane preparation
| Membrane | PES (wt%) | Ferrite NPs (wt%) | PVP (wt%) | DMAc (wt%) |
|---|---|---|---|---|
| M0 | 18 | 0 | 1 | 81.000% |
| M1 | 18 | 0.001% | 1 | 80.999% |
| M2 | 18 | 0.01% | 1 | 80.990% |
| M3 | 18 | 0.1% | 1 | 80.900% |
| M4 | 18 | 1% | 1 | 80.000% |
Characterization methods
FTIR was applied with a Bruker spectrometer (TENSOR 27) in the range of 400 to 4,000 cm−1 at the resolution of 1 cm−1 for each spectrum for the characterization of synthesized nanoparticles and membranes. FESEM (SU3500, USA) was employed to determine ferrite NPs and membrane morphology. Moreover, three-dimensional surface image was used to determine the surface morphology of the fabricated membranes in scanning area 6 × 6 μm by SPM software (version 6.4, Femtoscan).
X-ray diffraction pattern (model X’ Pert Pw 3373, λCu = 0.154°A, Philips, Holland) was used to characterize ferrite nanoparticles. The average size of ferrite crystals (D) was calculated using Debye Scherrer equation in Eq. 3:
where K as a dimensionless value is equal to 0.9, λ is the X-ray wavelength, and β is the full width at half maximum intensity of peak corresponding to 2θ.
The mean pore size and porosity of the membranes was determined by dry-wet weight method. Membranes immersed into the deionized water for 2 h and then weighted. After drying wet membranes, dry weight of membranes was measured. The membranes porosity (ɛ) and mean pore size of membrane (rm) were obtained by equations (BandehAli et al., 2019, 2021).
where Ww and Wd are the weight of the wet and dry membrane (g), Vm and ρf are membrane volume (cm3) and water density (g·cm−3), respectively. η is the water viscosity (8.9×10−4 Pa·s), Q is the volume of the permeated water flux (m3·s−1), ΔP is operating pressure (0.45 MPa), A is the membrane filtration area (m2), and L is the thickness of membranes (m).
The contact angle analyzer (G10, Kruss, Germany) was used to the measurement of water contact angle (θ) of membranes. Moreover, the water content of membranes were calculated according to base on wet and dry weight of membranes (BandehAli et al., 2019, 2021):
All experiments were repeated three times and their average values were reported for the decline of the experimental errors.
Funding information:
Authors state no funding involved.
Author contributions:
Davood Ghanbari: writing – review and editing, methodology, experimental analysis; Samaneh BandehAli: writing – original draft, experimental analysis; Abdolreza Moghadassi: writing, resources, project administration.
Conflict of interest:
Authors state no conflict of interest.
References
Ahmadian-Fard-Fini S., Ghanbari D., Amiri O., Salavati-Niasari M., Electro-spinning of cellulose acetate nanofibers/Fe/carbon dot as photoluminescence sensor for mercury (II) and lead (II) ions. Carbohyd. Polym., 2020, 229, 115428.10.1016/j.carbpol.2019.115428Search in Google Scholar
Ahmadian-Fard-Fini S., Ghanbari D., Salavati-Niasari M., Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: Hydrothermal synthesis of magnetic hollow NiFe2O4-carbon dots nanocomposite material. Compos. Part B-Eng., 2019, 161, 564–577.10.1016/j.compositesb.2018.12.131Search in Google Scholar
Ahmadian-Fard-Fini S., Salavati-Niasari M., Ghanbari D., Hydrothermal green synthesis of magnetic Fe3O4–carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. Acta A., 2018, 203, 481–493.10.1016/j.saa.2018.06.021Search in Google Scholar
Bandehali S., Sanaeepur H., Amooghin A.E., Shirazian S., Ramakrishna S., Biodegradable Polymers for Membrane Separation. Sep. Purif. Technol., 2021, 118731.10.1016/j.seppur.2021.118731Search in Google Scholar
Bandehali S., Moghadassi A., Parvizian F., Hosseini S.M., A new type of [PEI-glycidyl POSS] nanofiltration membrane with enhanced separation and antifouling performance. Korean J. Chem. Eng., 2019, 36 (10), 1657–1668.10.1007/s11814-019-0359-ySearch in Google Scholar
Cui W., Kerres J., Eigenberger G., Development and characterization of ion-exchange polymer blend membranes. Sep. Purif. Technol., 1998, 14, 145–154.10.1016/S1383-5866(98)00069-0Search in Google Scholar
Etminan M., Nabiyouni G., Ghanbari D., Preparation of tin ferrite–tin oxide by hydrothermal, precipitation and auto-combustion: photo-catalyst and magnetic nanocomposites for degradation of toxic azo-dyes. J. Mater. Sci.-Mater. El., 2018, 29, 1766–1776.10.1007/s10854-017-8085-xSearch in Google Scholar
