Abstract
Crystallography information files (CIF) were designed formed CoxCu1−xFe2O4 solid solution with the substitution factor x=0 to 1 with an increment of 0.1 depending on Vegard's law by using crystallography software. The effect of the substitution factor has been studied on some parameters and properties of the Co-Cu ferrite system, such as the effect of substitution factor on the lattice parameter, the volume of unit cell, and the density of the unit cell. Also, XRD patterns were estimated by crystallography software depending on the mathematical models of XRD. The XRD results showed a slight shift in the peak position varying with the substitution factor, these are due to the change in lattice parameter caused by the substitution of ions with different ionic radii. XRD also showed an increment in peak intensity varying with the substitution factor, that's due to an increase in the concentration of Cu which led to an increase in the density of electrons.
1 Introduction
Ferritesare ferromagnetic oxide have a wide range of applications in material science due to their magnetic properties [1, 2]. ferrite is classified as hard and soft ferrite depending on its permanent magnetization and the value of the coercive field [2, 3]. The hard ferrite usually has a hexagonal crystal structure (space group P63/mmc) [4, 5, 6], Hexa-ferrites have various technological and industrial applications in magnetic recording media, magnets, frequency devices, microwave devices due to their properties such as high Curie temperature and coercively, huge magnetization [5]. Strontium hex ferrite (SrFe12O19) and barium hexa-ferrite are the most famous material belong to this type [4, 7]. The soft ferrite is a material with a spinel structure with the formula AB2X4 where A and B are cations while X is an anion like oxygen, sulphur, selenium, and Tellurium [8, 9]. for the ferrite Spinel X is an oxygen O−2 and the trivalent anion is Iron Fe+3. The A-site is located inside the tetrahedral void while B-site is located inside the octahedral void. The spinel ferrite has a cubic crystal structure (space group) [10, 11]. the soft ferrite has low coercively and high magnetization, it has 4 octahedral voids and 8 tetrahedral voids. Type of spinel ferrite and the net magnetization are determined according to the orientation of the voids, the most popular spinel ferrite is ferrimagnetic and antiferro-magnetic which has the 180-degree domain [12, 13]. Also, spinel ferrite is classified as normal ferrite with the formula AFe2O4 and the inverse spinel ferrite with the formula [Fe+3]Tetrahedral void[Fe+3A+2]Octahedral voidO4 according to the Crystal field stabilization energy (CFSE)[14,15,16] and the spin state in the d orbital of the A and B sites of the spinel [15, 17]. In this study, it will focus on CoFe2O4 which has an inverse spinel and substitute of Cu with various concentration and its effect on the crystal structure, XRD, and density.
2 Method
In this work, crystallography information file(CIF) number COD#1533163 from crystallography open database was used to build CoxCu1−xFe2O4 solid solution by substitution of Cu+2 with Co+2 by factor x using crystallography software [18, 19] i.e. the occupancy of the Cobalt is changed by factor x and adding new Cu+2 at the same position of Cobalt by a factor (1-x). the lattice parameter was calculated according to Vegard's Law as shown in the following equation [20].
where a(AxB1−x) is the lattice parameter for the solid solution, aA is the lattice parameter for the A site atom, and aB is the lattice parameter for the B-site atom, and x is the substitution factor (for this study x=0, 0.1, 0.2 . . . 1). The solid solution for this study is CoxCu1−xFe2O4 of based on two compounds, the first is CoFe2O4 with lattice a=8.3806 Å [21] and the second was CuFe2O4 with lattice a=8.37 Å [22]. Also, both Cobalt ferrite and copper ferrite have the same crystal structure and the same space group (Fd-3m).
2.1 Estimation of X-ray diffraction pattern (XRD)
The XRD pattern was estimated using VESTA software [18] and Mercury software [23, 24, 25], the intensity of the XRD pattern was calculated using the following equation [24]
Where Ihkl is the intensity of peak diffracted from hkl lattice plane, hkl are the Miller indices, S is the scaling factor, Lp(2Θ) is the Lorentzian polarization factor, A is the X-ray absorption factor, Phkl is the preferred orientation factor, and Fhkl is the structural factor. The XRD pattern intensity depends on many factors as described in Eq. (2), it depends on the nature X-ray source and the technique of generation of X-ray and many factors but the intensity of the peak mainly depend on the structural factor as shown in Eq. (3) [24]
The structural factor depends on many factors; these are shown in Eq. (4) [24]
Where the summation is over all atoms (n), fn is the X-ray form factor which is a function of 2Θ and the atomic number Z, On is site occupation or the factor of the coordinate of atoms, Exp(2πi(hx+ky+lz) is the interface term which is a vector product between the position of atoms in the unit cell and a scatter vector of the hkl plane, and Wn is Debye-Waller factor or the atomic displacement factor which is caused by thermal vibration. All the previous terms affect the intensity of the XRD peak, so special crystal structure software were used to calculate the structural factor and the peak intensity and finally plotting the XRD pattern.
