Abstract
Rising environmental issues and huge disposal of rubber waste have resulted in an increased interest in the usage of reclaimed rubber (RR) to produce sustainable and environmental friendly applications. In this study, incorporation of butyl reclaimed rubber into carboxylated nitrile butadiene rubber (XNBR/BRR) was carried out where the loadings of each XNBR and BRR were varied. The rubber was cured with sulphur during the melt mixing using a two-roll mill. This study is aimed to investigate the applied BRR loading towards the physical and mechanical properties of the XNBRcompounds. The results appeared that the optimum cure time (T90) increased and curing rate index (CRI) showed that a faster curing reaction with the increase in the content of BRR where the T90 had an increment of 89% while the CRI was faster by 89%. As for the crosslink density which indicates the density of chains or segments in polymer network, it decreased about 20% with increasing level of BRR. Also, the compression set which refers to the ability of rubber to resist permanent deformation, had an increase of 73% as the loading ratio of BRR increased. These results were obtained due to the low molecular weight of the BRR where the high shear and temperature during the reclamation process severely breaks down the molecular chains of the BRR into shorter segments. With the results obtained, it is shown that reclaimed rubber has the potential for further development and could increase the interest of researchers all around the world in the incorporation of reclaimed rubber for footwear application.
1 Introduction
Polymer blending is one of the effective techniques to create new materials with better performance and properties than existing polymers. This is because polymer blending has a great influence on the mechanical properties of polymers. Polymer blending involves blending of two or more types of rubber where this is a widely used method to prepare materials with superior properties, which are not present in the component rubber [1, 2, 3]. Polymer blending is usually cheaper and consume less time for the production of polymeric materials with new properties compared to the development of new monomers and/or new polymerization methods. Another advantage of polymer blend is that the properties of the materials can be formulated by combining component polymers and changing the blend composition. The blends can be varied depending on the required properties and the specific application [4]. This is because the blending of different rubbers could achieve the required performance properties in the end products [5]. Products such as conveyor belts, tires and shoe soles would need optimized formulations made from different components as they are the sort of application which would acquire a wide range of properties [6]. There have been a few previous studies conducted on polymer blending. Examples of polymer blending are NR/TRR [7] and NBR/NRR [8]. In order to meet the demands and needs of consumers, research on synthetic elastomer materials including co- and terpolymers such as carboxylated nitrile butadiene rubber (XNBR) has increased rapidly and steadily [9]. Carboxylated nitrile butadiene rubber (XNBR) is a terpolymer composed of nitrile, butadiene and monomers which contains carboxyl groups such as methacrylic acids [10]. XNBR contains different active functional groups including nitrile groups (-CN), carboxylic groups (COOH) and alkene groups. All these groups can participate in the crosslinking reaction, which leads to the formation of different types of bonds, mainly covalent and ionic bonds. Lateral carboxyl functionalities (-COOH) provides more sites of curing and making it possible to use curing agents that can react with them which will then contribute to the formation of ionic bonds. Incorporation of carboxyl groups increases inter and intra molecular interactions, resulting in improved properties of the polymer where XNBR polymer is proven to have higher strength, tear, modulus and abrasion resistance. XNBR also has excellent oil and solvent resistance properties [11]. XNBR is often recommended for applications that require good heat, oils and abrasion resistance [12]. Improved mechanical and electromechanical properties of XNBR based dielectric elastomer actuator were achieved in a 2019 study where the XNBR composites had better filler dispersion and enhanced dielectric strength [13]. In another previous study, XNBR was simultaneously introduced to two types of nanofillers (aluminosilicate and carbon nanofillers) which resulted in excellent resistance to tearing, puncture and tensile strength [14]. Butyl reclaimed rubber is selected as one of the ingredients in this study as it is blended with XNBR to develop a rubber-based product such as for footwear application. This makes it sustainable as rubber was recycled to produce the product. Therefore this could help reduce abundance of polymer waste especially waste from rubber tubes which could be used for other sorts of useful applications. Environmental safety and pollution issues such as breeding of mosquitoes and air pollution created from the burning and disposal of rubber waste can be prevented [15, 16]. Looking at waste rubber from discarded tires alone, the amount of tire rubber waste has increased greatly and keeps rising until today. Rising rubber waste has become a worrying matter and a threat to our natural environment which leads to economical alternatives where used up rubber products are recycled [18]. This is due to rubber's very low decomposition rate as rubber contains presence of crosslinking agents, stabilizers and additives [19]. These rubber wastes are recycled to produce other beneficial products besides protecting the environment and renewable resources. Reclaimed rubber is a type of rubber that is obtained from vulcanized scrap rubber. Reclaimed rubber is in fact a mixture of rubber, carbon black, oil, zinc oxide, stearic acid and other compounding ingredients used in the original compounds [20, 21, 22]. It can be obtained from flashing or from recycled rubber products such as rubber tires and hoses by grinding and then treat them with alkali, oil and plasticizer to soften them again. In order to make the vulcanized rubber into a re-processable material, it is important that the links between and partly within the polymer are broken. Among many waste polymers, the utilization of reclaimed rubber has become one of the most prospective routes [23]. Judging from the economical and environmental advantages of reclaimed rubber, rubber goods manufacturers including the footwear and automotive industries have been rapidly utilizing reclaimed rubber in their productions [24]. Reclaimed rubber has been used in multiple studies before this. A study was done where application of natural rubber gloves reclaimed to replace virgin natural rubber [16]. Broad use of butyl rubber material in modern industries is attributed by its excellent damping properties and performance in air tightness [25]. Butyl reclaimed rubber is one of the types of reclaimed rubber that is obtained by reclaiming butyl inner tubes. Butyl reclaimed rubber is known for its low buildup during processing and high rate of curing properties. In this study, we exploited the addition of butyl reclaimed rubber (BRR) in carboxylated nitrile butadiene rubber (XNBR) to study the effect of varying reclaimed rubber loading on the final properties of the XNBR/BRR blend. This study aims to develop rubber blends with incorporation of reclaimed rubber which could be used to produce other types of rubber-based products at a cheaper cost, without diminishing the mechanical properties. Hence, providing an alternative in the efforts of rubber recycling and reducing ecological impact on the environment by incorporating reclaimed rubber as partial or full replacement of virgin rubber in formulations, the mechanical properties of the blend were then enhanced by reinforcing filler: carbon black. This is a simple, sustainable, environmentally friendly, low-cost, and effective way that provides a promising method to obtain a high-performance rubber blend with improved mechanical properties. Therefore, a suitable approach could be achieved to the circular economy of rubber waste's abundance.
