Abstract
In this work, new polymeric aniline-barium ferrite and aniline-strontium ferrite thin layers were synthesized. In the first step, hexaferrites nano-additives were prepared by applying ultra-sound and microwave irradiation. Then, hexaferrites were added to aniline electrolyte solution separately. The electrodeposition of aniline as a polymeric matrix and hexaferrite as nano-additives was performed in an electrochemical cell in the presence of various acids. Scanning electron microscopy images were applied for morphology investigation and measuring average particle size. Energy dispersive X-ray spectroscopy was applied for elemental detection and analysis, as well as presence confirmation of nanoparticles. Atomic force microscopy was applied for surface roughness analysis of thin films. Magnetic property of the nanoparticles and polymeric nanocomposites was checked and measured by vibrating sample magnetometer. The crystallinity, crystallite size, and phase of samples were confirmed by X-ray diffraction pattern analysis.
1 Introduction
Hexaferrite is usually a composition of divalent metals such as barium, strontium, or lead with iron oxide (general formula: MFe12O19), in terms of magnetic property they are part of ferromagnetic materials (Meng et al., 2016; Monsef and Salavati-Niasari, 2022; Xia et al., 2013). They are hard and brittle, and their color is gray or black. A natural magnet or magnetite is an example of a ferrite magnet that has been used to build a compass for centuries. They are widely used in a variety of industries for a variety of reasons, including their low price (Lahijani et al., 2018; Monsef et al., 2021). In terms of material structure, ferrites are polycrystalline materials. This means that they are composed of a large number of tiny crystals with different orientations (Ahmadian-Fard-Fini et al., 2018; Panahi-Kalamuei et al., 2015; Sadeghpour et al., 2022). In terms of crystal structure, ferrites are of different types, such as spinel, garnet, perovskite, and hexagonal ferrites. Three main families of ferrites are used to make magnets: hexagonal ferrites, spinel ferrites, and garnet ferrites. Their crystal structure is in the form of a hexagonal or hexagonal prism with a vertical axis (Hajian Karahroudi et al., 2020). The magnetization of the material along this vertical axis is easier than other axes. For this reason, these ferrites are magnetically considered to be hard magnetic materials, that is, the magnitude or direction of their magnetic field does not change easily, and they are therefore suitable for making permanent magnets. This family is also called “hexaferrite.” Hexaferrites are the main building blocks of permanent ferrite magnets (Masoumi et al., 2016; Gholami et al., 2017).
Electrodeposition is a controlled method for depositing different types of conductive and semiconductor layers. In this method, three electrodes are used, the work electrode is a local in which the target material is layered by gaining or losing electrons. A reference electrode is used to control the potential between the working electrode and the electrolyte, and a secondary electrode must be used to complete the electrical circuit (Davar et al., 2010; Sadeghi et al., 2018; Song et al., 2019; Zinatloo-Ajabshir et al., 2018).
In electrochemical polymerization technique, thickness and morphology of thin layers can be controlled by adjusting the density of current and potential. Electrochemical polymerization could be an economical and environmental technique because no toxic, complex, and expensive compounds are required (Hassanpour et al., 2017; Nautiyal and Parida, 2016; Zinatloo-Ajabshir and Salavati-Niasari, 2019). Various parameters will affect the electro-deposition, such as temperature, acidity, concentration, and crystal structure of substrate (Abdelraheem et al., 2018; Fleaca et al., 2016; He et al., 2015).
2 Results and discussion
Figure 1a illustrates scanning electron microscopy (SEM) images of barium hexaferrite that were achieved by sono-chemistry method irradiation under 200 W power for 25 min (on: 3 s, off: 3 s), as can be argued from the pictures, homogeneous nanoparticles of close size with an average diameter of 40 nm have been obtained. Figure 1b illustrates SEM images of barium ferrite synthesized with the help of microwave irradiation; as results confirm, uniform hexagonal plate of hexaferrite were achieved. Figure 1c depicts the morphology of strontium ferrite that were obtained applying ultrasound irradiation (100 W, 60 min), nano-products with an average size less than 100 nm were obtained. Figure 1d shows images of SrFe12O19 that were achieved by 600 W power microwave irradiation; it is perceived from the pictures that products with rods morphology were prepared. These images determine that all ferrites nanoparticles are in the nanoscale range.
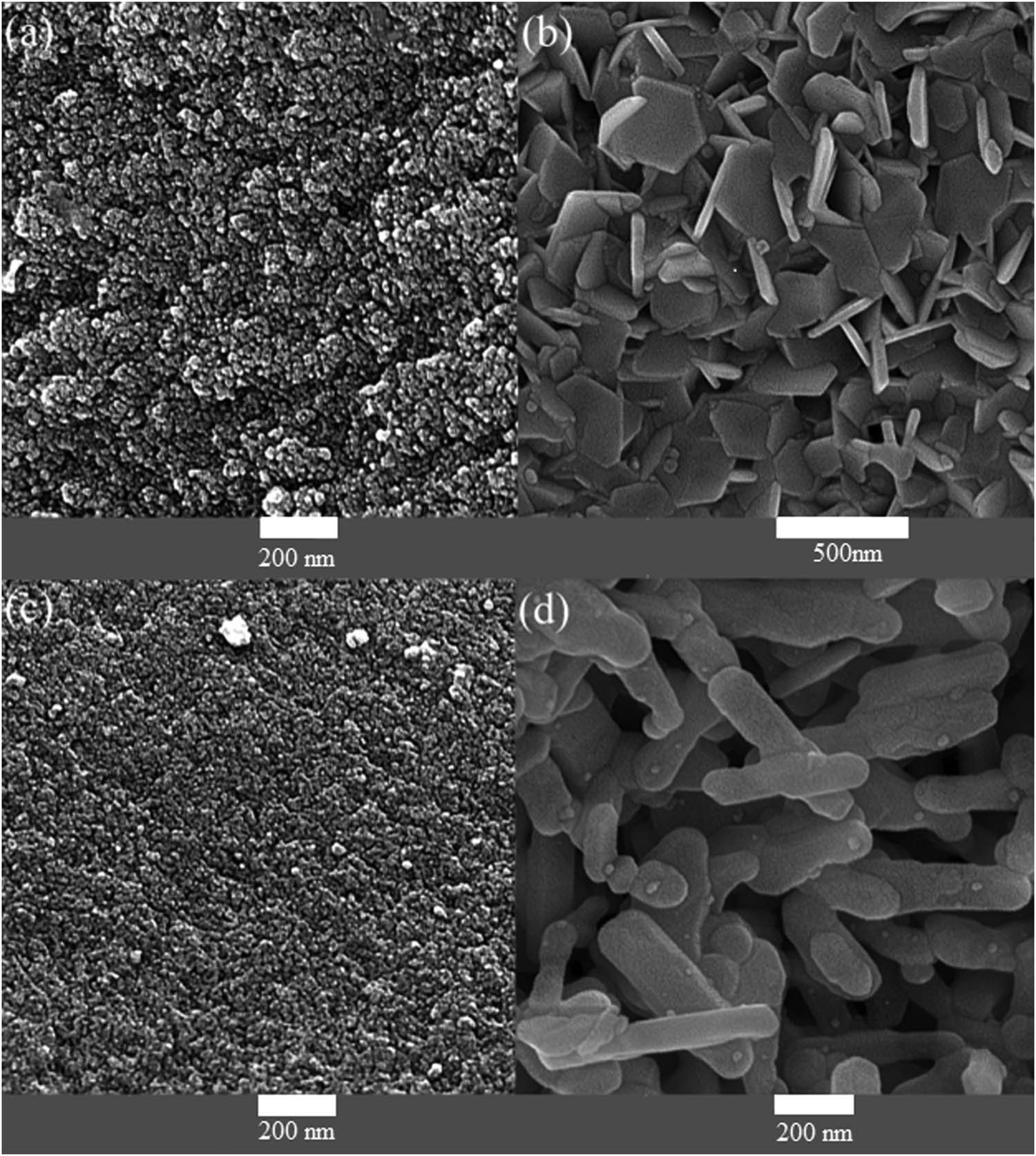
FESEM images of barium ferrite by (a) sono-chemical method and (b) microwave method. FESEM images of strontium ferrite nanoparticles by (c) sono-chemical method and (d) microwave method.