The relationship between the structural factor and the electron density in any plane is shown in Eq. (5). [18]
Where ρxyz is the density of electron in the plane causing the diffraction.
2.2 Estimation of density
The density of the built CIFs is calculated using crystal maker software according to Eq. (5) [26, 27]
Where ρ is the density in g/cm3, n is the number of atom per unit cell, M is the Molar Mass of the atoms, V is the volume of unit cell and Na is Avogadro's number (Na=6.022 × 1023 mol−1).
3 Result and discussion
The properties of solid solution CoxCu1−xFe2O4 depend on the value of the substitution factor, the CIF file for CoxCu1−xFe2O4 is shown in Figure 1.
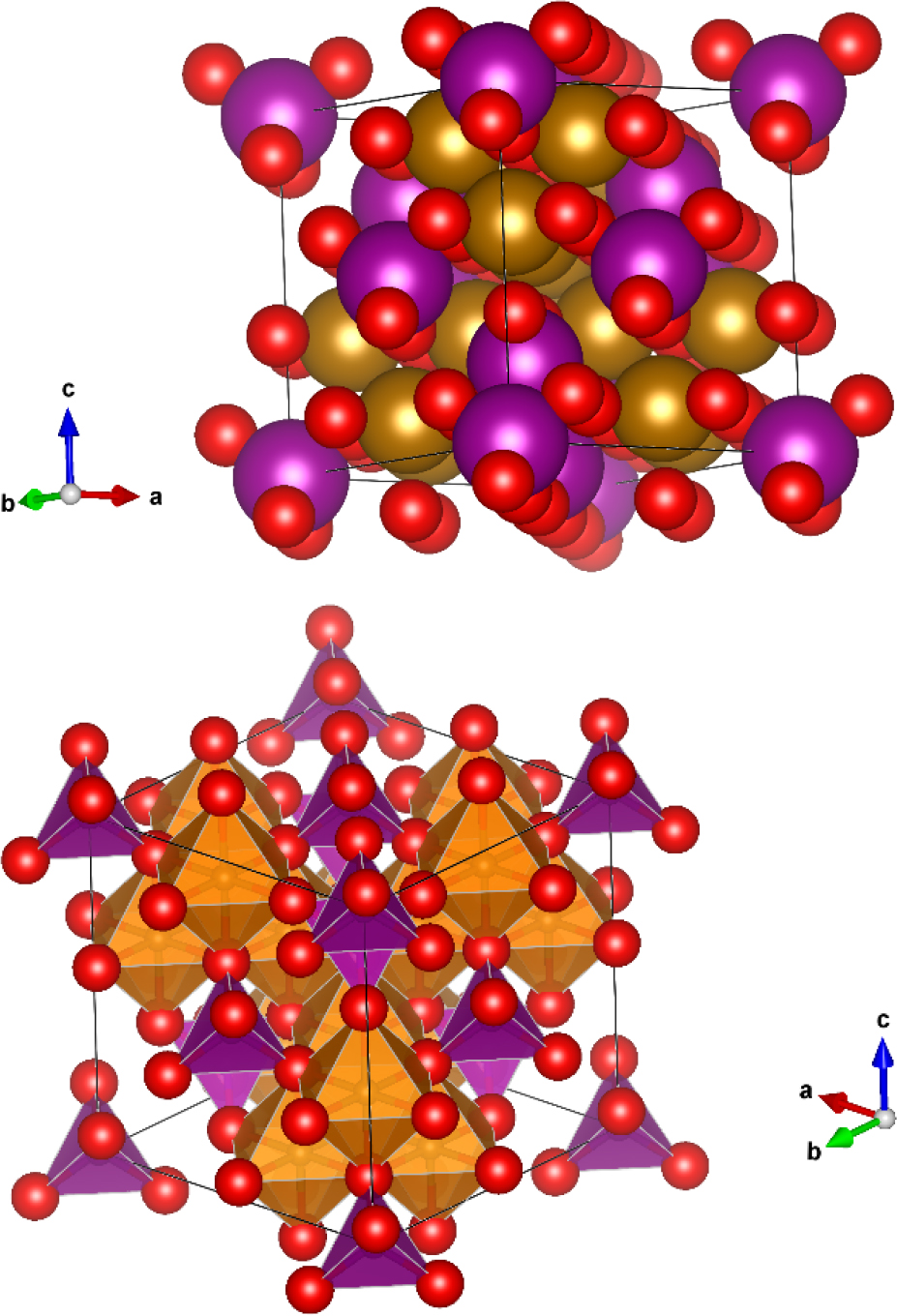
Crystal structure of CoxCu1−xFe2O4.