2 Materials and methods
2.1 Materials
There were a few materials used in this study which were carboxylated nitrile butadiene rubber (XNBR), butyl reclaimed rubber (BRR), zinc oxide (ZnO), stearic acid, benzothiazyl disulfide (MBTS), dipropylene glycol (DPG), tetramethylthiuram disulfide (TMTD), polymerized 2,2,4-trimethyl-1,1dihydroquinoline (TMQ) and sulphur. The carboxylated nitrile butadiene rubber XNBR used in this study is Krynac X 750 (7 wt% carboxyl groups content, 27 wt% nitrile contents). Carbon black used as the reinforcing filler in the polymer blend was high abrasion furnace (HAF), N330 with a particle size of 2836 nm and an average surface area of 76–80 m2/g. BRR800 was kindly supplied by Yong Fong Sdn. Bhd [3]. While XNBR was obtained from Aras Bakti Ventures. Both BRR and XNBR were used as received.
2.2 Preparation of XNBR/BRR blends
XNBR was blended with BRR in various proportions and were labelled as Blend 1, Blend 2, Blend 3, Blend 4, Blend 5, Blend 6, Blend 7, Blend 8 and Blend 9. The uniform blending of rubber is performed in an internal mixer and milling is carried out by using a two roll mill (Qingdao Shun Cheong Machinery Co., Ltd., Qingdao, China). Prior to vulcanization, the rubber blend is compounded with zinc oxide, calcium carbonate, stearic acid, and antioxidant TMQ. The blend was then mixed with sulphur, MBTS, DPG and TMTD, with a two-roll mill. The compounded rubber stocks are then placed in a mould and pressed between the heated platens of a hydraulic press at 150 °C to be cured at the specific time which are based on the cure characteristics results. After that, crosslink density and compression set are measured. The composition of each blends used for this study are presented in Table 1. The formulation was fabricated based on a previous study done in 2018 [20].
XNBR/BRR blend formulations.
| Ingredients | Amount (*phr) / Blend no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| XNBR | 100 | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 0 |
| BRR | 0 | 16 | 32 | 48 | 65 | 81 | 97 | 113 | 161 |
| CB | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Stearic acid | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| MBTS | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| DPG | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| TMTD | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| TMQ | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Sulphur | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
- *phr:
parts per hundred
2.3 Testing and characterization
2.3.1 TGA test
BRR 800 rubber sample was subjected to thermal analysis. The thermogravimetric analysis (TGA) was made using a TGA instrument STA 6000, from Perkin Elmer and heated in 50–850 °C temperature range at a rate of 20° C/min under a controlled dry nitrogen flow of 20ml/min.
2.3.2 Rheological test
A rheometer test was conducted and this was done before curing the blends in the press, to evaluate whether it was needed to carry out a readjustment of the prepared formulation. Rheological characteristics were studied at a temperature of 150 ± 2 °C and a pressure of 0.2 MPa using a Monsanto Moving Die Rheometer with about 4g of sample of the respective compounds.
2.3.3 Swelling measurement test
Each rubber blends in the presence of toluene and acetone are used for the determination of crosslink density. This test was carried out in accordance to ASTM D6814 (2018). Samples approximately 0.2–0.25 g are weighed, which was recorded as the initial weight and were then into bottles filled with 10 ml of pure toluene. The samples were allowed to swell in toluene for 24 hours at room temperature. The toluene was then replaced with a new set of toluene and put aside for another 24 hours. After 24 hours, the swollen samples were taken out and weighed. Acetone was next added into the bottle for about 30 minutes. The samples were left to dry until the next day. By using the Flory-Rehner equation, crosslink density was able to be calculated. The Flory-Rehner equation is shown below (Equation 1–3):
Note that ρr is the rubber density, Vs is the molar volume of toluene, Vr is the volume fraction of the rubber in the swollen specimen, Qm is the weight increase of the vulcanizate in toluene and κ is the interaction parameter of the rubber network-solvent, Vc is the crosslink density in mol per cubic centimeter (mol/cm3).
2.3.4 Compression set test
Compression set testing measures the ability of rubber to return to its original thickness after prolonged compressive stresses at a given temperature and deflection. Compression set test applies stresses to the sample over a specified period of time in order to measure elasticity. The compression set test was conducted in accordance with ISO 815 (2008) method where the samples were subjected to a prolonged compression at a constant strain of 25%, at a temperature of 70 °C, for about 22 hours. Compression set results for a material are expressed as a percentage. The lower the percentage, the better the material resists permanent deformation under a given deflection and temperature range.