Figure 2 shows the morphology of polymeric aniline-barium ferrite thin layer for the 5–20 cycles of electrodeposition, Figure 2a illustrates needle like surface morphology, the presence of nano-additives in all three polyaniline-barium ferrite thin layer are approved. As shown in Figure 2b and c the grain and thickness of layers enhance with increase in the number of cycles.
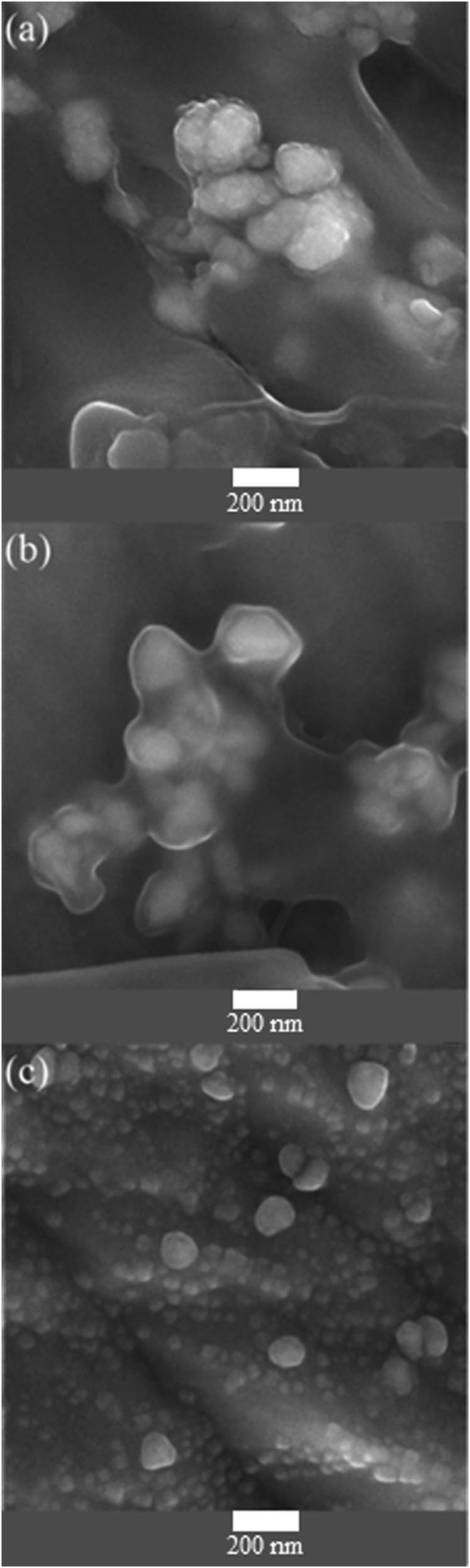
FESEM images of polyaniline-barium ferrite with (a) 5, (b) 10, and (c) 20 cycles of electrodeposition.
Figure 3a–c shows surface roughness of the polyaniline-SrFe12O19 thin layer composite for the 5–20 cycles of electrodeposition. In Figure 3, can be seen composite with more circles have more particles rather to composite. On examining the morphology, it is seen that the SrFe12O19 particles are uniform in the polyaniline films and the thicker layers have bigger grains in the polyaniline.
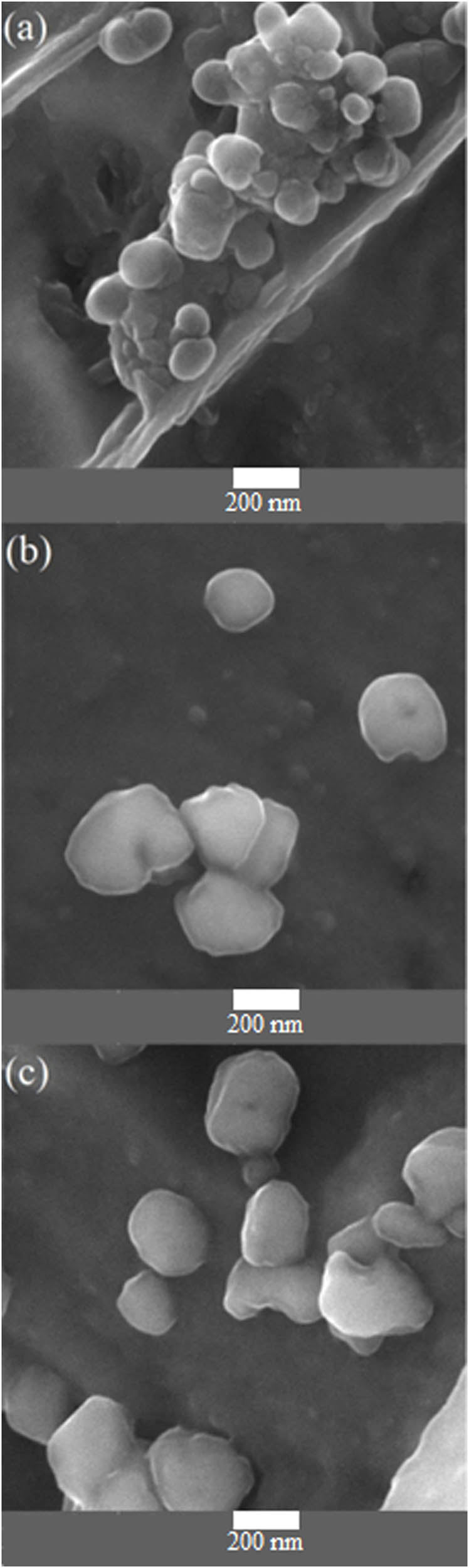
FESEM images of polyaniline-strontium ferrite with (a) 5, (b) 10, and (c) 20 cycles of electrodeposition.