The substitution of copper ion was performed depending on Vegard's Law, the occupancy of both A and B sites of the composition (i.e. Co and Cu in AB2O4) because the cobalt ferrite is an inverse spinel of Co+2 occupied in both A and B sites, Table 1 shows the process of substitution of Cu+2 ion with Co+2 with their Wyckoff position and coordinates of atoms.
Substitution of Cu+2 with Co+2 in AB2X4 system to build CoxCu1−xFe2O4.
| Site | Atom | Composition | Occupancy | Wyckoff position | Coordinate | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A-Site | Fe;Co;Cu | 0.627Fe+3 +(0.373 x).Co+2 +(0.373[1-x]).Cu+2 | 1 | ||||
| Fe+3 | 0.627 | 16d | 0.5 | 0.5 | 0.5 | ||
| Co+2 | 0.373 . x | 16d | 0.5 | 0.5 | 0.5 | ||
| Cu+2 | 0.373. (1-x) | 16d | 0.5 | 0.5 | 0.5 | ||
| B-site | Fe;Co;Cu | 0.745Fe+3 +(0.255x).Co+2 +(0.255[1-x]).Cu+2 | 1 | ||||
| Fe+3 | 0.745 | 8a | 0.125 | 0.125 | 0.125 | ||
| Co+2 | 0.255 . x | 8a | 0.125 | 0.125 | 0.125 | ||
| Cu+2 | 0.255 . (1-x) | 8a | 0.125 | 0.125 | 0.125 | ||
| X-site | O−2 | O−2 | 1 | 32e | 0.2566 | 0.2566 | 0.2566 |
Both Co+2 and Cu+2 have the same position in the crystal because both of them have the same oxidation state (i.e. +2) with different ionic radii, For Co+2 the ionic radii are 0.58 Å for the A-site (for Co+2 inside the tetrahedral void with coordination number IV) while for the Co+2 in B-site is 0.745 Å (for Co+2 inside the octahedral void with coordination number VI) [28, 29]. The ionic radii for Cu+2 is 0.57 Å for the A-site while for Cu+2 in B-site is 0.73 Å [28, 30]. In general, Co+2 radius is slightly greater than Cu+2 in about 0.01–0.012 Å, this is the reason for variation in the lattice parameter of the solid solution, the lattice parameter of CoFe2O4 is a=8.3806 Å which is slightly greater than for CuFe2O4 a=8.37 Å (i.e. the difference is about 0.01 Å). Figure 2. Shows the variation of the lattice parameter and lattice volume with the substitution factor.

a) Variation of lattice parameter with substitution factor(x); b) Variation of lattice volume with substitution factor(x).
The lattice parameter and volume of unit cell increase slightly with the substitution factor x due to the difference in ionic radii. The volume of unit cell varying with x is estimated in Eq. (7).
3.1 X-ray diffraction pattern (XRD)
The calculated XRD patterns showed that there is a small shift in the peak position for all peaks. The shifting in peak position increase slightly with decreasing the substitution factor x, that's because the increasing of lattice parameter leads to an increase in the d spacing, and the increase in d space leads to a decrease in the 2Θ position according to Bragg's law [31]. Figure 3: Shows the XRD patterns calculated using Mercury software.