3 Results and discussion
3.1 Thermal behavior of BRR
Thermogravimetric analysis (TGA) was carried out to evaluate the amount of polymer content, filler content and the ash content of uncompounded BRR 800 alone. Thermogravimetric curve of BRR 800 is presented in Figure 1 and this curve represents the percentage weight of sample's contents as a function of time. The remarkable stability of BRR800 at high temperatures was observed. The decomposition occurs in three steps. BRR 800 showed more than one degradation stages due to the comparable presence of polymer, carbon black and ash residues [26, 27]. The step at approximately 436.3 °C with 62.2 % of weight loss can be attributed to degradation of butyl rubber which corresponds to thermal decomposition of BRR [1], [28]. The second step at about 871.6 °C with 33.7 % of weight loss, correspond to thermal decomposition of carbon black. The remaining residues such as ash content represent about 4.0 % in the mass of the starting material. Reclaimed rubber contains carbon black that would stay as residue in an atmosphere filled with nitrogen [29]. Based on the TGA result reflected in Figure 1, the filler content of the reclaimed rubber which refers to carbon black are leached out. Therefore, leading to thermal stability loss of the reclaimed rubber network. This proves that when high temperature is subjected to the BRR800, a great quantity of thermal energy is being provided to the BRR800 sample which is enough and sufficient for rubber decomposition process. Since we are dealing with butyl reclaimed rubber, it is noteworthy to know that butyl rubber is known for having low permeability to gases, which could hinder the interaction of polymeric matrix with its surrounding. This supports the usage of high temperature for thermal degradation of the BRR800. At temperature range of approximately 350–500 °C, steady weight percentage loss of BRR800 sample was observed. This could be due to the gaseous components formed as a result of decomposition process [30]. A previous study was conducted to study the degradation of butyl rubber under different conditions which are thermal, thermos-oxidative and induced radiation [28]. It is said to be important to have the knowledge of a polymer's limitation, especially when it comes to the degradation under multiple conditions so that the full potential of the polymer's application can be known. Esmizadeh also reported that blends with higher loading of reclaimed rubber would degrade at earlier stages compared to blends containing lower loading of reclaimed rubber [31]. The bond formed between the polymer molecular chain and fillers restricts the movement of rubber molecular backbone and prevents the development of degradation, hence causing the process of thermal degradation to be slowed down [32]. Inorganic residue would gradually increase when the reclaimed rubber content increases. High weight percentage of inorganic residue could be due to the high amount of remaining ashes present in the reclaimed rubber. According to the results of thermal analysis done on BRR800 sample, BRR800 has a polymer content of 62.2%, filler content of 33.7% and ash content of 4%. Since BRR800 is not entirely made of rubber, therefore the proportion of reclaimed rubber (BRR) incorporated for each blends needed to be higher compared to the proportion of virgin rubber (XNBR) which explains the formulation of XNBR/BRR blends in Table 1.
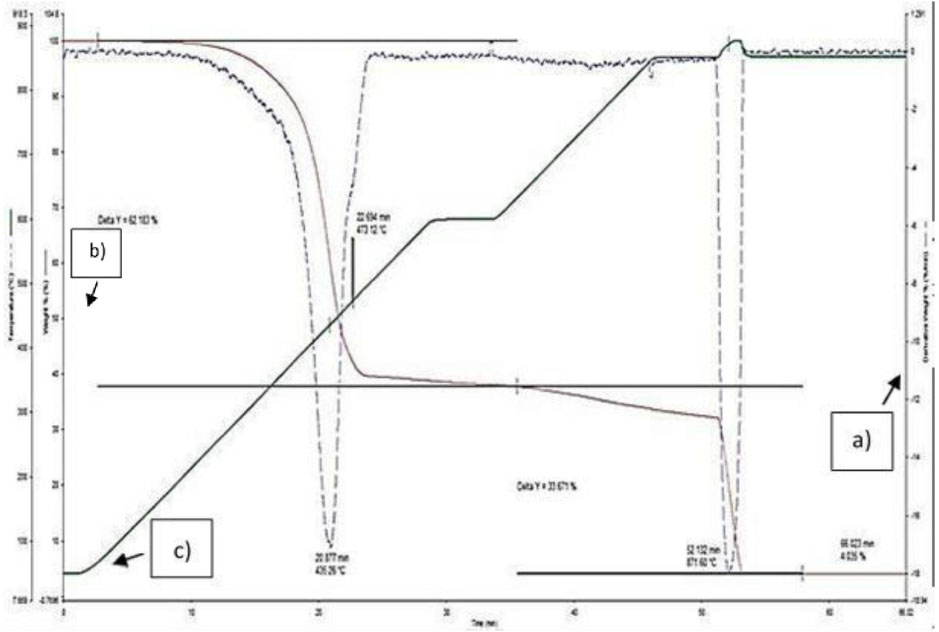
TGA curve of BRR800, a) Derivative weight percentage of the BRR800 sample (%/min), b) Weight percentageof the BRR800 sample (%), c) Temperature (°C): Held at 50 °C for 1 minute, heated from 50 °C to 600 °C at 20 °C/min, held at 600 °C for 5 minutes, heated from 600 °C to 850 °C at 20 °C/min, held at 850 °C for 20 minutes.
3.2 Rheological characteristics of XNRB/BRR blends
Table 2 shows the cure characteristics of XNBR/BRR blends while Figure 2 presents the variation in the normalized elastic (S’) components of the torque versus time. The data and curves were obtained from a MDR rheometer at 150°C. From the rheometer test, there were a few data obtained including S’min, S’max, delta torque (ΔS’), TS2, T90, T95 and cure rate index (CRI). Referring to Table 2, no results were obtained for Blend 1, Blend 2 and Blend 3. Due to having high ratio of XNBR, the end compound was not successful as it got too hard. From a previous study, XNBR is said to be a high wearing polymer where it naturally has chemical resistance, high abrasion and most importantly high hardness. Due to XNBR's nature, higher levels of XNBR in blends will results in higher modulus, tensile strength and hardness. However, this can be a limitation as the blends can be too hard causing them to be difficult for compression and testing. According to the rheometer results, the increase in BRR800 content in XNBR/BRR blends result in an increased value of minimum torque, S’ min and value of maximum torque, S’ max. The increase of S’ min values indicate that the processing of XNBR/BRR blends become harder. From the values of T90 it can be concluded that the optimum curing time slightly increases with the increase in the amount of BRR800. The cure rate index (CRI) shows that a faster curing reaction was achieved as the content of BRR800 increases. Blend 4 has the highest maximum torque with the value of 10.53 dNm. Maximum torque can be expressed as measure of stiffness of rubber, hence Blend 4 has the most stiffness compared to other blends. As for the scorch time, Ts2, each blends has almost similar scorch time except for Blend 9 which consist of 161 phr of BRR. Since Blend 6 is only BRR alone, a longer scorch time is needed so that the BRR to be partly vulcanized. Scorch time is the period before vulcanization and it is necessary that vulcanization does not start until scorching process is complete. Blend 4 also has the highest cure rate index (CRI) among the blends, hence Blend 4 would require the most amount of time for complete curing process. The rate of cure is where the crosslinking and development of modulus of the compound occur after scorch point.