Figure 4a shows Energy dispersive X-ray (EDX) analysis for barium ferrite and Figure 4b shows strontium ferrite nano-powder. In the EDX spectra, there are related elements of each ferrite. Figure 5a shows the elemental map of polyaniline film via ten cycles of electrodeposition. The map shows the elements N, O, and C monotonic dispensation in polymer. Figure 5b shows the map of polyaniline film on copper substrate.
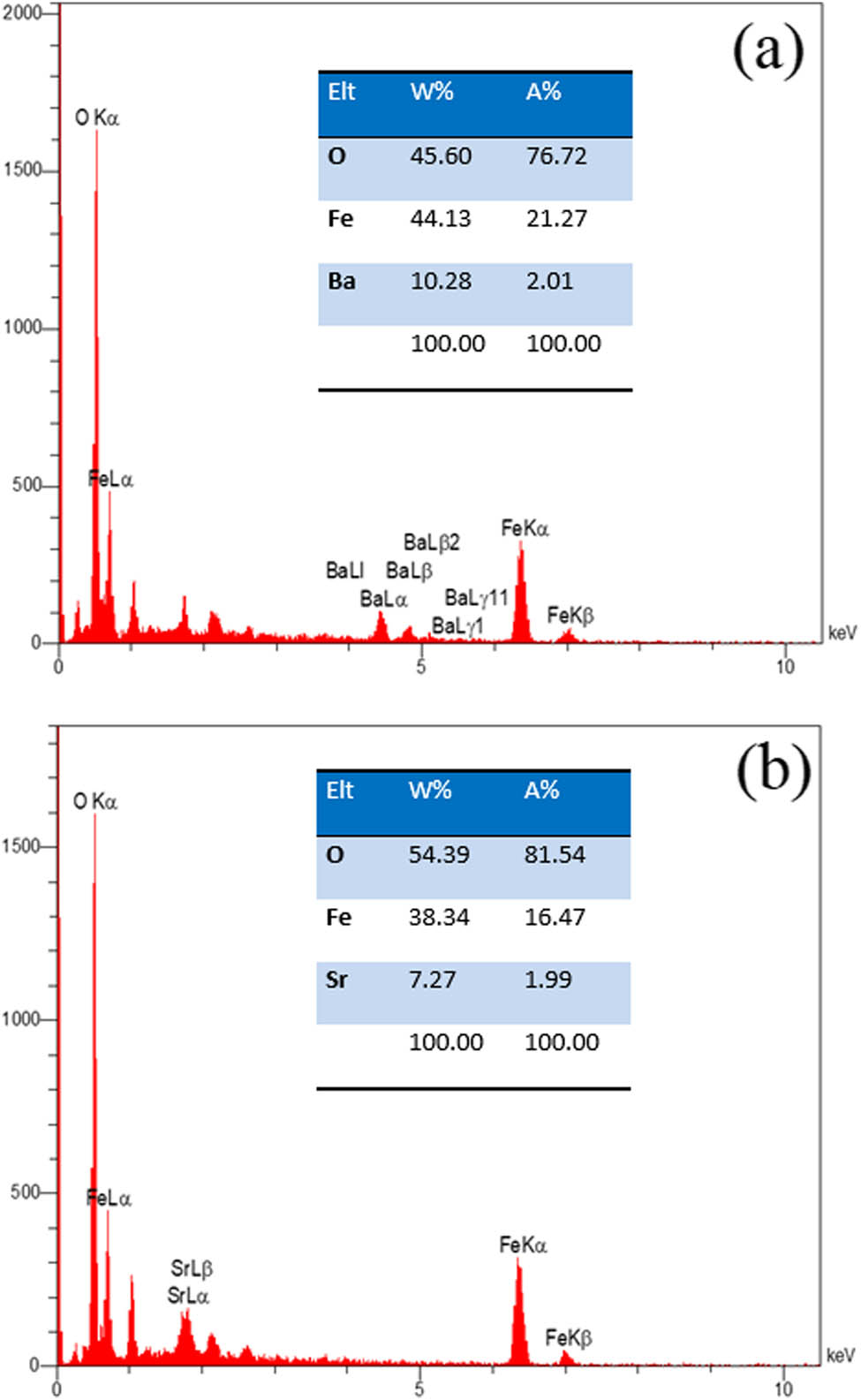
XED analysis of (a) barium ferrite and (b) strontium ferrite.
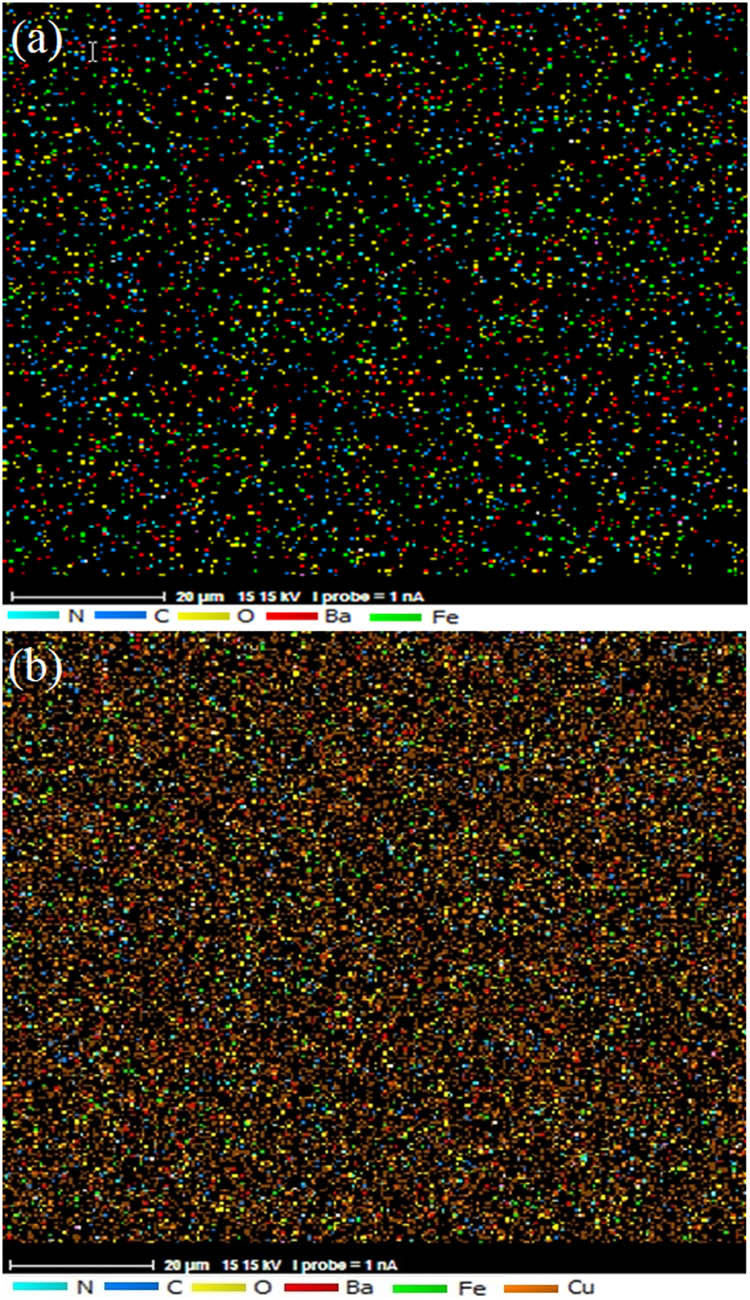
X-ray element distribution maps (MAP) analysis of (a) polyaniline/barium ferrite nanocomposite and (b) polyaniline-barium ferrite on the copper substrate.