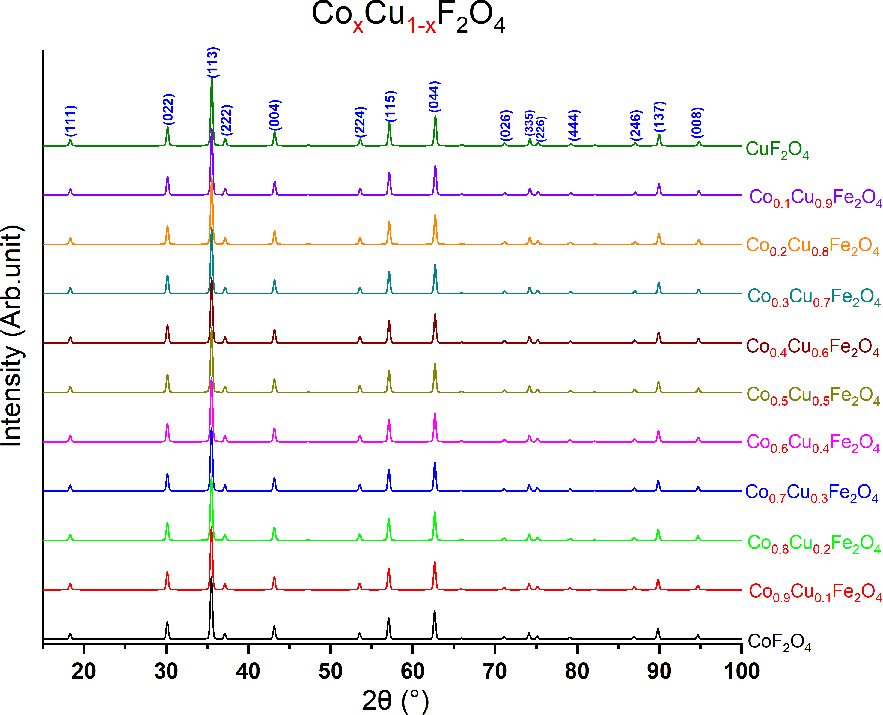
XRD patterns for CoxCu1−xFe2O4.
The second result of the XRD is a very slight shift in the value of the intensity varying with the substitution factor. Figures 4a–q shows the variation of peak intensity with the substitution factor on the y-axis. It's known that the X-ray is scattered or diffracted from electronic clouds, which means for any peak the more intensity, the more electron density in that plane caused the diffraction. By decreasing the substitution factor the peak intensity increase i.e. increasing Cu+2 concentration lead to increase peak intensity, which means more density of electrons or the probability of finding electrons in this plane increase [11, 30, 32]. By comparing the last result with the atomic number of Co which has an atomic number equal to 27 while for Cu is equal to 29, that means 27 electrons for Co and 29 electrons for Cu [33]. The variation of intensity is affected by the ratio of electron density of both Co/Cu i.e. 27/29=0.931 multiplied by the substitution factor the last result is also agreed with Vegard's Law [30, 34].

Intensity of for CoxCu1−xFe2O4 peaks (a): 111 peak; (b): 022 peak; (c): 113 peak; (d): 222 peak; (e): 004 peak; (f): 224 peak; (g): 115 peak; (h): 044 peak; (i): 135 peak; (j): 026 peak; (k): 335 peak; (l): 444 peak; (m): 155 peak; (n): 246 peak; (o): 137 peak; (p): 008 peak; (q): 337 peak
The XRD peak intensity is directly proportion with the square of the structure factor (Ihkl ~ F2hkl), also the structural factor is direct proportion to the density of electron of the plane cased the diffraction (Fhkl ~ ρxyz) so the intensity of the peak is proportional to the density of electrons for any plane caused the diffraction (Ihkl ~ (ρxyz)2) [18]. The distribution of the density of electrons is shown in Figure 5a–q for the lattice planes caused the diffraction. The distribution of electrons inside the lattice depends on many factors such as the type of bonds between atoms, the position of atoms in the plane of lattice, the atomic number of the basis, and the type of the lattice. In Figure 5 the density distribution occurs in the red region with a probability of 100%. The scale bares in Figure 5. Is between 0–1 from blue to red which refers to the probability of finding electrons from 0% at blue to 100% at the red region, i.e. XRD occurs from the red region only, and the intensity of the XRD peak indicates that the diffraction occurs from the red region of the crystal planes of the lattice rather than from that completely plane.

Electron density of different planes for CoxCu1−xFe2O4 peaks (a):111 plane; (b): 022 plane; (c): 113 plane; (d):222 plane; (e):004 plane; (f): 224 plane; (g): 115 plane; (h):044 plane; (i):135 plane; (j): 026 plane; (k): 335 plane; (l):444 plane; (m): 155 plane; (n):246 plane; (o): 137 plane; (p):008 plane; (q):337 plane.