Rheometric properties of XNBR/BRR blends.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| S’ min (dNm) | – | – | – | 1.97 | 2 | 2.06 | 2.79 | 2.17 | 0.94 |
| S’ max (dNm) | – | – | – | 10.53 | 9.56 | 8.6 | 7.72 | 6.59 | 6.22 |
| ΔS’ (dNm) | – | – | – | 8.56 | 7.56 | 6.54 | 4.93 | 4.42 | 5.28 |
| Ts2 (min) | – | – | – | 0.89 | 0.86 | 0.89 | 1.3 | 1.28 | 8.38 |
| T90 (min) | – | – | – | 2.58 | 2.81 | 4.51 | 4.58 | 4.51 | 24.67 |
| T95 (min) | – | – | – | 8.15 | 8.65 | 10.03 | 10.1 | 9.93 | 27.2 |
| CRI (min−1) | – | – | – | 59.17 | 51.28 | 27.62 | 30.49 | 30.96 | 6.14 |

Normalized elastic component (S’) of the torque as a function of time for XNBR/BRR blends crosslinked at 150 °C.
This is when the polymer chains are connected firmly and modulus increases as more crosslinks are formed. As for Figure 2, it shows the influence of an increase in the amount of loading of BRR800 on the curing curves of the XNBR/BRR blends.
Based on Table 2, the difference between maximum torque S’max and minimum torque S’min which is also known as the torque increment, gradually decreases with the increasing content of BRR800. This is the reflection on the reduction of crosslink density, hence this will cause the mechanical properties of the blend to be affected where the blends would have low mechanical properties. However, it can be seen in Figure 2 that a plateau was reached faster for Blend 4 where the XNBR/BRR blend is in the ratio of 70/48 during the analyzed reaction time. As curing starts, the torque increases proportionately. After an amount of time, the torque typically attains maximum value and it plateaus out. This is called a plateau curve. An increasing torque could indicate that crosslinking dominates and when the torque reaches plateau, this means that the curing of the blend is completed and stable network is formed in the blend itself.
3.3 Crosslink density test
The test failed for Blend 1, Blend 2 and Blend 3 due to the reasons stated earlier. Based on the results on Figure 3, it can be seen that as the loading of BRR800 increases, the crosslink density seems to be decreasing. The crosslink density results show the poor reinforcement effect of high BRR800 contents in the XNBR/BRR blends. The blends with higher ratio of reclaimed rubber have low crosslink density due to the fact that chain scission during the reclaiming process of BRR800 has lowered the molecular weight of the reclaimed rubber itself [33]. The breakdown of crosslink and polymer chains cause this to occur and affecting the molecular-weight distribution. Hence crosslink density is dependent to the loading of reclaimed rubber added to the blends. The difference of crosslink density of blends was manifold reflecting the high strength of the blend with lower reclaimed rubber loading. For the filler-filled rubbers, it is expected that the rubber blends to be less stiff and more penetrable by solvent due to the reduced crosslink density effect. This explains the rheometric properties obtained in Table 2 where the variation of (maximum minimum) torque gradually decreases with the increase of BRR800 loading. This indicates that the interaction between the blend components did not improve due to the decrease in the crosslink density of the blends [34]. This also corresponds with the lower magnitude of elastic torque plateau shown in Figure 2, which foresees a lower crosslink density level. Therefore, a softer material. The lower level of crosslinking at higher reclaimed rubber loading can also be because of the reclaimed rubber is already partially crosslinked.
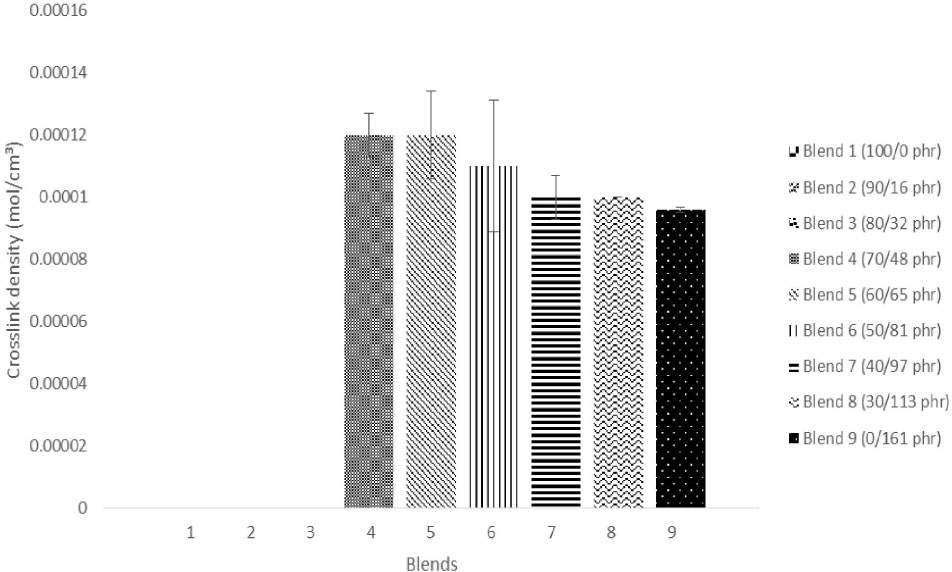
Crosslink density of XNBR/BRR blends.