The phase structure was approved by X-ray diffraction (XRD) (Philips, X’Pert Pro), (CuKα radiation, λ = 0.154 nm). Figure 6a shows the XRD pattern of BaFe12O19 nanocrystals. The XRD diagram showing the BaFe12O19 peaks has a good appraise by BaFe12O19 standard peaks (Ebrahimi et al., 2017).
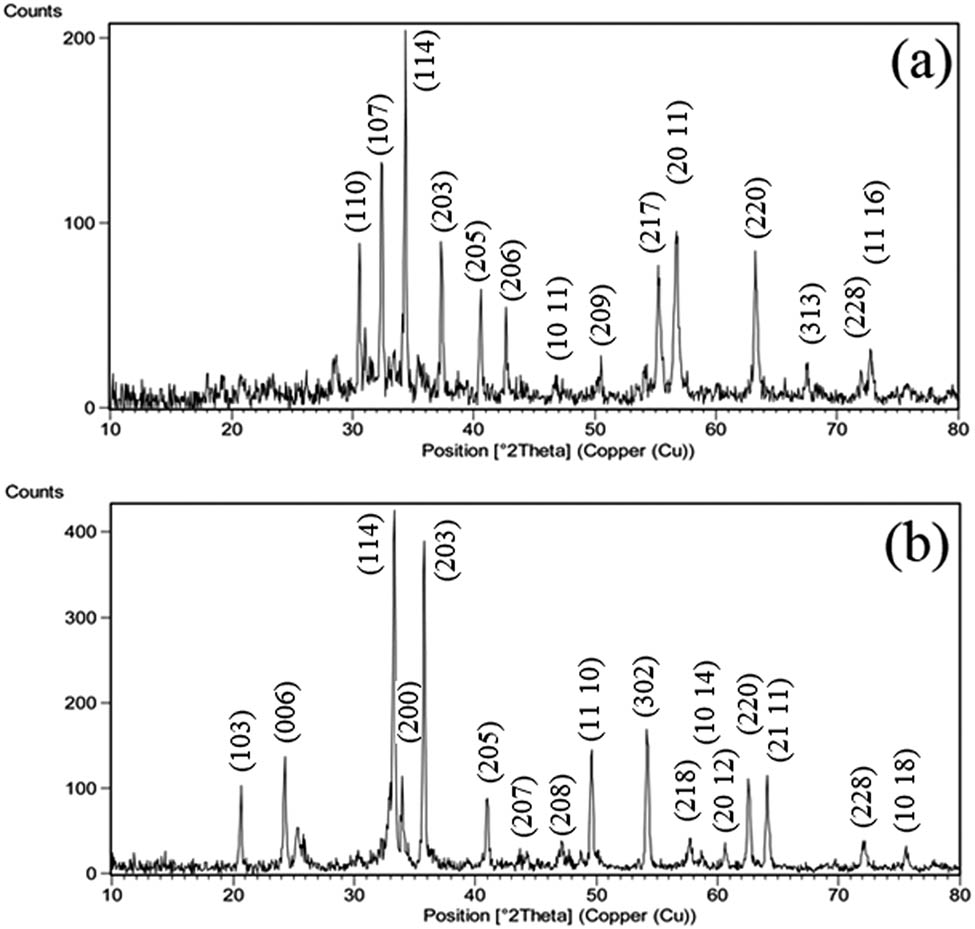
XRD pattern for (a) barium ferrite and (b) strontium ferrite nanoparticles.
Figure 6b indicates the XRD peaks of SrFe12O19 nanocrystals. The XRD diagram complies with the standard samples (Hajian Karahroudi et al., 2020).
The crystallite size of ferrites calculated via Debye–Scherrer equation (Eq. 1):
where β is the width of peaks in half maximum intensity, λ is 1.54 Å, θ is the angle of diffraction, and t is the size of crystallite (Abbasi et al., 2021). The crystallite size of SrFe12O19 nanoparticles was calculated to be 34 nm and BaFe12O19 nanoparticles was calculated to be 28 nm.
The Williamson–Hall formula calculates the crystallite size and strain of the crystal lattice:
where ε is lattice strain and β, θ, and λ are the same as in Eq. 1 (Abbasi et al., 2021). The crystallite size of BaFe12O19 and SrFe12O19 nanoparticles using Williamson–Hall method was calculated to be 25 and 32, respectively. The lattice strain of BaFe12O19 and SrFe12O19 was calculated to be 0.011 and 0.015, respectively.
Roughness is checked on the surface of many materials and thin layers. The roughness or kinetic roughening for layers depended to time of deposition or thickness of films. The roughness (w) is indicated by Eq. 3:
where h is the height of different points on the sample surface. In Family-Vicsek scaling assumption, roughness is expressed during short and long scan lengths with the following relations (Eqs. 4 and 5):
where H, l c , and β are Hurst coefficient, crossover length, and growth exponent, respectively. The anomalous scaling systems are explained via the following relations (Eqs. 6 and 7):
where β loc is the local roughness exponent (Hedayati, 2015).
The roughness of polyaniline/(SrFe12O19 and BaFe12O19) films was investigated by atomic force microscopy (AFM). The AFM images of BaFe12O19 and polyaniline composites in cycles of 5, 10, and 20 are shown in Figure 7a–c.