3.2 Density estimation
The density of the CIF files was calculated using crystal maker software, it is known that the density of the unit cell is in direct proportion to the mass and inversely proportional to the volume of unit cell. The density of the solid solution depends on the reduced mass of solid solution (dependent on Vegard's law). The volume of unit cell varied with the substitution factor because V=a3 for the cubic lattice and the lattice also varied with the substitution factor according to dependence on Vegard's law. The results for the density of the CoxCu1−xFe2O4 is shown in Figure 6. The results showed that the density decrease the substitution factor, this behavior of the density of the solid solution is due to the increment in volume of unit cell with the substitution factor (ρ ~ 1/V, V ~ x, so increase x lead to decrease density). Also, the density of solid solution depends on the variation of molar masses with the substitution factor (ρ ~ M, M ~ 1/x, so increase x leads to a decrease in the density). The equation for the density of the CoxCu1−xFe2O4 solid solution is shown in Eq. (8).

Density of CoxCu1−xFe2O4 solid solution.
4 Conclusion
The XRD showed a slight shift in peak position due to the change in the lattice size, and a slight change in the peak intensity due to the change in concentration of Cu that leads to an increase in the density of electrons. Also, the density of the solid solution decreases linearly with the substitution factor. These results must be taken into consideration in studying the solid solution and in the experimental design of the Co-Cu ferrite solid solution.
Funding information:
The authors state no funding involved.
Author contributions:
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Conflict of interest:
The authors state no conflict of interest.
References
[1] Hadjipanayis GC, editor. Magnetic Hysteresis in Novel Magnetic Materials. Springer Netherlands; 1997. https://doi.org/10.1007/978-94-011-5478-9.10.1007/978-94-011-5478-9Search in Google Scholar
[2] Narlikar AV, editor. Frontiers in Magnetic Materials. Springer Berlin Heidelberg; 2005. https://doi.org/10.1007/b138873.10.1007/b138873Search in Google Scholar
[3] Drujba I. Solid State Magnetic Sensors, 1st Editio. Elsevier Science Ltd; 1994.Search in Google Scholar
[4] Pullar RC. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog Mater Sci. 2012;57(7):1191–334.10.1016/j.pmatsci.2012.04.001Search in Google Scholar
[5] Soria GD, Jenus P, Marco JF, Mandziak A, Sanchez-Arenillas M, Moutinho F, et al. Strontium hexaferrite platelets: a comprehensive soft X-ray absorption and Mössbauer spectroscopy study. Sci Rep. 2019;9(1):11777.10.1038/s41598-019-48010-wSearch in Google Scholar PubMed PubMed Central
[6] Li Q, Song J, Saura-Múzquiz M, Besenbacher F, Christensen M, Dong M. Magnetic Properties of Strontium Hexaferrite Nanostructures Measured with Magnetic Force Microscopy. Sci Rep. 2016;6(1):25985.10.1038/srep25985Search in Google Scholar PubMed PubMed Central
[7] Nga NK, Hong PT, Lam TD, Huy TQ. A facile synthesis of nanostructured magnesium oxide particles for enhanced adsorption performance in reactive blue 19 removal. J Colloid Interface Sci. 2013;398:210–6.10.1016/j.jcis.2013.02.018Search in Google Scholar PubMed
[8] Xiong Z, Huang L, Peng J, Hou Y, Ding Z, Wang S. Spinel-Type Mixed Metal Sulfide NiCo2S4 for Efficient Photocatalytic Reduction of CO2 with Visible Light. ChemCatChem. 2019;11(22):5513–8.10.1002/cctc.201901379Search in Google Scholar
[9] Beal JH, Prabakar S, Gaston N, Teh GB, Etchegoin PG, Williams G, et al. Synthesis and comparison of the magnetic properties of iron sulfide spinel and iron oxide spinel nanocrystals. Chem Mater. 2011;23(10):2514–7.10.1021/cm2002868Search in Google Scholar
[10] Al-Tabbakh AA, Karatepe N, Al-Zubaidi AB, Benchaabane A, Mahmood NB. Crystallite size and lattice strain of lithiated spinel material for rechargeable battery by X-ray diffraction peak-broadening analysis. Int J Energy Res. 2019;43(5):1903–11.10.1002/er.4390Search in Google Scholar
[11] Abdel Maksoud MI, El-Sayyad GS, Abd Elkodous M, Awed AS. Controllable synthesis of Co1−xMxFe2O4 nanoparticles (M = Zn, Cu, and Mn; x = 0.0 and 0.5) by cost-effective sol–gel approach: analysis of structure, elastic, thermal, and magnetic properties. J Mater Sci Mater Electron. 2020;31(12):9726–41.10.1007/s10854-020-03518-0Search in Google Scholar