Ghanbari D., Salavati-Niasari M., Khaghani S., Beshkar F., Preparation of Polyvinyl Acetate (PVAc) and PVAc–Ag–Fe3O4 Composite Nanofibers by Electrospinning Method. J. Clust. Sci., 2016, 27(4), 1317–1333.10.1007/s10876-016-1002-2Search in Google Scholar
Ghanbari D., Salavati-Niasari M., Beshkar F., Amiri O., Electro-spinning of cellulose acetate nanofibers: Microwave synthesize of calcium ferrite nanoparticles and CA–Ag–CaFe2O4 nanocomposites. J. Mater. Sci.-Mater. El., 2015, 26(11), 8358–8366.10.1007/s10854-015-3502-5Search in Google Scholar
Goodarzi M., Joukar S., Ghanbari D., Hedayati K., CaFe2O4–ZnO magnetic nanostructures: photo-degradation of toxic azo-dyes under UV irradiation. J. Mater. Sci.-Mater. El., 2017, 28(17), 12823–12838.10.1007/s10854-017-7110-4Search in Google Scholar
Hedayati K., Azarakhsh., Saffari J., Ghanbari D., Photo catalyst CoFe2O4–CdS nanocomposites for degradation of toxic dyes: investigation of coercivity and magnetization. J. Mater. Sci.-Mater. El., 2016a, 27(8), 8758–8770.10.1007/s10854-016-4900-zSearch in Google Scholar
Hedayati K., Azarakhsh S., Ghanbari D., Synthesis and magnetic investigation of cobalt ferrite nanoparticles prepared via a simple chemical precipitation method. J. Nanostruct., 2016b, 6(2), 127–131.Search in Google Scholar
Hedayati K., Synthesis and characterization of nickel zinc ferrite nanoparticles. J. Nanostruct., 2015, 5(1), 13–16.Search in Google Scholar
Heidary F., Khodabakhshi A.R, Ghanbari D., A novel sulfonated poly phenylene oxide-poly vinylchloride/ZnO cation-exchange membrane applicable in refining of saline liquids. J. Clust. Sci., 2017, 28, 1489–1507.10.1007/s10876-017-1156-6Search in Google Scholar
Kavousi F., Goodarzi M., Ghanbari D., Hedayati K., Synthesis and characterization of a magnetic polymer nanocomposite for the release of metoprolol and aspirin. J. Mol. Struct., 2018, 1183, 324–330.10.1016/j.molstruc.2019.02.003Search in Google Scholar
Khodabakhshi A.R., Heidary F., Ghanbari D., Cation Exchange Nanocomposite Membrane Containing Mg(OH)2 Nanoparticles: Characterization and Transport Properties. J. Nanostruct., 2018, 8(2), 191–201.Search in Google Scholar
Kiani A., Nabiyouni G., Masoumi S., Ghanbari D., A novel magnetic MgFe2O4–MgTiO3 perovskite nanocomposite: Rapid photodegradation of toxic dyes under visible irradiation. Composite part B: Engineering, 2019, 175, 107080.10.1016/j.compositesb.2019.107080Search in Google Scholar
Moradi B., Nabiyouni G., Ghanbari D., Rapid photodegradation of toxic dye pollutants: green synthesis of mono-disperse Fe3O4–CeO2 nanocomposites in the presence of lemon extract. J Mater Sci: Mater Electron., 2018; 29 (13), 11065–1108010.1007/s10854-018-9189-7Search in Google Scholar
Nabiyouni G., Boroojerdian P., Hedayati K., Ghanbari D., A simple method for synthesis of PbS nanoparticles using 2-mercaptoethanol as the capping agent, High. Temp. Mater. Process. 2012, 31 (6), 723–725.10.1515/htmp-2011-0155Search in Google Scholar
Rana D., Bag K., Bhattacharyya S.N., Mandal B.M., Miscibility of poly(styrene-co-butyl acrylate) with poly(ethyl methacrylate): Existence of both UCST and LCST, J. Polym. Sci., Polym. Phys. Ed., 2000, 38(3), 369–375.10.1002/(SICI)1099-0488(20000201)38:3<369::AID-POLB3>3.0.CO;2-WSearch in Google Scholar
Rana D., Mandal B.M., Bhattacharyya S.N., Analogue calorimetry of polymer blends: poly(styrene-co-acrylonitrile) and poly (phenyl acrylate) or poly(vinyl benzoate). Polymer., 1996a; 37(12): 2439–2443.10.1016/0032-3861(96)85356-0Search in Google Scholar
Rana D., Mandal B.M., Bhattacharyya S.N., Analogue Calorimetric Studies of Blends of Poly(vinyl ester)s and Polyacrylates, Macromolecules 1996b, 29(5), 1579–1583.10.1021/ma950954nSearch in Google Scholar
Rana D., Mandal B.M., Bhattacharyya S.N., Miscibility and phase diagrams of poly(phenyl acrylate) and poly(styrene-co-acrylonitrile) blends. Polymer., 1993; 34(7): 1454–1459.10.1016/0032-3861(93)90861-4Search in Google Scholar
© 2022 Davood Ghanbari et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure
Articles in the same Issue
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure