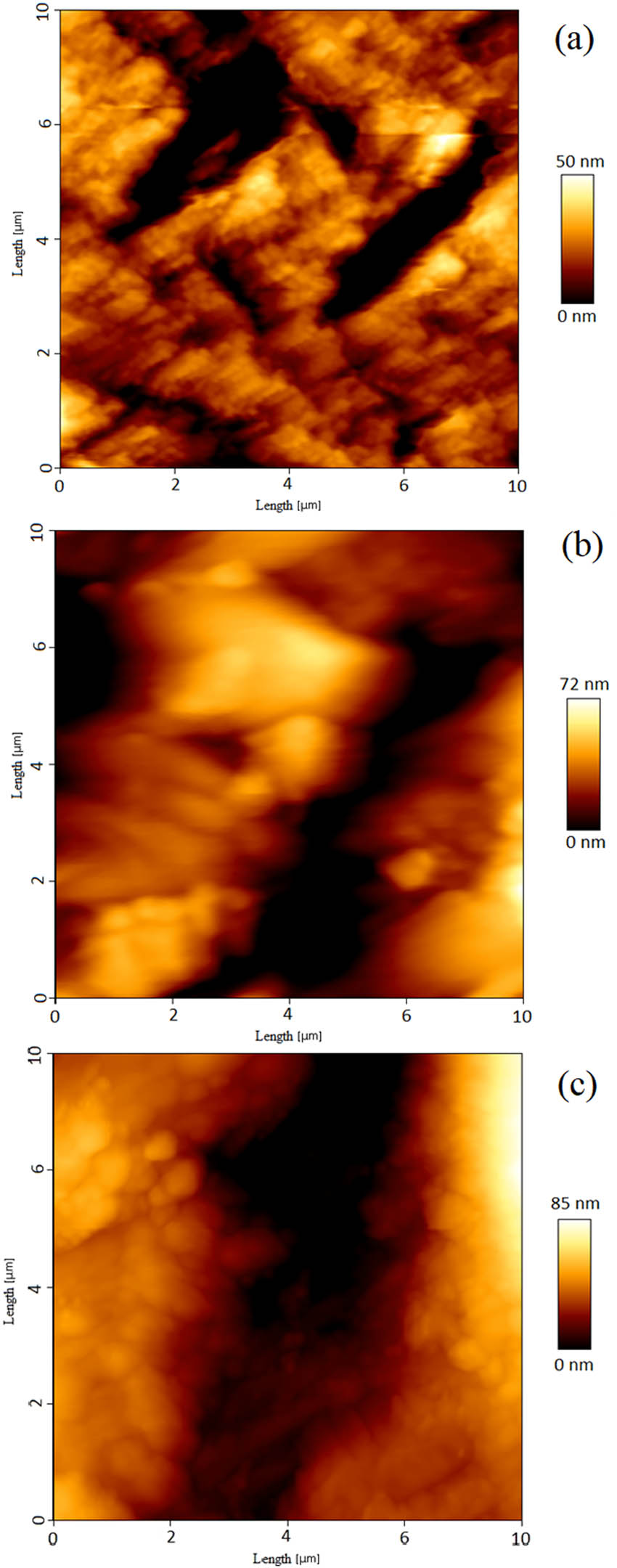
AFM images of thin layer of polyaniline/barium ferrite nanocomposite with (a) 5, (b) 10, and (c) 20 cycles of deposition.
Figure 8a–c show the AFM image for polyaniline-SrFe12O19 thin layer for 5,10, and 20 cycles, respectively. The logarithmic curve of roughness vs length scale of deposited BaFe12O19-polyaniline thin films with 5–20 cycles is depicted in Figure 9a.
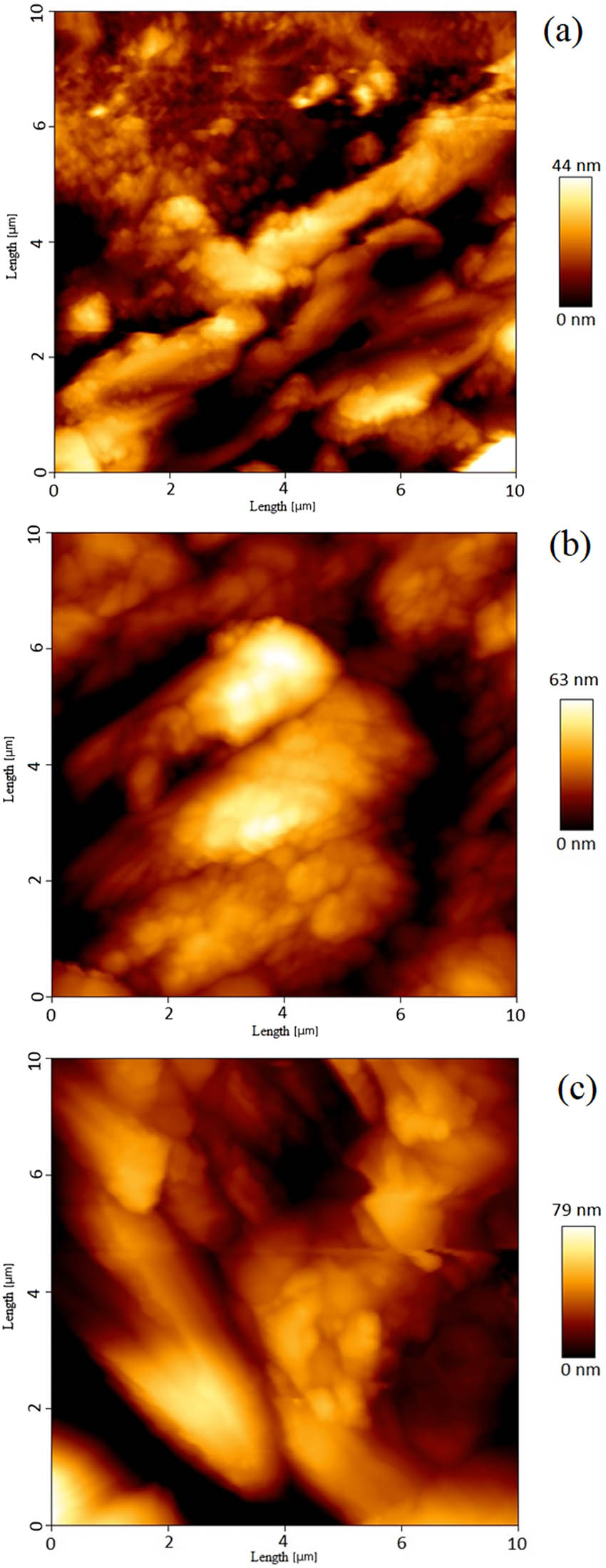
AFM images of thin layer of polyaniline/strontium ferrite nanocomposite with (a) 5, (b) 10, and (c) 20 cycles of deposition.