[12] Raghavender AT. How to Make Zinc Ferrites Become Ferromagnetic. Nano-Sized Multifunct Mater Synth Prop Appl. 2018;165–205.10.1016/B978-0-12-813934-9.00008-6Search in Google Scholar
[13] Mørup S, Hansen MF, Frandsen C. Magnetic nanoparticles. Compr Nanosci Nanotechnol. 2019;89–140.10.1016/B978-0-12-374396-1.00036-2Search in Google Scholar
[14] Wang T, Zheng Y, Yu G, Chen X. Glycolysis of polyethylene terephthalate: magnetic nanoparticle CoFe2O4 catalyst modified using ionic liquid as surfactant. Eur Polym J. 2021;155:110590.10.1016/j.eurpolymj.2021.110590Search in Google Scholar
[15] Hou YH, Zhao YJ, Liu ZW, Yu HY, Zhong XC, Qiu WQ, et al. Structural, electronic and magnetic properties of partially inverse spinel CoFe2O4: A first-principles study. J Phys D Appl Phys. 2010;43(44):445003.10.1088/0022-3727/43/44/445003Search in Google Scholar
[16] Hashim M, Alimuddin S, Kumar S, Koo BH, Shirsath SE, Mohammed EM, et al. Kumar, B.H. Koo, S.E. Shirsath, E.M. Mohammed, J. Shah, R.K. Kotnala, H.K. Choi, H. Chung, R. Kumar, Structural, electrical and magnetic properties of Co-Cu ferrite nanoparticles. J Alloys Compd. 2012;518:11–8.10.1016/j.jallcom.2011.12.017Search in Google Scholar
[17] Choudary GS, Prameela P, Varma MC, Kumar AM, Rao KH. Contribution to Analysis of Co/Cu Substituted Ni-Zn Ferrites, Indian. J Mater Sci. 2013;2013:1–7.10.1155/2013/350707Search in Google Scholar
[18] Momma K, Izumi F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J Appl Cryst. 2008;41(3):653–8.10.1107/S0021889808012016Search in Google Scholar
[19] Izumi F, Momma K. Three-dimensional visualization in powder diffraction. Diffus Defect Data Solid State Data Pt B Solid State Phenom. 2007;130:15–20.10.4028/3-908451-40-x.15Search in Google Scholar
[20] Jacob KT, Raj S, Rannesh L. Vegard's law: A fundamental relation or an approximation? Int J Mater Res. 2007;98(9):776–9.10.3139/146.101545Search in Google Scholar
[21] Ferreira TA, Waerenborgh JC, Mendonça MH, Nunes MR, Costa FM. Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 2003;5(2):383–92.10.1016/S1293-2558(03)00011-6Search in Google Scholar
[22] Samadashvili ID, Varazashvili VS, MacHaladze TE, Pavlenishvili TA. Thermodynamic functions of Cu1−XZnxFe2O4 ferrite solid solutions from 300 to 900 K. Inorg Mater. 2002;38(11):1186–8.10.1023/A:1020987104575Search in Google Scholar
[23] Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, et al. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr B. 2002;58(Pt 3 Pt 1):389–97.10.1107/S0108768102003324Search in Google Scholar
[24] Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, et al. Mercury: visualization and analysis of crystal structures. J Appl Cryst. 2006;39(3):453–7.10.1107/S002188980600731XSearch in Google Scholar
[25] Macrae CF, Sovago I, Cottrell SJ, Galek PT, McCabe P, Pidcock E, et al. Mercury 4.0: from visualization to analysis, design and prediction. J Appl Cryst. 2020;53(Pt 1):226–35.10.1107/S1600576719014092Search in Google Scholar
[26] Ellner M, Reule H, Mittemeijer EJ. Unit cell parameters and densities of the gadolinium dihydride GdH2+x. J Alloys Compd. 1998;279(2):179–83.10.1016/S0925-8388(98)00681-1Search in Google Scholar
[27] Plocher J, Panesar A. Effect of density and unit cell size grading on the stiffness and energy absorption of short fibre-reinforced functionally graded lattice structures. Addit Manuf. 2020;33:101171.10.1016/j.addma.2020.101171Search in Google Scholar
[28] Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A. 1976;32(5):751–67.10.1107/S0567739476001551Search in Google Scholar
[29] Balavijayalakshmi J, Suriyanarayanan N, Jayaprakash R, Gopalakrishnan V. Effect of concentration on dielectric properties of Co-Cu ferrite nano particles. Phys Procedia. 2013;49–57.10.1016/j.phpro.2013.10.010Search in Google Scholar
[30] Rani BJ, Saravanakumar B, Ravi G, Ganesh V, Ravichandran S, Yuvakkumar R. Structural, optical and magnetic properties of CuFe2O4 nanoparticles. J Mater Sci Mater Electron. 2018;29(3):1975–84.10.1007/s10854-017-8108-7Search in Google Scholar