3.4 Compression set test
Compression set is the ability of cured rubber to return back to its original shape after given a certain deformation and it can be determined as the ratio of elastic to viscous part of a rubber resulting from the deforming force acted upon it [37]. The compression set of the XNBR/BRR blends increased progressively as the loading of BR800 was raised which can be seen in Figure 4. When being tested, the blends containing higher reclaimed rubber loading flow more, causing higher compression set. When the crosslink density has been previously determined in Figure 3, as expected there was a correlation between crosslink density and compression set. The determination of crosslink density is important as crosslink density affects mechanical, dynamic and performance properties. Properties such as hardness and modulus will decrease when crosslink density decrease while compression set will increase. Such compression set values was obtained due to insufficient crosslinking where less reaction sites are available for further crosslinking to take place [34]. The presence of the crosslink could have limited the magnitude of equilibrium swelling. The usage of diluent during crosslinking test is only able to partially disentangle a part of the polymer chain especially if higher loading of reclaimed rubber is incorporated. Hence lower crosslink density results is obtained [35]. Based on a previous study, it was observed that good values of mechanical properties could be achieved when the crosslink density is approximately 1 × 10−4 mol/cm2 [36]. These results also correspond with data reported in another study showing that the vulcanizates or blends that contain butyl rubber exhibit around the same value of crosslink density [37].

Compression set of XNBR/BRR blends.
Since the number of crosslinks responsible for compression resistance is less, therefore the rubber blends do not recover perfectly to its original shape and form. Higher value of compression set in blends is the result of the low crosslink density [38]. The higher crosslink density and amount of stable crosslinks enhanced the compression set of blend due to its stable structure. Based on a previous study, indentation measurement can be high dependent to crosslinking degree, especially when it comes to unfilled compounds. It can be said that a reduction in crosslink density results in a less rigid rubber matrix, hence lower hardness and high compression set percentage [39]. This result is within the expectations of mechanical property loss, where high compression set percentage is obtained, which supports the reduction of crosslink density of the blends. The poor formed crosslink network is not strong enough to uphold and resist the mechanical force applied to the blend sample [40]. Moreover, when subjected to a mechanical force, there are relative slippage between molecules. Hence, it can be said a low crosslink density contributes to poor mechanical properties. From Figure 4, it can be seen that the increase of compression set percentage of the XNBR/BRR blends could be a result from the formation of new crosslinks by free curative residues in the vulcanizate. When a rubber compound is subjected to deformation, the rubber chains are either strained or compressed causing a change in their arrangements. If new crosslinking is formed during the time of the rearrangement, this could lead to a permanent deformation [41]. Although there is presence of carbon black in the BRR800 beforehand and carbon black is the reinforcing filler used in this study, the increasing loading of BRR800 resulted in decreasing value of compression set where the mobility of rubber chains in the blends is reduced and stiffness is induced in the filled blends [42]. The resulted compression set values coincide with the generally accepted trends of properties due to the changing of crosslink density as commonly reported by previous studies [43]. Another reason that can cause this to happen is the poor strength of the low molecular weight of BRR800. Rubber compounds that have low compression set would generally be excellent in elastic recovery [44]. In addition, based on a previous study, the maximum recommended value for compression set percentage of vulcanized rubber for shoe sole application is 30% [20]. Referring to the results in Figure 4, half of the blends comply with the maximum recommended value. Findings of Blend 4, 5 and 6 are far below the maximum value by approximately 43%, 37% and 20%.
4 Conclusions
In conclusion, it can be deduced that reclaimed rubber content affects the curing characteristics, crosslink density and compression set of the blends due to its low molecular weight. Moreover, the chain scission during the reclaiming process of reclaimed rubber that causes the decrease of molecular weight. Among the blends, Blend 4 is the best blend as it shows high crosslink density and low compression set which might be suitable for a shoe sole application. Hence, if Blend 4 were to be introduced into the market, the cost of production could be reduced by approximately 43%. With further development, reclaimed rubber can not only be used to make various applications such as playground safety floorings, car mat and conveyor belts too. These reclaimed rubbers can be future materials for the production of environmental friendly applications.
Acknowledgement
The authors would like to thank all the staff at the Rubber Research Institute of the Malaysian Rubber Board for the assistance received in carrying the preparation of the samples and the tests. And also thank Yong Fong Rubber Industries to supply the reclaimed rubbers.
Patents:
This research has been awarded a patent by the Intellectual Property Corporation of Malaysia (MyIPO).
Funding information:
The authors state no funding involved.
Author contributions:
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Conflict of interest:
The authors state no conflict of interest.
Patents:
Some data that supports the findings of this study are deposited in a data repository and will be made available by submitting the request to the corresponding author.