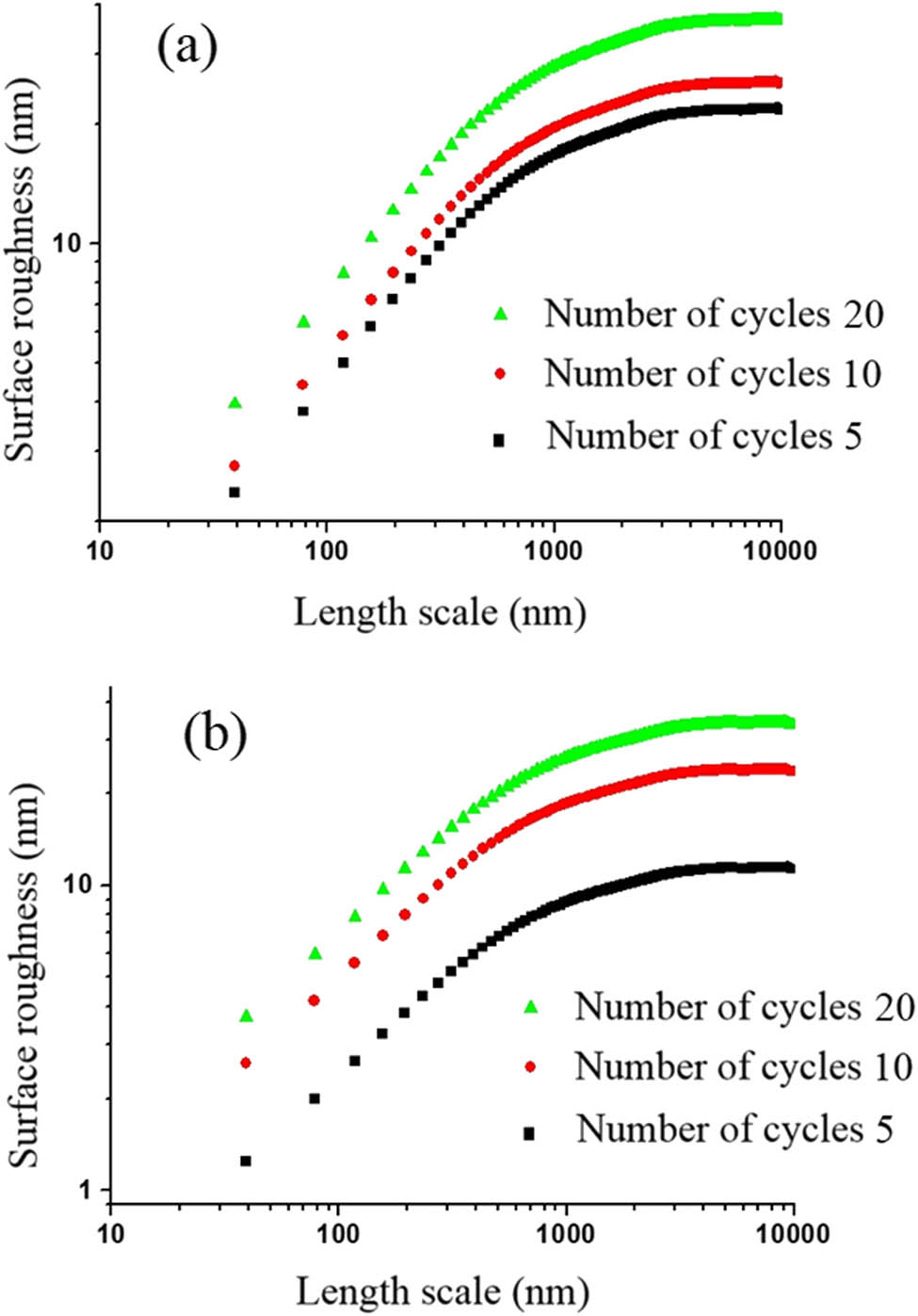
The roughness-scan length diagram in logarithmic scale for thin layer of (a) polyaniline/barium ferrite and (b) polyaniline/strontium ferrite nanocomposite with 5, 10, and 20 cycles of deposition.
Figure 9b shows logarithmic curve of the roughness and length scale deposited SrFe12O19-polyaniline layers via 5, 10, and 20 cycles. The diagrams of Figure 9a and b show that both SrFe12O19- and BaFe12O19-polyaniline layers have anomalous scaling treatment. According to these images, with increase in the deposition cycles, the roughness increases.
The magnetic hysteresis curve of composite films investigated via vibrating sample magnetometer (VSM) at room temperature. Figure 10a and b indicate the VSM loop for BaFe12O19 and SrFe12O19 nanocrystals, respectively. The remanence and saturation magnetization and coercivity of nano powders are described in Table 1. These loops demonstrate that BaFe12O19 and SrFe12O19 are ferromagnetic and BaFe12O19 is harder than SrFe12O19.
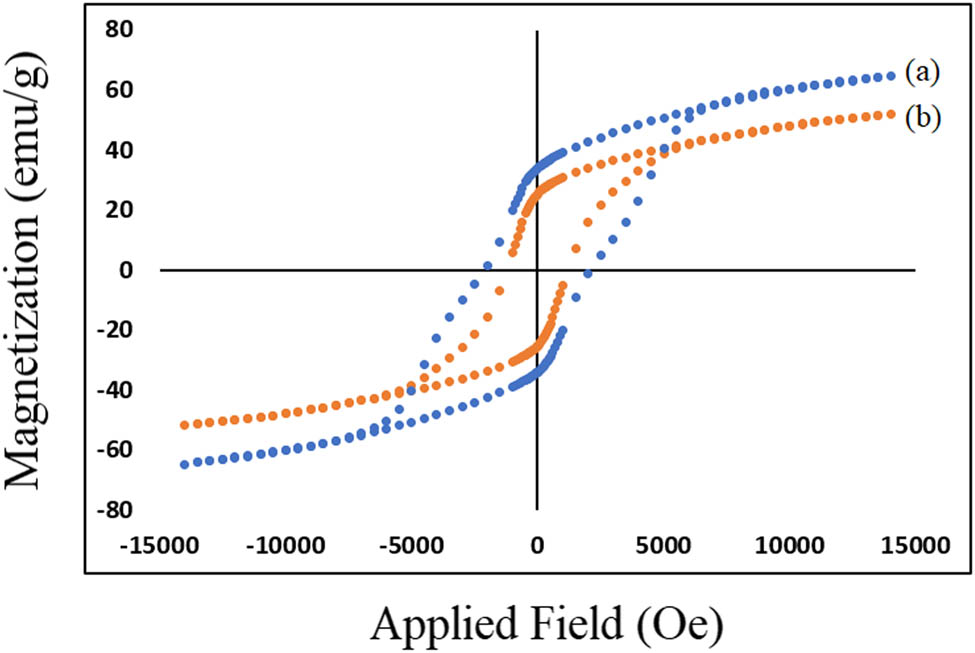
Hysteresis loop for (a) barium ferrite and (b) strontium ferrite nanoparticles.