[31] Mahmood NB, Al-Shakarchi EK. Dielectric properties of BNT-x BT prepared by hydrothermal process. J Adv Dielectr. 2017;07(3):1750019.10.1142/S2010135X17500199Search in Google Scholar
[32] Hamodi A, Hökelek T, Hamodi YI, Mahmood NB, Nakamori N. Restorations of fresh surfaces for topological materials by de-capping Te. Appl Surf Sci. 2020;530:147225.10.1016/j.apsusc.2020.147225Search in Google Scholar
[33] Dippong T, Deac IG, Cadar O, Levei EA, Petean I. Impact of Cu2+ substitution by Co2+ on the structural and magnetic properties of CuFe2O4 synthesized by sol-gel route. Mater Charact. 2020;163:110248.10.1016/j.matchar.2020.110248Search in Google Scholar
[34] Singh C, Bansal S, Kumar V, Tikoo KB, Singhal S. Encrustation of cobalt doped copper ferrite nanoparticles on solid scaffold CNTs and their comparison with corresponding ferrite nanoparticles: A study of structural, optical, magnetic and photo catalytic properties. RSC Advances. 2015;5(49):39052–6110.1039/C5RA03330FSearch in Google Scholar
© 2021 Natheer B. Mahmood et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The elastic-plastic properties of an anti-icing coating on an aluminum alloy: Experimental and numerical approach
- Optimization of recycled slag-fresh flux mixture based upon weld bead quality for submerged arc welding of stainless steel
- Design and optimization of differential capacitive micro accelerometer for vibration measurement
- Mechanical performance of abrasive sandpaper made with palm kernel shells and coconut shells
- Experimental investigation of WEDM process through integrated desirability and machine learning technique on implant material
- Mechanical properties and microstructural characteristics of rotating arc-gas metal arc welded carbon steel joints
- Assessment of cement replacement with fine recycled rubber particles in sustainable cementitious composites
- Structural response and sensitivity analysis of granular and asphaltic overlayment track considering linear viscoelastic behavior of asphalt
- Unmanned aerial vehicle evasion manoeuvres from enemy aircraft attack
- Effect of corrosion on surface degradation of galvanized steel in poultry dung, pig dung and urea solutions using rice straw as an inhibitor
- Mathematical modeling of AZ30 magnesium alloys at high temperature using the ring compression test and genetic algorithm method
- Study on hot deformation behavior and workability of stir-cast Al6063-6wt.% steelp based composites
- The effects of processing parameters on the formation of oxide layers in aluminium alloys using plasma electrolytic oxidation technique
- Behavior of green reactive powder mortar reinforced with steel fibers
- On the hygrothermal properties of sandcrete blocks produced with sawdust as partial replacement of sand
- Mechanical behavior of thin-walled steel under hard contact with rigid seabed rock: Theoretical contact approach and nonlinear FE calculation
- Mechanical properties and microstructural characteristics of rotary friction welded dissimilar joints of rolled homogeneous armor steel and medium carbon steel
- Studies of carboxylated nitrile butadiene rubber/butyl reclaimed rubber (XNBR/BRR) blends for shoe soles application
- Mechanical properties of wire arc additive manufactured carbon steel cylindrical component made by gas metal arc welding process
- Synthesis and mechanical characterization of Si3N4 reinforced copper-tin matrix composites
- Analysis of plated-hull structure strength against hydrostatic and hydrodynamic loads: A case study of 600 TEU container ships
- Mechanical performance investigation of lignocellulosic coconut and pomegranate / LDPE biocomposite green materials
- Special Issue MICAP-2021
- Double hydrothermal synthesis of iron oxide/silver oxide nanocomposites with antibacterial activity**
- Enhanced photocatalytic activity of TiO2-CdS composite nanofibers under sunlight irradiation**
- Structural properties of CoxCu1−xFe2O4 solid solution**
- Green-synthesis of Ag2O nanoparticles for antimicrobial assays**
- Effect of current density on the porous silicon preparation as gas sensors**
- A mechanochemical preparation, properties and kinetic study of kaolin–N, P fertilizers for agricultural applications**
- Impact strength of surface treated SS316L wires reinforced PMMA**