References
[1] Karmanova OV, Tikhomirov SG, S. Kayushnikov SN, Shashok ZS, Polevoy PS. Obtaining and using of reclaimed butyl rubber with the use of ionizing radiation. Radiation Physics and Chemistry. 2019; 159:154–8.10.1016/j.radphyschem.2019.02.038Suche in Google Scholar
[2] Mamauod SNL, Romli AZ, Rizuan MIR. Synergistic effect of nano calcium carbonate (NCC)/carbon black (CB) on the cure characteristics and physico-mechanical properties of NR/SBR blends. AIP Conference Proceedings. 2017; 1885:020028.10.1063/1.5002222Suche in Google Scholar
[3] Abidin ZZ, Mamauod SNL, Sarkawi SS, Saimi NSM. Influence of filler system on the cure characteristics and mechanical properties of butyl reclaimed rubber. BioResources. 2020; 15:6045–60.10.15376/biores.15.3.6045-6060Suche in Google Scholar
[4] Saiwari S, Lohyi E, Nakason C. Application of NR Gloves Reclaim: Cure and Mechanical Properties of NR/Reclaim Rubber Blends. Advanced Materials Research. 2013; 844:437–40.10.4028/www.scientific.net/AMR.844.437Suche in Google Scholar
[5] Kaliyathan AV, Varghese K, Nair AS, Thomas S. Rubber-rubber blends: A critical review. Progress in Rubber, Plastics and Recycling Technology. 2019; 36:196–242.10.1177/1477760619895002Suche in Google Scholar
[6] Şaşmaz S, Karaağaç B, Uyanık N. Utilization of chrome-tanned leather wastes in natural rubber and styrene-butadiene rubber blends. Journal of Material Cycles and Waste Management. 2018; 21:166–75.10.1007/s10163-018-0775-9Suche in Google Scholar
[7] Rattanasom N, Poonsuk A, Makmoon T. Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polymer Testing. 2005; 24:728–32.10.1016/j.polymertesting.2005.04.008Suche in Google Scholar
[8] Morsi SM, Mohamed HA, El-Sabbagh S H. Polyesteramidesulfone as novel reinforcement and antioxidant nanofiller for NBR blended with reclaimed natural rubber. Materials Chemistry and Physics. 2019; 224:206–16.10.1016/j.matchemphys.2018.12.017Suche in Google Scholar
[9] Mali MN, Arakh AA, Dubey K, Mhaske S. Influence of triallyl cyanurate as co-agent on gamma irradiation cured high density polyethylene/reclaimed tire rubber blend. Radiation Physics and Chemistry. 2019; 131: 66–72.10.1016/j.radphyschem.2016.10.020Suche in Google Scholar
[10] Pal K, Pal SK, Das CK, Kim JK. Effect of fillers on morphological properties and wear characteristics of XNBR/NR blends. Journal of Applied Polymer Science. 2010; 120:710–8.10.1002/app.33234Suche in Google Scholar
[11] Paran SM, Naderi G, Mosallanezhad H, Movahedifar E, Formela K, Saeb MR. Microstructure and Mechanical Properties of Carboxylated Nitrile Butadiene Rubber/Epoxy/XNBR-grafted Halloysite Nanotubes Nanocomposites. Polymers. 2020; 12:1192.10.3390/polym12051192Suche in Google Scholar
[12] Ibarra L, Alzorriz M. Ionic elastomers based on carboxylated nitrile rubber (XNBR) and zinc peroxide: Influence of carboxylic group content on properties. Journal of Applied Polymer Science. 2002; 84:605.10.1002/app.10313Suche in Google Scholar
[13] Yang D, Kong X Ni Y, Ruan M, Huang S, Shao P, Zhang L. Improved mechanical and electrochemical properties of XNBR dielectric elastomer actuator by poly(dopamine) functionalized graphene nano-sheets. Polymers. 2019; 11:218.10.3390/polym11020218Suche in Google Scholar
[14] Krzemińska S, Lipińska L, Woluntarski M, Oleksy M, Ślusarczyk C, Biniaś W, Smejda-Krzewicka A. Hybrid XNBR composites with carbon and aluminosilicate nanofillers. Polymer Bulletin. 2019; 77:1749–80.10.1007/s00289-019-02825-9Suche in Google Scholar
[15] Movahed SO, Ansarifar A, Estagy S. Review of the reclaiming of rubber waste and recent work on the recycling of ethylene–propylene–diene rubber waste. Rubber Chemistry and Technology. 2016; 89:54–78.10.5254/rct.15.84850Suche in Google Scholar
[16] Chin KP, Wan NY, Mt Saad CS. Microcellular Rubber: A Study on Reclaimed Natural Rubber (NR) Latex Gloves/Standard Malaysian Rubber (SMR) 20 Blends. Pertanika J Sci & Technol. 2011; 19:171–6.Suche in Google Scholar
[17] Fang Y, Zhan M, Wang Y. The status of recycling of waste rubber. Materials & Design. 2001; 22:123–8.10.1016/S0261-3069(00)00052-2Suche in Google Scholar
[18] Zedler L, Przybysz M, Klein M, Saeb MR, Formela K. Processing, physico-mechanical and thermal properties of reclaimed GTR and NBR/reclaimed GTR blends as function of various additives. Polymer Degradation and Stability. 2017; 143:186–95.10.1016/j.polymdegradstab.2017.07.004Suche in Google Scholar
[19] Zhang X, Saha P, Cao L, Li H, Kim J. Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent Waste Management. 2018; 78:980–91.10.1016/j.wasman.2018.07.016Suche in Google Scholar PubMed
[20] Yuvaraj P, Rao JR, Fathima NN, Natchimuthu N, Mohan R. Complete replacement of carbon black filler in rubber sole with CaO embedded activated carbon derived from tannery solid waste. Journal of Cleaner Production. 2018; 170:446–50.10.1016/j.jclepro.2017.09.188Suche in Google Scholar
[21] Mandal SK, Alam N, Debnath SC. Reclaiming of ground rubber tire by safe multifunctional rubber additives: I. tetra benzyl thiuram disulfide. Rubber Chemistry and Technology. 2012; 85:629–44.10.5254/rct.12.88949Suche in Google Scholar
[22] Isayev AI, Kim SH, Levin VY. Superior Mechanical Properties of Reclaimed SBR with Bimodal Network. Rubber Chemistry and Technology. 1996; 70:194–201.10.5254/1.3538424Suche in Google Scholar
[23] A Hejna, M Klein, MR Saeb, K Formela. Towards understanding the role of peroxide initiators on compatibilization efficiency of thermoplastic elastomers highly filled with reclaimed GTR. Polymer Testing. 2019; 73:143–51.10.1016/j.polymertesting.2018.11.005Suche in Google Scholar
[24] X Zhang, TK Sinha, J Lee, Y Ahn, JK Kim. Temperature dependent amphoteric behavior of bis [3-(triethoxysilyl) propyl] tetrasulfide towards recycling of waste rubber: a triboeleltric investigation. Journal of Cleaner Production. 2019; 213:569–76.10.1016/j.jclepro.2018.12.149Suche in Google Scholar
[25] Xiang K, Huang G, Zheng J, Wang X, Huang J. Investigation on the thermal oxidative aging mechanism and lifetime prediction of butyl rubber. Macromolecular Research. 2012; 21: 10–16.10.1007/s13233-012-0174-3Suche in Google Scholar