Mr, Ms, and Hc for magnetic nanoparticles
| Coercivity (Oe) | Saturation magnetization (emu‧g−1) | Remanence magnetization (emu‧g−1) | Nanoparticles |
|---|---|---|---|
| 2,000 | 64.66 | 33.94 | Barium ferrite |
| 1,000 | 51.79 | 25.36 | Strontium ferrite |
| 3,000 | 2.09 | 0.87 | Polyaniline/barium ferrite |
| 0 | 3.75 | 0.01 | Polyaniline/strontium ferrite |
Figure 11a represents magnetic loop of BaFe12O19-polyaniline (ten cycles) composite in parallel field. The hysteresis loop of SrFe12O19-polyaniline (ten cycles) is shown in Figure 11b. For polyaniline-BaFe12O19, amount of Ms and Mr decreased compared to that of BaFe12O19 but Hc increased. In comparison to SrFe12O19-polyaniline vs SrFe12O19, amount of Ms, Mr, and Hc is decreased and SrFe12O19-polyaniline have super-paramagnetic behavior.
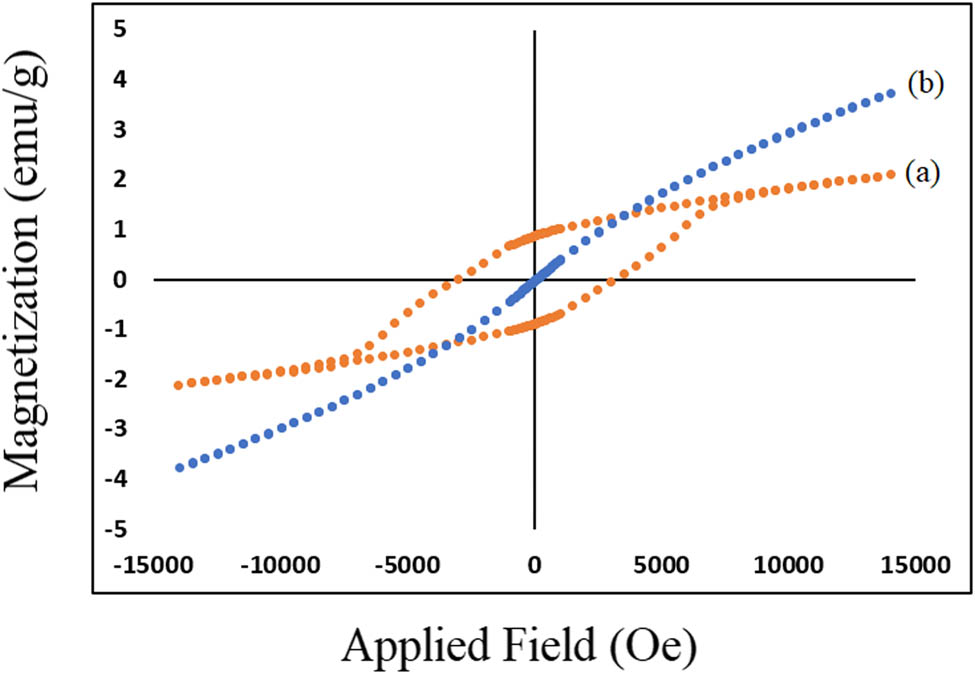
Hysteresis loop for (a) polyaniline/barium ferrite and (b) polyaniline/strontium ferrite thin layer composite in parallel field.
3 Conclusion
BaFe12O19 and SrFe12O19 nanoparticles were manufactured by both microwave irradiation and sono-chemical method. The ferrites-polyaniline nanocomposite films were grown by electrodeposition technique. SEM images showed that the grain size increased via increasing the film thickness. The EDX spectra indicated related peaks of BaFe12O19- and SrFe12O19-polyaniline. XRD analysis illustrated the crystalline structure of BaFe12O19 and SrFe12O19. The strain of lattice and crystallite size of BaFe12O19 and SrFe12O19 were computed via Debye–Scherrer equation and Williamson–Hall method. The computed crystallite size of BaFe12O19 and SrFe12O19 nanoparticles is in agreement with the observed SEM grain size. Kinetic roughening of BaFe12O19- and SrFe12O19-polyaniline composite films was anomalous scaling treatment. The magnetic loop indicated that BaFe12O19, SrFe12O19, and BaFe12O19-polyaniline are ferromagnetic, but SrFe12O19-polyaniline is super-paramagnetic.
Experimental method
Preparation of hexaferrite nanoparticles
Strontium nitrate, barium nitrate, iron nitrate, aniline, and sodium hydroxide were purchased from Sigma-Aldrich and Merck (Germany) and all materials have a purity above 99%. For synthesized strontium ferrite, 0.01 mol of strontium sulfate hydrate and 0.12 mol of iron nitrate were dissolved in 200 mL of deionized water. The solution was placed under microwave irradiation (900 W, on: 30 s, off: 30 s) for 30 min. Then, the pH of the prepared solution was increased to 10 by 20 mL of sodium hydroxide (1 M) solution. After that the solution was microwave irradiated for 10 min. 0.01 mol of Ba(NO3)2 and 0.12 mol of Fe(NO3)3‧9H2O were added to 50 mL of deionized water. By adding 15 mL of 1 M NaOH solution, alkalinity was increased to 10. The solution was placed under microwave irradiation (900 W, on: 30 s, off: 30 s) for 30 min. After precipitation and washing, the product was placed at 800°C for 2 h.
For sono-chemical synthesis, all chemical precursors were used as the same as microwave irradiation with the difference that ultra sound irradiation (400 W, 30 min) was used instead of microwave irradiation.
Polyaniline/barium ferrite and strontium ferrite composites
Aniline thin layers were deposited on copper substrate by cyclic voltammetry technique in various number of cycles from 5 to 20. Sulfuric acid (0.2 M) solution and aniline (0.1 M) were used as electrolyte. The voltage applied at this stage ranged from −0.2 to 1.2 V. The substrates were prepared by two-step mechanical and electrochemical polishing. For electroporation, 0.1 g of each ferrite were added to 100 mL of electrolyte
-
Funding information: The project was financially supported by the Ministry of Science, Research and Technology, under grant number 299901000108.
-
Author contributions: Farzaneh Sharifi: writing – original draft, and formal analysis; Kambiz Hedayati: writing – original draft, writing – review and editing, formal analysis, visualization, and project administration; Davood Ghanbari: writing – original draft, writing – review and editing, methodology, and formal analysis.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All relevant data are included in the article.