- Computational studies on electronic and optical properties of dopamine derivatives structure: A DFT study**
- Multilayer coating effects on the thermal conductivity of tools using an electric furnace technique**
- The positron and mechanical parameters of a cold-worked aluminum alloy (3004) Using PALT, PADBT and HV**
- Effect of thermal annealing on the structural and optical properties of TiO2 nanostructures**
- Improvement of forging die life by failure mechanism analysis**
Articles in the same Issue
- Research Articles
- The elastic-plastic properties of an anti-icing coating on an aluminum alloy: Experimental and numerical approach
- Optimization of recycled slag-fresh flux mixture based upon weld bead quality for submerged arc welding of stainless steel
- Design and optimization of differential capacitive micro accelerometer for vibration measurement
- Mechanical performance of abrasive sandpaper made with palm kernel shells and coconut shells
- Experimental investigation of WEDM process through integrated desirability and machine learning technique on implant material
- Mechanical properties and microstructural characteristics of rotating arc-gas metal arc welded carbon steel joints
- Assessment of cement replacement with fine recycled rubber particles in sustainable cementitious composites
- Structural response and sensitivity analysis of granular and asphaltic overlayment track considering linear viscoelastic behavior of asphalt
- Unmanned aerial vehicle evasion manoeuvres from enemy aircraft attack
- Effect of corrosion on surface degradation of galvanized steel in poultry dung, pig dung and urea solutions using rice straw as an inhibitor
- Mathematical modeling of AZ30 magnesium alloys at high temperature using the ring compression test and genetic algorithm method
- Study on hot deformation behavior and workability of stir-cast Al6063-6wt.% steelp based composites
- The effects of processing parameters on the formation of oxide layers in aluminium alloys using plasma electrolytic oxidation technique
- Behavior of green reactive powder mortar reinforced with steel fibers
- On the hygrothermal properties of sandcrete blocks produced with sawdust as partial replacement of sand
- Mechanical behavior of thin-walled steel under hard contact with rigid seabed rock: Theoretical contact approach and nonlinear FE calculation
- Mechanical properties and microstructural characteristics of rotary friction welded dissimilar joints of rolled homogeneous armor steel and medium carbon steel
- Studies of carboxylated nitrile butadiene rubber/butyl reclaimed rubber (XNBR/BRR) blends for shoe soles application
- Mechanical properties of wire arc additive manufactured carbon steel cylindrical component made by gas metal arc welding process
- Synthesis and mechanical characterization of Si3N4 reinforced copper-tin matrix composites
- Analysis of plated-hull structure strength against hydrostatic and hydrodynamic loads: A case study of 600 TEU container ships
- Mechanical performance investigation of lignocellulosic coconut and pomegranate / LDPE biocomposite green materials
- Special Issue MICAP-2021
- Double hydrothermal synthesis of iron oxide/silver oxide nanocomposites with antibacterial activity**
- Enhanced photocatalytic activity of TiO2-CdS composite nanofibers under sunlight irradiation**
- Structural properties of CoxCu1−xFe2O4 solid solution**
- Green-synthesis of Ag2O nanoparticles for antimicrobial assays**
- Effect of current density on the porous silicon preparation as gas sensors**
- A mechanochemical preparation, properties and kinetic study of kaolin–N, P fertilizers for agricultural applications**
- Impact strength of surface treated SS316L wires reinforced PMMA**
- Computational studies on electronic and optical properties of dopamine derivatives structure: A DFT study**
- Multilayer coating effects on the thermal conductivity of tools using an electric furnace technique**
- The positron and mechanical parameters of a cold-worked aluminum alloy (3004) Using PALT, PADBT and HV**
- Effect of thermal annealing on the structural and optical properties of TiO2 nanostructures**
- Improvement of forging die life by failure mechanism analysis**