[26] Singh RK, Ruj B, Jana A, Mondal S, Jana B, Sadhukhan AK, Gupta P. Pyrolysis of three different categories of automotive tyre wastes: Product yield analysis and characterization. Journal of Analytical and Applied Pyrolysis. 2018; 135:379–89.10.1016/j.jaap.2018.08.011Suche in Google Scholar
[27] Zedler L, Kowalkowska-Zedler D, Vahabi H, Saeb MR, Colom X, Cañavate J, Formela K. Preliminary Investigation on Auto-Thermal Extrusion of Ground Tire Rubber. Materials. 2019; 12:2090.10.3390/ma12132090Suche in Google Scholar PubMed PubMed Central
[28] Scagliusi SR, Cardoso EC, Lugao AB. Radiation induced degradation of butyl rubber vulcanized by three different crosslinking systems. Radiation Physics and Chemistry. 2012; 81:991–4.10.1016/j.radphyschem.2012.01.011Suche in Google Scholar
[29] Hejna A, Zedler L, Przybysz-Romatowska M, Cañavate J, Colom X, Formela K. Reclaimed Rubber/Poly (ɛ-caprolactone) Blends: Structure, Mechanical, and Thermal Properties. Polymers. 2020; 12:1204.10.3390/polym12051204Suche in Google Scholar PubMed PubMed Central
[30] Onoji SE, Iyuke SE, Igbafe AI, Daramola MO. Transesterification of Rubber Seed Oil to Biodiesel over a Calcined Waste Rubber Seed Shell Catalyst: Modeling and Optimization of Process Variables. Energy & Fuels. 2017; 31:6109–19.10.1021/acs.energyfuels.7b00331Suche in Google Scholar
[31] Esmizadeh E, Naderi G, Bakhshandeh GR, Fasaie MR, Ahmadi S. Reactively compatibilized and dynamically vulcanized thermoplastic elastomers based on high-density polyethylene and reclaimed rubber. Polymer Science, Series B. 2017; 59:362–71.10.1134/S1560090417030046Suche in Google Scholar
[32] Han R, Wang Z, Zhang Y, Niu K. Thermal stability of CeO2/graphene/phenyl silicone rubber composites. Polymer Testing. 2019; 75:277–83.10.1016/j.polymertesting.2019.02.027Suche in Google Scholar
[33] Qiu Y, Zhang A, Wang L. Carbon Black–Filled Styrene Butadiene Rubber Masterbatch Based on Simple Mixing of Latex and Carbon Black Suspension: Preparation and Mechanical Properties. Journal of Macromolecular Science, Part B. 2015; 54:1541–53.10.1080/00222348.2015.1103434Suche in Google Scholar
[34] Nelson PA, Kutty SKN. Effect of Silane Coupling Agent on Cure Characteristics and Mechanical Properties of Chloroprene Rubber/Reclaimed Rubber Blend. Polymer Plastics Technology and Engineering. 2004; 43:1141–56.10.1081/PPT-200030065Suche in Google Scholar
[35] Howse S, Porter C, Mengistu T, Pazur RJ. Experimental determination of the quantity and distribution of chemical crosslinks in unaged and aged natural rubber, part 1: Peroxide vulcanization. Polymer Testing. 2018; 70:263–74.10.1016/j.polymertesting.2018.07.002Suche in Google Scholar
[36] Stelescu M, Airinei A, Manaila E, Craciun G, Fifere N, Varganici C, Doroftei F. Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers. 2018; 10:1206.10.3390/polym10111206Suche in Google Scholar PubMed PubMed Central
[37] Allcock HR: Introduction to Materials Chemistry, Wiley, Hoboken, NJ, USA (2008).Suche in Google Scholar
[38] Movahed SO, Ansarifar A, Mirzaie F. Effect of various efficient vulcanization cure systems on the compression set of a nitrile rubber filled with different fillers. Journal of Applied Polymer Science. 2014; 132.10.1002/app.41512Suche in Google Scholar
[39] Lu Y, Zhang J, Chang P, Quan P, Chen Q. Effect of filler on the compression set, compression stress-strain behavior, and mechanical properties of polysulfide sealants. Journal of Applied Polymer Science. 2010; 120:2001–7.10.1002/app.33298Suche in Google Scholar
[40] Sutanto P, Picchioni F, Janssen LP, Dijkhuis KA, Dierkes WK, Noordermeer JW. EPDM rubber reclaim from devulcanized EPDM. Journal of Applied Polymer Science. 2006; 102:5948–57.10.1002/app.25153Suche in Google Scholar
[41] Bornstein D, Pazur R. The sulfur reversion process in natural rubber in terms of crosslink density and crosslink density distribution. Polymer Testing. 2020; 88:106524.10.1016/j.polymertesting.2020.106524Suche in Google Scholar
[42] Wang J, Pan S, Zhang Y, Guo S. Crosslink network evolution of BIIR/EPDM blends during peroxide vulcanization. Polymer Testing. 2017; 59:253–61.10.1016/j.polymertesting.2016.12.034Suche in Google Scholar
[43] Lee YS, Park SH, Lee JC, Ha K. Influence of microstructure in nitrile polymer on curing characteristics and mechanical properties of carbon black-filled rubber composite for seal applications. Journal of Elastomers & Plastics. 2016; 48:659–76.10.1177/0095244315613621Suche in Google Scholar
[44] Mostafa A, Abouel-Kasem A, Bayoumi M, El-Sebaie M. Effect of carbon black loading on the swelling and compression set behavior of SBR and NBR rubber compounds. Materials & Design. 2019; 30:1561–8.10.1016/j.matdes.2008.07.043Suche in Google Scholar
© 2021 Zafirah Zainal Abidin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- The elastic-plastic properties of an anti-icing coating on an aluminum alloy: Experimental and numerical approach
- Optimization of recycled slag-fresh flux mixture based upon weld bead quality for submerged arc welding of stainless steel
- Design and optimization of differential capacitive micro accelerometer for vibration measurement
- Mechanical performance of abrasive sandpaper made with palm kernel shells and coconut shells
- Experimental investigation of WEDM process through integrated desirability and machine learning technique on implant material
- Mechanical properties and microstructural characteristics of rotating arc-gas metal arc welded carbon steel joints
- Assessment of cement replacement with fine recycled rubber particles in sustainable cementitious composites
- Structural response and sensitivity analysis of granular and asphaltic overlayment track considering linear viscoelastic behavior of asphalt
- Unmanned aerial vehicle evasion manoeuvres from enemy aircraft attack
- Effect of corrosion on surface degradation of galvanized steel in poultry dung, pig dung and urea solutions using rice straw as an inhibitor
- Mathematical modeling of AZ30 magnesium alloys at high temperature using the ring compression test and genetic algorithm method
- Study on hot deformation behavior and workability of stir-cast Al6063-6wt.% steelp based composites
- The effects of processing parameters on the formation of oxide layers in aluminium alloys using plasma electrolytic oxidation technique
- Behavior of green reactive powder mortar reinforced with steel fibers
- On the hygrothermal properties of sandcrete blocks produced with sawdust as partial replacement of sand
- Mechanical behavior of thin-walled steel under hard contact with rigid seabed rock: Theoretical contact approach and nonlinear FE calculation
- Mechanical properties and microstructural characteristics of rotary friction welded dissimilar joints of rolled homogeneous armor steel and medium carbon steel
- Studies of carboxylated nitrile butadiene rubber/butyl reclaimed rubber (XNBR/BRR) blends for shoe soles application
- Mechanical properties of wire arc additive manufactured carbon steel cylindrical component made by gas metal arc welding process
- Synthesis and mechanical characterization of Si3N4 reinforced copper-tin matrix composites
- Analysis of plated-hull structure strength against hydrostatic and hydrodynamic loads: A case study of 600 TEU container ships
- Mechanical performance investigation of lignocellulosic coconut and pomegranate / LDPE biocomposite green materials
- Special Issue MICAP-2021
- Double hydrothermal synthesis of iron oxide/silver oxide nanocomposites with antibacterial activity**
- Enhanced photocatalytic activity of TiO2-CdS composite nanofibers under sunlight irradiation**
- Structural properties of CoxCu1−xFe2O4 solid solution**
- Green-synthesis of Ag2O nanoparticles for antimicrobial assays**
- Effect of current density on the porous silicon preparation as gas sensors**
- A mechanochemical preparation, properties and kinetic study of kaolin–N, P fertilizers for agricultural applications**
- Impact strength of surface treated SS316L wires reinforced PMMA**
- Computational studies on electronic and optical properties of dopamine derivatives structure: A DFT study**
- Multilayer coating effects on the thermal conductivity of tools using an electric furnace technique**
- The positron and mechanical parameters of a cold-worked aluminum alloy (3004) Using PALT, PADBT and HV**
- Effect of thermal annealing on the structural and optical properties of TiO2 nanostructures**
- Improvement of forging die life by failure mechanism analysis**
Artikel in diesem Heft
- Research Articles
- The elastic-plastic properties of an anti-icing coating on an aluminum alloy: Experimental and numerical approach
- Optimization of recycled slag-fresh flux mixture based upon weld bead quality for submerged arc welding of stainless steel
- Design and optimization of differential capacitive micro accelerometer for vibration measurement
- Mechanical performance of abrasive sandpaper made with palm kernel shells and coconut shells
- Experimental investigation of WEDM process through integrated desirability and machine learning technique on implant material
- Mechanical properties and microstructural characteristics of rotating arc-gas metal arc welded carbon steel joints
- Assessment of cement replacement with fine recycled rubber particles in sustainable cementitious composites
- Structural response and sensitivity analysis of granular and asphaltic overlayment track considering linear viscoelastic behavior of asphalt
- Unmanned aerial vehicle evasion manoeuvres from enemy aircraft attack
- Effect of corrosion on surface degradation of galvanized steel in poultry dung, pig dung and urea solutions using rice straw as an inhibitor
- Mathematical modeling of AZ30 magnesium alloys at high temperature using the ring compression test and genetic algorithm method
- Study on hot deformation behavior and workability of stir-cast Al6063-6wt.% steelp based composites
- The effects of processing parameters on the formation of oxide layers in aluminium alloys using plasma electrolytic oxidation technique
- Behavior of green reactive powder mortar reinforced with steel fibers
- On the hygrothermal properties of sandcrete blocks produced with sawdust as partial replacement of sand
- Mechanical behavior of thin-walled steel under hard contact with rigid seabed rock: Theoretical contact approach and nonlinear FE calculation
- Mechanical properties and microstructural characteristics of rotary friction welded dissimilar joints of rolled homogeneous armor steel and medium carbon steel
- Studies of carboxylated nitrile butadiene rubber/butyl reclaimed rubber (XNBR/BRR) blends for shoe soles application
- Mechanical properties of wire arc additive manufactured carbon steel cylindrical component made by gas metal arc welding process
- Synthesis and mechanical characterization of Si3N4 reinforced copper-tin matrix composites
- Analysis of plated-hull structure strength against hydrostatic and hydrodynamic loads: A case study of 600 TEU container ships
- Mechanical performance investigation of lignocellulosic coconut and pomegranate / LDPE biocomposite green materials
- Special Issue MICAP-2021
- Double hydrothermal synthesis of iron oxide/silver oxide nanocomposites with antibacterial activity**
- Enhanced photocatalytic activity of TiO2-CdS composite nanofibers under sunlight irradiation**
- Structural properties of CoxCu1−xFe2O4 solid solution**
- Green-synthesis of Ag2O nanoparticles for antimicrobial assays**
- Effect of current density on the porous silicon preparation as gas sensors**
- A mechanochemical preparation, properties and kinetic study of kaolin–N, P fertilizers for agricultural applications**
- Impact strength of surface treated SS316L wires reinforced PMMA**
- Computational studies on electronic and optical properties of dopamine derivatives structure: A DFT study**
- Multilayer coating effects on the thermal conductivity of tools using an electric furnace technique**
- The positron and mechanical parameters of a cold-worked aluminum alloy (3004) Using PALT, PADBT and HV**
- Effect of thermal annealing on the structural and optical properties of TiO2 nanostructures**
- Improvement of forging die life by failure mechanism analysis**