References
Abbasi L., Hedayati K., Ghanbari D., Magnetic properties and kinetic roughening study of prepared polyaniline: lead ferrite, cobalt ferrite and nickel ferrite nanocomposites electrodeposited thin films. J Mater Sci: Mater Electron., 2021, 32, 14477–14493.10.1007/s10854-021-06006-1Search in Google Scholar
Abdelraheem A., El-Shazly A.H., Elkady M.F., Characterization of atypical polyaniline nano-structures prepared via advanced techniques. J. Alex. Eng. J., 2018, 57, 3291–3297.10.1016/j.aej.2018.01.012Search in Google Scholar
Ahmadian-Fard-Fini S., Salavati-Niasari M., Ghanbari D., Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim. Acta A., 2018, 203, 481–493.10.1016/j.saa.2018.06.021Search in Google Scholar PubMed
Davar F., Salavati-Niasari M., Fereshteh Z., Synthesis and characterization of SnO2 nanoparticles by thermal decomposition of new inorganic precursor. Journal of Alloys and Compounds, 2010, 496(1–2), 638–643.10.1016/j.jallcom.2010.02.152Search in Google Scholar
Ebrahimi Z., Hedayati K., Ghanbari D., Preparation of hard magnetic BaFe12O19–TiO2 nanocomposites: applicable for photo-degradation of toxic pollutants. Journal of Materials Science: Materials in Electronics, 2017, 28, 13956–13969.10.1007/s10854-017-7245-3Search in Google Scholar
Fleaca C.T., Dumitrache F., Morjan I., Niculescu A.M., Sandu I., Ilie A., et al., Synthesis and characterization of polyaniline–Fe@C magnetic nanocomposite powder. Appl. Surf. Sci., 2016, 374, 213–221.10.1016/j.apsusc.2015.11.043Search in Google Scholar
Gholami T., Salavati-Niasari M., Varshoy S., Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method, International Journal of Hydrogen Energy, 2017, 42(8), 5235–5245.10.1016/j.ijhydene.2016.10.132Search in Google Scholar
Hajian Karahroudi Z., Hedayati K., Goodarzi M., Green synthesis and characterization of hexaferrite strontium-perovskite strontium photocatalyst nanocomposites. Main Group Met. Chem., 2020, 43, 26–42.10.1515/mgmc-2020-0004Search in Google Scholar
Hassanpour M., Safardoust-Hojaghan H., Salavati-Niasari M., Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite. Journal of Molecular Liquids, 2017, 229, 293–299.10.1016/j.molliq.2016.12.090Search in Google Scholar
He Z., Qi S., Zhong X., Ma H., Wang P., Qiu H., Preparation and microwave-absorbing properties of silver-coated strontium ferrite with polyaniline via in situ polymerization. Journal of Alloys and Compounds, 2015, 621, 194–200.10.1016/j.jallcom.2014.09.187Search in Google Scholar
Hedayati K., Structural and magnetic characterization of electrodeposited Ni–Cu/Cu and Fe–Ni–Cu/Cu multilayer. Applied Physics A., 2015, 118, 975–979.10.1007/s00339-014-8851-zSearch in Google Scholar
Lahijani B., Hedayati K., Goodarzi M., Magnetic PbFe12O19–TiO2 nanocomposites and their photocatalytic performance in the removal of toxic pollutants. Main Group Met. Chem., 2018, 41(3–4), 53–62.10.1515/mgmc-2017-0055Search in Google Scholar
Masoumi, S., Nabiyouni, G., Ghanbari, D., Photo-degradation of azo dyes: photo catalyst and magnetic investigation of CuFe2O4–TiO2 nanoparticles and nanocomposites. J. Mater. Sci. Mater.Electron., 2016, 27, 9962–9975.10.1007/s10854-016-5067-3Search in Google Scholar
Meng X., Xu S., Zhou J., Tang Q., Monodisperse hexagonal SrFe12O19 nanoflake with enchanced magnetic properties. Ceram. Int., 2016, 42(5), 6025–6032.10.1016/j.ceramint.2015.12.156Search in Google Scholar
Monsef R., Salavati-Niasari M., Electrochemical sensor based on a chitosan-molybdenum vanadate nanocomposite for detection of hydroxychloroquine in biological samples. J. Colloid Interface Sci., 2022, 613, 1–14.10.1016/j.jcis.2022.01.039Search in Google Scholar PubMed
Monsef R., Ghiyasiyan-Arani M., Salavati-Niasari M., Design of Magnetically Recyclable Ternary Fe2O3/EuVO4/g-C3N4 Nanocomposites for Photocatalytic and Electrochemical Hydrogen Storage. ACS Appl. Energy Mater., 2021, 4(1), 680–695.10.1021/acsaem.0c02557Search in Google Scholar
Nautiyal A., Parida S., Comparison of polyaniline electrodeposition on carbon steel from oxalic acid and salicylate medium. J. Progr. Org. Coat., 2016 94, 28–33.10.1016/j.porgcoat.2016.01.014Search in Google Scholar
Panahi-Kalamuei M., Alizadeh S., Mousavi-Kamazani M., Salavati-Niasari M., Synthesis and characterization of CeO2 nanoparticles via hydrothermal route. J. Ind. Eng. Chem., 2015, 21, 1301–1305.10.1016/j.jiec.2014.05.046Search in Google Scholar
Sadeghi M. M., Shokuhi Rad A., Ardjmand M., Mirabi A., Preparation of magnetic nanocomposite based on polyaniline/Fe3O4 towards removal of lead (II) ions from real samples. J. Synth. Met., 2018, 245, 1–9.10.1016/j.synthmet.2018.08.001Search in Google Scholar
Sadeghpour F., Nabiyouni G., Ghanbari D., Simple synthesis of conductive poly aniline/cobalt ferrite magnetic nanocomposite: its radio waves absorption and photo catalyst ability. J. Cluster Sci., 2022, 33, 1257–1266.10.1007/s10876-021-02057-wSearch in Google Scholar
Song Y., Liu S., Wang B., Shang M., Lin L., Su Y., Continuous and controllable preparation of polyaniline with different reaction media in microreactors for supercapacitor applications. J. Chem. Eng. Sci., 2019, 207, 820–828.10.1016/j.ces.2019.07.008Search in Google Scholar
Xia A., Zuo C., Chen L., Jin C., Lv Y., Hexagonal SrFe12O19 ferrites: hydrothermal synthesis and their sintering properties. J. Magn. Magn. Mater., 2013, 332, 186–191.10.1016/j.jmmm.2012.12.035Search in Google Scholar
Zinatloo-Ajabshir S., Mortazavi-Derazkola S., Salavati-Niasari M., Nd2O3–SiO2 nanocomposites: A simple sonochemical preparation, characterization and photocatalytic activity. Ultra Sonochem., 2018, 42, 171–182.10.1016/j.ultsonch.2017.11.026Search in Google Scholar PubMed
Zinatloo-Ajabshir S., Salavati-Niasari M., Preparation of magnetically retrievable CoFe2O4@SiO2@Dy2Ce2O7 nanocomposites as novel photocatalyst for highly efficient degradation of organic contaminants. Comp Part B: Eng., 2019, 174, 106930.10.1016/j.compositesb.2019.106930Search in Google Scholar
© 2022 Farzaneh Sharifi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure
Articles in the same Issue
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure

